Abstract
This study provides new information on the effects of various concentrations of the trace metals copper, lead, zinc, cadmium, and nickel on fertilization success of gametes from the scleractinian reef corals Goniastrea aspera,Goniastrea retiformis, Acropora tenuis, and Acropora longicyathus. The EC50 values (the concentration that reduces the fertilization rate by 50% relative to the control fertilization) for copper effects on fertilization success of these coral species range from 15 to 40 µg/L, which is similar to responses of other marine invertebrates. The EC50 values for lead were 1450–1800 µg/L for the Acropora species, and >2400 µg/L for G. aspera gametes, which indicates that lead was much less toxic than copper. Fertilization responses to zinc and nickel were variable and a significant reduction in fertilization success for A. tenuis gametes was found only at very high cadmium concentrations. The data from this study and other recent research clearly demonstrate that some trace metals impair the fertilization success of gametes from faviid and acroporiid reef corals. Trace metal inputs into reef waters should be limited and controlled to avoid potential interference with sexual reproductive processes of reef corals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fertilization success in broadcast spawning corals is dependant upon gamete interaction within the water column, and thus can be vulnerable to changes in water quality. Repeated successful spawning years are necessary for the long-term maintenance of reef coral populations (Harrison et al. 1984; Harrison and Wallace 1990), and therefore, determining the effects of pollutants on fertilization success is important for the management of coral reefs. A few previous studies have used coral fertilization success as a tool for determining stress effects on corals (Heyward 1988; Richmond 1993; Harrison 1994, 1995; Gulko 1996; Reichelt-Brushett and Harrison 1999; Harrison and Ward 2001; Negri and Heyward 2001). The use of fertilization success to determine the effects of trace metals on corals is limited to three previous studies. Heyward (1988) studied the effects of copper and zinc sulphates on fertilization rates in Favites chinensis and Platygyra ryukyuensis and provided the first published data on the effects of trace metals on fertilization success of scleractinian corals. Reichelt-Brushett and Harrison (1999) investigated the effects of copper and lead on the fertilization success of Goniastrea aspera gametes. Negri and Heyward (2001) subsequently investigated the effects of copper and tributyltin on the fertilization success of Acropora millepora.
The fertilization toxicity test method developed by Harrison for studies on oil pollution (1994) and used by Harrison and Ward for studies on nutrient contamination (2001), and Reichelt-Brushett and Harrison (1999) for pilot studies on trace metals in 1994 was also used in this study. The results provide new information on the effects of various concentrations of the trace metals copper, lead, zinc, cadmium, and nickel on fertilization success of gametes from species in two families of scleractinian reef corals (Faviidae and Acroporidae). Corals of these families occur in many tropical regions throughout the Indo-Pacific (Veron 2000), and there are few ecotoxicological studies of tropical organisms compared to studies on temperate organisms (Peters et al. 1997). Coral reefs are found in many tropical countries including some of the poorest nations in the world (Lacher and Goldstein 1997). These tests provide ecotoxicological data for environmentally sensitive species that occur in many of these locations.
Trace metals enter the marine waters of tropical environments naturally from riverine input (Oȁ9Neill 1985; Mance 1987). There are many anthropogenic sources of trace metals including mining operations (e.g. Brown 1987), urban discharge (e.g. Gonzàlez 1990; Scott 1990; Guzmàn and Jiménez 1992), agricultural runoff (e.g. Guzmàn and Jiménez 1992), desalination plants (e.g. Johannes 1975), dredging (e.g. Reichelt and Jones 1994), marinas and ports (e.g. Reichelt and Jones 1994), and toxic waste and marine dumping (e.g. Richmond 1993). Since scleractinian reef corals are the dominant structuring organisms of coral reefs, it is important to understand the potential effects of trace metals on their health and reproductive success.
Methods
Location and spawning dates
The fertilization toxicity tests in this study were completed during 1995 and 1996 at Magnetic Island in the Central section of the Great Barrier Reef Marine Park, Australia. In 1996, further tests were completed at One Tree Island, in the Capricorn Bunker Group of islands in the Southern section of the Great Barrier Reef Marine Park, Australia. This study follows on from the pilot studies completed in 1994 by Reichelt-Brushett and Harrison (1999). Table 1 shows the coral species, locations, and metals used for these 12 fertilization experiments.
The methods of gamete collection for corals differ from established early life stage toxicity tests, mature organisms of many species can be routinely collected from the field, maintained in aquaria and induced to spawn when gametes are required (e.g. McGibbon and Moldan 1986; Neiheisel and Young 1992; Ringwood 1992). The gametogenic cycle in faviid and acroporid corals is normally from 5 to 9 months long (Harrison and Wallace 1990), and corals do not survive well in aquaria. Therefore, corals were collected a few hours prior to spawning during annual coral spawning events in October (nearshore Magnetic Island) and November (offshore One Tree Island). This is an effective method that provides access to large numbers of gametes that have fully matured in natural conditions.
