Abstract
Diadema antillarum was once ubiquitous in the Caribbean, but mass mortality in 1983–84 reduced its numbers by >97%. We measured Diadema abundance on back reefs and patch reefs that have been well studied for >25 years. From June 2000 to June 2001, populations on back reefs have increased >100% (June 2001 mean densities 0.004–0.368/m2), while patch reef populations increased >350% (June 2001 densities 0.236–0.516/m2). Populations are dominated by small urchins, suggesting high recent recruitment. Increased Diadema densities appear to be affecting macroalgae abundance. The general spatio-temporal pattern of recovery around St. Croix seems to be following that of the die-off, suggesting that the same oceanographic features that spread Diadema's pathogen are now carrying urchin larvae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1983–84, Diadema antillarum, the Caribbean long-spined sea urchin, experienced one of the most devastating mortalities ever recorded in a marine animal. Almost certainly caused by an unknown pathogen, the mass mortality reaped >97% of the total Caribbean population, and was not followed by rapid recovery (Lessios 1995).
Its prolonged absence has affirmed that Diadema was a major controller of macroalgae on Caribbean reefs, as experiments had suggested earlier [reviewed in Lessios (1988)]. Intensive ecological studies of Diadema on St. Croix reefs began in the 1970s, and