The gamete collection and experimental design was the same as that used by Reichelt-Brushett and Harrison (1999). Spawned egg sperm bundles were collected from several colonies of corals of each species after spawning periods in October or November 1995 and 1996. Eggs and sperm from each colony were separated using gentle rinsing through plankton mesh. The spermatozoa concentration was determined using a haemocytometer (final concentration in the test containers of ∼ ∼2×106 mL−1), then each experiment was set up using 20 mL vials for each replicate. Stock solutions of CuCl2, ZnSO4, Cd(NO3)2, Pb(NO3)2, and NiCl2 were diluted with sperm-free seawater (SFSW) to twice the desired concentration, so the final metal concentration in the 20 mL vials was as desired. Treatments consisted of a seawater control, and various concentrations of trace metals, and each treatment level had five replicate vials. In each experiment the sperm from one coral colony was crossed with the eggs from a different coral colony. For each metal treatment, groups of approximately 100 eggs were carefully pipetted into 8-mm diameter wells filled with SFSW and photographed (actual egg number manually counted at a later date using the photographic image), and then added to 5 mL of SFSW and placed in 20 mL vials. The eggs are buoyant and sit in the wells in one layer on the water surface. Approximately 100 eggs were estimated when the eggs covered about 20% of the surface area of each well. 5 mL of the spermatozoa dilution was placed in separate 20 mL vials. At set time intervals each set of eggs and sperm were dosed with 5 mL of the appropriate metal concentration, or in the control treatments, 5 mL of SFSW was added to the vials. After a 30-min period of metal exposure to eggs and sperm separately, the 10 mL of dosed spermatozoa was added to the dosed eggs and the vials were sealed, placed in a mesh bag and tied to a mooring buoy on the reef. After a 5 h development period, the vials were retrieved from the buoy and examined under an Olympus stereo microscope. Percentage fertilization was determined by counting the total number of divided embryos and undivided eggs present in each vial. Eggs that showed no sign of cleavage formation were counted as unfertilized.
The results for each experiment were compared by calculating the percentage fertilization of the original egg number counted from each photographic image. The data were analysed by one-way ANOVA, which identified significant differences (p≤0.05) in fertilization success among treatments (Sokal and Rohlf 1995). Where the variances about the treatment means were homogeneous, post hoc 'Tukeyȁ9 comparisons were used. However, if the variances were non-homogeneous, then a post hoc' Tamhaneȁ9 method was applied using the SPSS statistics package. The EC50 (the concentration that reduces the fertilization rate by 50% relative to the control fertilization) was estimated by using the Spearman–Karber method for estimating median lethal concentrations in toxicity tests (Hamilton et al. 1977, 1978). The no observed effect concentration (NOEC) was determined for each test by calculating the highest concentration in which percentage fertilization was not significantly different from the control response (Anderson et al. 1991; Hoffman et al. 1995; Wu et al. 1997).
Nominal concentrations from calculated dilutions of trace metals were used in 1995. Samples of each metal dose from all experiments in 1996 were acidified and analysed by Varian 6400Z graphite furnace atomic absorption spectrophotometry (GFAAS) using modifiers and platforms as outlined in the standard analytical methods (Clesceri et al. 1995) after each sample had been diluted five times. The dilution of each sample reduced the potential for interference from the salt water, while the expected metal concentrations in each dose were still within the range of detection by the Varian GFAAS.
Results
The effect of copper on fertilization success
Copper effects on Goniastrea retiformis gametes
Mean fertilization success of G. retiformis gametes in nominal copper concentrations of 2, 5, and 10 µg/L were not significantly different from the control treatments, and mean fertilization success ranged from 91 to 97% (Fig. 1). Copper concentrations of 20 µg/L and above, all had significantly lower mean fertilization success than the control treatments (Table 2). Mean fertilization success was slightly greater in 50 and 100 µg/L copper treatment than in the 20 µg/L copper treatment, however, the lowest fertilization success rate occurred in the highest (250 µg/L) copper treatment.
Copper effects on Goniastrea aspera gametes
Nine different copper concentrations were used to provide more data points for determining the dose/response effect of copper on fertilization success of G. aspera gametes. Figure 1 shows the measured copper concentrations that were determined by GFAAS. Mean fertilization rates in the 4.8 and 12.8 µg/L copper treatments did not differ significantly from the controls, with fertilization rates between 59 and 67%, compared to 67% fertilization success in the controls. The mean fertilization success was significantly lower than the controls in copper treatments of 20.4 µg/L and in all higher copper concentrations. As copper concentrations increased, the fertilization success decreased until at 74.8 µg/L of copper and above, the mean fertilization rate was 1% or less.
Copper effects on Acropora tenuis gametes
The mean fertilization success of A. tenuis gametes appeared to be less affected by the lower concentrations of copper used, compared with other species (Fig. 1). Fertilization success in measured copper concentrations of 4.5, 7.4, 15.6, and 33.5 µg/L did not differ significantly from the control treatments, and mean fertilization success ranged between 73 and 88% in these treatments. A significant reduction in mean fertilization rate compared with controls was not evident until 41.9 µg/L copper. Less than 5% fertilization success occurred at 66.6 µg/L copper, which is similar to the results from copper tests on other coral species (Fig. 1).
Copper effects on Acropora longicyathus gametes
The effect of copper on the fertilization success of A. longicyathus (Fig. 1) was similar to the effects of copper on G. aspera gametes. There was a clear pattern in the results, which showed a reduction in mean fertilization success with increased copper concentrations. The mean fertilization success in copper treatments of 5 and 10 µg/L were not significantly different from the controls. There was a significant (Table 2) reduction in fertilization success in 23.6 µg/L copper, and at all concentrations above this. Copper concentrations of 60.5 µg/L and above had less than 3% mean fertilization success.
The effect of lead on fertilization success
The initial (1995) lead toxicity experiments were done using G. aspera gametes. The results showed that the fertilization success of G. aspera gametes was not lower than 78%, following dosing with lead concentrations up to 1000 µg/L (Fig. 2). Higher concentrations of lead were used in subsequent (1996) experiments, and dose response curves, using measured concentrations of lead, were produced from experiments using gametes from G. aspera, A. tenuis, and A. longicyathus (Fig. 2). A variable response among species was evident. A. longicyathus was the most sensitive species tested, as a significant (Table 2) reduction in mean fertilization success occurred at a lead concentration of 855 µg/L (Fig. 2), compared with controls. A. tenuis had a significant (Table 2) reduction in mean fertilization success compared with controls, at a lead concentration of 1982 µg/L (Fig. 2). In contrast, the mean fertilization success of G. aspera was significantly (Table 2) reduced only at lead concentrations of 6409 µg/L and above.
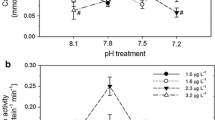
The effect of zinc on fertilization success
The mean fertilization success of A. tenuis gametes in 1996 was significantly reduced (Table 2) at zinc concentrations of 10, 100, and 1000 µg/L, with no fertilization occurring in 5000 µg/L (Fig. 3). Mean fertilization success of A. tenuis gametes in the control treatments was 91%, in the 10 µg/L zinc treatment it was 70%, and in the 100 µg/L zinc treatment the mean fertilization rate dropped to 1%.
The effect of nickel on fertilization success
In the first experiment (1995), mean fertilization success of G. aspera gametes was above 83% in all treatments, which included the controls, 5, 100, 1000, and 2000 µg/L of nickel (Fig. 4a). A significantly (Table 2) lower fertilization rate occurred at 100 µg/L, which was still 91% of the control treatment. No trend of decreased fertilization success occurred with increased nickel concentrations in this experiment. A second experiment was completed on G. aspera gametes in 1996 using the same nickel concentrations (Fig. 4b). In the second test, a significantly (Table 2) lower mean fertilization rate occurred in nickel treatments of 100 µg/L and higher, although fertilization success did not fall below 60% at concentrations up to 2000 µg/L.
The effect of cadmium on fertilization success
A significantly (Table 2) lower mean fertilization rate occurred at nominal cadmium concentrations of 5000 and 10,000 µg/L, compared to the controls (Fig. 5). Mean fertilization success of A. tenuis gametes in the controls was 78%, in the 5000 µg/L cadmium treatment it was 52%, and in the 10,000 µg/L cadmium treatment it was 53%.
Calculated EC50 and NOEC values
The EC50 values for fertilization success of gametes of the coral species tested in this study for copper and lead are shown in Table 3. The EC50 values for copper effects on the various coral species range from 15 to 40 µg/L, which indicates a similar response to copper among the four species tested. EC50 values for lead were two orders of magnitude higher than for copper, which indicates that lead was much less toxic than copper. The EC50 values for lead were similar in both of the Acropora species, and were higher for G. aspera gametes. The EC50 values for other metals tested (nickel, cadmium, and zinc) could not be calculated because the results did not meet the conditions of the EC50 test.
The NOEC values can only be determined if there is a significant trend in the measured effect caused by increased concentrations. Therefore, NOEC values are available only for those experiments that exhibit a significant decrease in fertilization success with increased metal concentration (Table 3). The copper NOEC values for G. aspera, G. retiformis, and A. longicyathus were 12.8, 10.0, and 15.3 µg/L, respectively. The NOEC value for A. tenuis was higher at 33.5 µg/L, however, repeated experiments would be required to verify whether gametes of this species were less sensitive to copper. The lead NOEC values for G. aspera are much greater than for copper and more variable among species. A. longicyathus and A. tenuis had lead NOEC values of 451.0 and 790.0 µg/L, respectively. In contrast the lead NOEC value for G. aspera was 5455 µg/L. The NOEC values for nickel, zinc, and cadmium are preliminary and further experiments should be done to verify these values. Cadmium appears to be less toxic to A. tenuis gametes than all other metals tested.
Discussion
The effects of copper on the fertilization success of coral gametes
Copper is an essential element for all living organisms as it acts as a catalyst for many enzyme systems (Depledge and Rainbow 1990), and is also important as an electron carrier in intracellular structures (Hay 1984). Copper is required in relatively low concentrations compared with other essential elements such as zinc. Higher concentrations of copper are the basis for common fungicides and molluscicides, and it is thought that these higher concentrations disrupt protein-binding systems and interfere with metabolism (Hay 1984).
Copper concentrations in seawater range from 8×10−4 µg/L in pristine open oceans, up to 29.2 µg/L at highly polluted sites (Sadiq 1992). The toxicity of copper to marine organisms is fairly well documented, as a result of its widespread use in anti-foulant coatings as well as its presence in industrial, urban and agricultural discharges (Mance 1987). In the ANZECC and ARMCANZ (2000) Australian and New Zealand water quality guidelines, the trigger value estimated to protect 99% of species in marine waters is 0.3 g/L copper, and to protect 95% of species the trigger value is 1.3 g/L copper. If higher copper concentrations are found in seawater it 'triggers' a response for action.
The effect of copper on coral fertilization success was similar among most species (EC50 values between 15.2 and 39.7 µg/L) tested during this study, and for earlier fertilization studies which showed EC50 values of 14.5 and 17.4 µg/L for G. aspera (Reichelt-Brushett and Harrison 1999) and A. millepora (Negri and Heyward 2001), respectively. The NOEC values are also similar among species (Table 3). Studies completed on F. chinensis gametes (Heyward 1988) and A. millepora gametes (Negri and Heyward 2001) also showed similar dose-response effects to those found in the present study. At 100 µg/L copper, mean fertilization success of G. retiformis gametes was 44% (this study) compared with 46% in F. chinensis (Heyward 1988). G. aspera gametes showed 1% fertilization successat 96.8 µg/L of copper in the present study. This response was similar to Heywardȁ9s (1988) results using gametes from P. ryukyuensis, which had <10% fertilization success at 100 µg/L. Similarly, Negri and Heywardȁ9s (2001) results using A. millepora gametes showed <30% fertilization success at about 70 µg/L copper.
There were some differences in the experimental design between this study and Heywardȁ9s (1988) study. Heyward (1988) combined the gametes immediately prior to treatment; whereas, we dosed the gametes 30 min prior to combining eggs and sperm, to allow time for any potential toxic effects on the gametes prior to fertilization. Also, in this study, sperm concentrations were controlled to avoid the potential problem of high sperm concentrations masking the effects of sub-acute toxicity. Negri and Heyward (2001) also controlled sperm concentration in their study, but it is uncertain if the sperm or eggs were dosed prior to combining the gametes. Furthermore, Negri and Heyward (2001) filtered the seawater used in the experiments, which may influence the copper speciation by limiting the complexation of copper with particulate organics under normal seawater conditions.
The effects of lead on the fertilization success of coral gametes
Lead has no known biological function and is not an essential element, but it is known to be toxic at relatively low concentrations (Zakrzewski 1991). The main sources of lead to the marine environment are from stormwater and sewage dischage (González 1990; Peters et al. 1997). The ANZECC and ARMCANZ (2000) trigger value for lead to protect 99% of species in marine waters is 2.2 µg/L lead, and to protect 95% of species it is 4.4 µg/L. Concentrations of lead as high as 26.9 µg/L have been found in marine waters (Brugmann 1981).
These are the first quantitative data on the effects of lead on fertilization rates in corals. No other studies on lead toxicity have been conducted on corals so no comparisons can be made with the results from this study. The data in this study show that relatively high concentrations of lead can disrupt fertilization processes in three species of reef corals from the Great Barrier Reef. A. longicyathus was the most sensitive species tested, as a significant (p≤0.05) reduction in mean fertilization success occurred compared with controls, at a lead concentration of 855 µg/L. The EC50 values show that lead had a variable effect on gametes of the three coral species tested.
The effects of zinc on the fertilization success of coral gametes
Zinc is an essential element for all biological systems (Depledge and Rainbow 1990) and zinc requirements for organisms are usually higher than for copper. Zinc can also be more abundant in the environment than other trace metals, therefore organisms have developed several mechanisms for bioregulation (Depledge and Rainbow 1990; Morrison et al. 1989). Zinc concentrations recorded throughout the worldȁ9s open oceans average 5 µg/L (Riley and Chester 1989). Sewage discharge and mining waste are sources of zinc pollution, with average concentrations of zinc in sewage sludge being 3000 mg/kg (Oȁ9Neill 1985). The ANZECC and ARMCANZ (2000) trigger value for zinc to protect 99% of species in marine waters is 7.0 µg/L zinc, and to protect 95% of species it is 15.0 µg/L zinc.
The effect of zinc on gametes from A. tenuis was significant at 10 µg/L, and minimal fertilization occurred at 100 and 1000 µg/L. The fertilization success of G. aspera gametes was not affected at zinc concentrations up to 500 µg/L in the study by Reichelt-Brushett and Harrison (1999). Heyward (1988) found that 50–60% fertilization occurred in P. ryukyuensis gametes in 500 µg/L zinc treatments, and 20–30% fertilization occurred in 1000 µg/L zinc. Heyward (1988) also tested the effects of zinc on the coral F. chinensis and found minimal fertilization at 1000 µg/L, similar to the fertilization rate found in this study for A. tenuis gametes at the same zinc concentrations. No lower zinc concentrations were used in the study by Heyward (1988), therefore the effect of zinc on F. chinensis gametes at lower concentrations is not known. Given the variable responses recorded among coral species, further experiments should be done to assess the variability in the effects of zinc on fertilization success.
The effects of nickel on the fertilization success of coral gametes
Nickel has no known essential biological function, however, unlike many non-essential elements, nickel does not tend to be highly toxic. The ANZECC and ARMCANZ (2000) trigger value for nickel to protect 99% of species for in marine waters is 7.0 µg/L nickel, and to protect 95% of species it is 70.0 µg/L nickel. Concentrations of nickel in marine waters are generally below 0.1 µg/L. Concern has previously been raised over the potential toxicity of nickel to corals because a nickel ore processing plant is located on the coast in North Queensland adjacent to the Great Barrier Reef Marine Park (Knauer 1977).
In the first test conducted on G. aspera gametes in 1995, mean fertilization success did not fall below 83% at nickel concentrations up to 2000 µg/L. However, the results of the second test in 1996 showed that nickel concentrations of 100, 1000, and 2000 µg/L significantly reduced fertilization success to as low as 60%. These results show that there is some degree of variability between experiments, either due to inter annual or inter colony variability in the response of spawned gametes to nickel pollution. No comparisons of these results can be made because no other published studies were found on the effects of nickel on the fertilization success of other marine species.
The effects of cadmium on the fertilization success of coral gametes
Cadmium is a relatively rare element, with concentrations in seawater ranging from 2×10−4 to 2.9 µg/L (Sadiq 1992). However, in polluted estuaries or harbors and ports, values up to 50 µg/L have been recorded (Chester 1990). The ANZECC and ARMCANZ (2000) trigger value for cadmium to protect 99% of species in marine waters is 0.7 µg/L cadmium, and to protect 95% of species it is 5.5 µg/L cadmium.
Cadmium was considered an important metal to study because there is a continuous source of this metal to the Great Barrier Reef from the phosphates that are deposited onto the reef from land runoff. According to labelling on packages and independent analyses by the authors, phosphate fertilizers contain up to 300 µg/g of cadmium and the cadmium concentration is directly correlated with the amount of total phosphorus in the fertilizer (Loganathan and Hedley 1997). CRC (2003) estimate that 9000 tonnes of phosphorsus are discharged into the Great Barrier Reef per year. Therefore, if cadmium concentrations in the phosphorus load are estimated at an average of 150 µg/g, over 1 tonne of cadmium is also discharged into the Great Barrier Reef annually.
Concentrations of cadmium, up to 10,000 µg/L, were tested using gametes from A. tenuis and a significant reduction in fertilization success was found at cadmium concentrations of 5000 and 10,000 µg/L. These results suggest that cadmium is less toxic to coral fertilization success than all the other metals tested including copper, lead, zinc, and nickel. The NOEC values indicate that concentrations of 2500 µg/L did not significantly affect the fertilization success of A. tenuis. Reichelt-Brushett and Harrison (1999) also found that concentrations of cadmium of 200 and 1000 µg/L did not affect the fertilization success of G. aspera and Oxypora lacera, respectively. The NOEC values for cadmium were high compared to those determined for other metals tested. It is unlikely that these levels of cadmium would be found in any coral reef systems.
The effects of metals on the fertilization success of other marine organisms
There are few other published studies on marine invertebrates that examine fertilization success as an endpoint for trace metal toxicology, and of these, the vast majority use sea urchins, including: Arbacia punctulata, Arbacia spatuligera, Echinometra mathaei,Strongylocentrotus purpuratus, Temnopleurus toreumaticus, Dendraster excentricus,Peronella japonica, Heliocidaris erythrogramma, and Diadema setosum (Table 3). In most of these studies, the sperm were dosed and then crossed with the undosed eggs (Kobayashi 1980; Pagano et al. 1982; Nacci et al. 1986; Dinnel et al. 1987; Dinnel et al. 1989; Neiheisel and Young 1992; Ringwood 1992; Bailey et al. 1995; Zùniga et al. 1995; Warnay et al. 1996; Ramachandran et al. 1997), or the eggs and sperm were dosed prior to crossing (Lee and Xu 1984). Few other studies examine the effects of trace metals on fertilization success of other marine organisms. Such studies include germination of the kelp, Macrocystis pyrifera (Anderson et al. 1990); fertilization of gametes from the hard clam, Mercenaria mercenaria (Stiles et al. 1991); fertilization of gametes from the bivalve, Isognomon californicus (Ringwood 1992), and fertilization of gametes from the fish, Atherinops affinis (Anderson et al. 1991). Only some of the above studies provided EC50 or NOEC data (Table 3) allowing comparisons to be made with the results from this study.
Copper is by far the most toxic element for the organisms tested in seawater, with EC50 and NOEC values all within the same range as the values found for the coral species tested in this study (Table 3). The EC50 values for copper on A. punctulata, E. mathaei, S. purpuratus and C. gigas are between 12.0 and 45.7 µg/L, and the values recorded for different species of coral are similar; G. aspera 18.5 µg/L, A. longicyathus 15.2 µg/L, G. retiformis 24.8 µg/L, and A. tenuis 39.7 µg/L. The EC50 values for some other species including Isognoman californicus (55 µg/L), and A. affinis (109 µg/L) are higher than those found for corals in this study. The fertilization success of corals seems be more sensitive to lead than the fertilization success of other tropical marine species including A. punctulata, S. purpuratus, and C. gigas (Table 3). The decreasing order of toxicity of the elements tested is generally copper, zinc, lead then cadmium, and a similar order was found for the toxicity of metals tested in this study (Table 3).
Sensitivity of corals to trace metals
Some trace metals can stress and affect the early life stages of corals, including fertilization success (Heyward 1988; Reichelt-Brushett and Harrison 1999; Negri and Heyward 2001), larval motility (Reichelt-Brushett and Harrison 2004), and larval settlement success (Esquivel 1986; Goh 1991; Reichelt-Brushett and Harrison 2000). In all of the fertilization or sub lethal studies that test copper effects, the EC50 values are similar and range between 14.5 and 39.7 µg/L. The effects of copper on coral larval survival are described in Reichelt-Brushett and Harrison (2004), and the comparisons of this test of lethality with the sub lethal tests show that measurements of survival exhibit a delayed toxic response to copper compared to the fertilization and sub lethal measurements. Lead impairs the motility of larvae and inhibits fertilization at much lower concentrations than those that are toxic to larval survival (Reichelt-Brushett and Harrison 1999, 2000, 2004). The metals lead, zinc, cadmium, TBT, and nickel are generally less toxic than copper to the early life stages of corals (Esquivel 1986; Heyward 1988; Goh 1991; Reichelt-Brushett and Harrison 1999, 2000; Negri and Heyward 2001; and this study). The NOEC and EC50 values reported in these studies also show that the sublethal tests are more sensitive to copper than the test on larval survival.
Trace metal toxicological studies on adult corals include investigations of stress by quantifying zooxanthellae loss (Howard et al. 1986; Jones 1997), histopathological studies (Glynn et al. 1989), growth rate (Howard et al. 1986; Scott 1990), respiration (Howard et al. 1986), and oxygen consumption using a perturbation index (Nystrom et al. 1997). None of these studies on adult corals identified an EC50 value or a NOEC value, and only one paper identified an LC50 value for corals (Howard et al. 1986). Howard et al. (1986) determined a copper 96 h LC50 of 48.0 µg/L for the adult coral Montipora verrucosa, and stressed the difficulty in determining LC50 values for adult colonial organisms. Other studies on the effects of trace metals on corals do not provide ecotoxicological values, however, these studies show significant sublethal effects of zooxanthellae loss (Jones 1997) and changes in oxygen consumption via a perturbation index (Nystrom et al. 1997) from exposure to copper concentrations of 20 and 10 µg/L, respectively. These are similar concentrations to those found to significantly inhibit the fertilization success of corals examined in this study.
Our results demonstrate that trace metals exert variable, but generally pronounced effects on the fertilization success of corals. Copper was the most toxic of the trace metals tested, followed by lead, zinc, cadmium, and nickel. Ecological risk assessment is increasingly dependant on the use of ecotoxicological results to determine pollutant toxicities (Logan and Wilson 1995). There are minimal data on ecotoxicology for tropical marine organisms (Peters et al. 1997), and hence the fertilization toxicity test used in this study provides a useful tool that is relevant to, and can aid in, future ecological risk assessments for coral reef ecosystems.
References
Anderson BS, Hunt JW, Turpen SL, Coulon AR, Martine M (1990) Copper toxicity to microscopic stages of giant kelp Macrocystis pyrifera: interpopulation comparisons and temporal variability. Mar Ecol Prog Ser 68:174–156
Anderson BS, Middaugh DP, Hunt JW, Turpen SL (1991) Copper toxicity to sperm, embryos and larvae of topsmelt Atherinops affinis, with notes on induced spawning. Mar Environ Res 31:17–35
Australian and New Zealand Environment and Conservation Council (ANZECC) and Agriculture and Resource Management Council of Australia and New Zealand (ARMCANZ) (2000) Australian and New Zealand water quality guidelines for fresh and marine waters. Environment Australia
Bailey HC, Miller JL, Miller MJ, Dhaliwal BS (1995) Application of toxicity identification procedures to the echinoderm fertilization assay to identify toxicity of a municipal effluent. Arch Environ Contam Toxicol 18(5):747–755
Brown BE (1987) Heavy metal pollution on coral reefs. In: Salvat B (ed) Human impacts on coral reefs: facts and recommendations. French Polynesia, Antenne Museum, pp 119–134
Brugmann L (1981) Heavy metals in the Baltic Sea. Mar Pollut Bull 12:214–218
Chester R (1990) Marine Geochemistry. Allen Unwin Australia
Clesceri LS, Greenberg AE, Eaton AD (eds) (1995) Standard methods for the examination of water and wastewater, 19th edn. United Book Press Inc., Baltimore, MD
CRC (2003) Land use and the great barrier reef. CRC Reef Research Centre, Townsville
Depledge MH, Rainbow PS (1990) Models of regulation and accumulation of trace metals in marine invertebrates. Comp Biochem Physiol 97C:1–7
Dinnel PA, Link JM, Stober QJ (1987) Improved methodology for a sea urchin sperm cell bioassay for marine waters. Arch Environ Contam Toxicol 16:23–32
Dinnel PA, Link JM, Stober QJ, Letourneau MW (1989) Comparative sensitivity of sea urchin sperm bioassays to metals and pesticides. Arch Environ Contam Toxicol 18(5):748–755
Esquivel IF (1986) Short term bioassay on the planula of the reef coral Pocillopora damicornis. In: Jokiel PL, Richmond RH, Rogers RA (eds) Coral reef population biology. Hawaii Inst Mar Biol Tech Rep 37:465–472
Glynn PW, Szmant AM, Corcoran EF, Cofer-Shabica SV (1989) Conditions of coral reef cnidarians from the Northern Florida reef tract: pesticides, heavy metals and histopathological examination. Mar Pollut Bull 20:568–576
Goh B (1991) Mortality and settlement success of Pocillopora damicornis planula larvae during recovery from low levels of nickel. Pac Sci 45:276–286
González H (1990) Heavy metals in sediments around a sewage outfall at Havana, Cuba. Mar Pollut Bull 21(5):253–255
Gulko D (1996) Effects of ultraviolet radiation on fertilization and production of planula larvae in the Hawaiian coral Fungia scutaria. Ultra-violet Radiation and Coral Reefs. Hawaii Inst Mar Biol Tech Rep 41:135–147
Guzmàn HM, Jiménez CE (1992) Contamination of coral reefs by heavy metals along the Caribbean coast of Central America. Mar Pollut Bull 24:554–561
Hamilton MA, Russo RC, Thurston RV (1977) Trimmed Spearman-Karber method for estimating median lethal concentration in toxicity bioassays. Environ Sci Technol 11:714–719
Hamilton MA, Russo RC, Thurston RV (1978) Correction. Environ Sci Technol 12:417
Harrison PL (1994) The effect of oil pollutants on fertilization rates in the scleractinian coral Acropora tenuis. Abstract: joint scientific conference on science, management and sustainability of marine habitats in the 21st century. James Cook University, Townsville
Harrison PL (1995) Support to the Kuwait institute for scientific research for the project on the ecology of coral reefs in Kuwait and effects of stressors on corals. UNIDO/UNDP
Harrison PL, Ward S (2001) Elevated levels of nitrogen and phosphorus reduce fertilization success of gametes from scleractinian reef corals. Mar Biol 139:1057–1068
Harrison PL, Babcock RC, Bull GD, Oliver JK, Wallace CC, Willis BL (1984) Mass spawning in tropical reef corals. Science 223:1186–1189
Harrison PL Wallace CC (1990) Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky Z (ed) Coral reefs. Ecosystems of the World, Elsevier Science Publishers, Amsterdam
Hay RW (1984) Bio-inorganic chemistry. Ellis Horwood Ltd. Camelot Press, Great Britain
Heyward AJ (1988) Inhibitory effects of copper and zinc sulphates on fertilization in corals. Proc 6th Int Coral Reef Symp 2:299–303
Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr (1995) Handbook of ecotoxicology. CRC Press Inc, Boca Raton
Howard LS, Crosby DG, Alino P (1986) Evaluation of some methods for quantitatively assessing the toxicity of heavy metals to corals. In: Jokiel PL, Richmond RH, Rogers RA (eds) Coral reef population biology Hawaii. Inst Mar Biol Tech Rep vol 37, pp 452–464
Johannes RE (1975) Pollution and degradation of coral reef communities. In: Ferguson Wood EJ, Johannes RE (eds) Tropical marine pollution. Elsevier, Amsterdam
Jones RJ (1997) Zooxanthellae loss as a bioassay for assessing stress in corals. Mar Ecol Prog Ser 149:163–171
Knauer GA (1977) Immediate industrial effects on sediment metals in a clean coastal environment. Mar Pollut Bull 8:249–254
Kobayashi N (1980) Comparative sensitivity of various developmental stages of sea urchins to some chemicals. Mar Biol 58:163–171
Lacher TE, Goldstein MI (1997) Tropical ecotoxicology: status and needs. Environ Toxicol Contam 16(1):100–111
Lee HH, Xu CH (1984) Effects of metals on sea urchin development: a rapid bioassay. Mar Pollut Bull 15(1):18–21
Logan DT, Wilson HT (1995) An ecological risk assessment method for species exposed to contaminant mixtures. Environ Toxicol Chem 14(2):351–359
Loganathan P, Hedley MJ (1997) Downward movement of cadmium and phosphorus from phosphatic fertilizers in a pasture soil in New Zealand. Environ Pollut 95(3):319–324
Mance G (1987) Pollution threat of heavy metals in aquatic environments. Elsevier Applied Science Publishers, London
McGibbon S, Moldan AGS (1986) Routine toxicity testing of toxicants using a sea urchin gamete bioassay. Mar Pollut Bull 17(2):68–72
Morrison G P, Batley GE, Florence TM (1989) Metal speciation and toxicity. Chem Britain 791–796
Nacci DE, Jackim E, Walsh R (1986) Comparative evaluation of three rapid marine toxicity tests. Sea urchin early embryo growth test, sea urchin sperm toxicity test and microtox. Environ Toxicol Chem 5:521–525
Negri AP, Heyward AJ (2001) Inhibition of coral fertilisation and larval metamorphosis by tributyltin and copper. Mar Environ Res 51:17–27
Neiheisel TW, Young ME (1992) Use of three artificial sea salts to maintain fertile sea urchins (A. punctulata) and conduct fertilization tests with copper and sodium dodecyl sulfate. Environ Toxicol Chem 11:1179–1185
Nystrom M, Moberg F, Tedengren M (1997) Natural and anthropogenic disturbance of reef corals in the inner gulf of Thailand: physiological effects of reduced salinity, copper, and siltation. Proc 8th Int Coral Reef Symp 2:1893–1898
Oȁ9Neill (1985) Environmental chemistry. George Allen and Unwin Ltd. London, p 232
Pagano G, Esposito A, Giordano GG (1982) Fertilization and larval development in sea urchins following exposure of gametes and embryos to cadmium. Arch Environ Toxicol Contam 11:47–55
Peters EC, Gassman NJ, Firman JC, Richmond RH, Power EA (1997) Ecotoxicology of tropical marine ecosystems. Environ Toxicol Chem 16(1):12–40
Ramachandran S, Patel TR, Colbo MH (1997) Effect of copper and cadmium on three Malaysian tropical estuarine invertebrate larvae. Ecotox Environ Safety 36:183–188
Reichelt AJ, Jones GB (1994) Trace metal as tracers of dredging activities in Cleveland Bay-Field and laboratory studies. Aust J Mar Freshw Res 45:1237–1257
Reichelt-Brushett AJ, Harrison PL (1999) The effect of copper, zinc and cadmium on fertilization success of the scleractinian coral Goniastrea aspera. Mar Pollut Bull 38:182–187
Reichelt-Brushett AJ, Harrison PL (2000) The effect of copper on the settlement success of larvae from the scleractinian coral Acropora tenuis. Mar Pollut Bull 41:385–391
Reichelt-Brushett AJ, Harrison PL (2004) Development of a sublethal test to determine the effects of copper and lead on scleractinian coral larvae. Arc Environ Contam Toxicol 47:40–55
Richmond RH (1993) Coral reefs: present problems and future concerns resulting from anthropogenic disturbance. Am Zool 33:524–536
Riley JP, Chester R (1989) Introduction to marine chemistry. St Edmundsbury Press Ltd, Great Britain
Ringwood A (1992) Comparative sensitivity of gametes and early developmental stages of a sea urchin (E. mathaei) and a bivalve species (Isognomon californicus) during metal exposure. Arch Environ Contam Toxicol 22:288–295
Sadiq M (1992) Toxic metal chemistry in marine environments. Marcel Dekker, New York
Scott PJB (1990) Chronic pollution recorded in coral skeletons in Hong Kong. J Exp Mar Biol Ecol 139:51–64
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W. H. Freeman and Company, New York
Stiles S, Choromanski J, Nelson D, Greig R, Sennefelder G (1991) Early reproductive success of the hard clam (Mercenaria mercenaria) from five sites in Long Island Sound. Estuaries 14(3):332–342
Veron JEN (2000) Corals of Australia and the Indo-Pacific. University of Hawaii Press, Hawaii, Singapore
Warnay M, Iaccarino M, De-Biase A, Temara A, Jangoux M, Dubois P, Pagona, G (1996) Spermiotoxicity and embryotoxicity of heavy metals in the echinoid Paracentrotus lividus. Environ Toxicol Chem 15(11):1931–1936
Wu RSS, Lam PKS, Zhou BS (1997) A phototaxis inhibition assay using barnacle larvae. Environ Toxicol Water Qual 12:231–236
Zakrzewski SF (1991) Principles of environmental toxicology. American Chemical Society, Washington
Zùniga M, Roa R, Larrain A (1995) Sperm cell bioassays with the sea urchin A. spatuligera on samples from two polluted chilean coastal sites. Mar Pollut Bull 30(5):313–319
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Environmental Editor B.G. Hatcher
Rights and permissions
About this article
Cite this article
Reichelt-Brushett, A.J., Harrison, P.L. The effect of selected trace metals on the fertilization success of several scleractinian coral species. Coral Reefs 24, 524–534 (2005). https://doi.org/10.1007/s00338-005-0013-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-005-0013-5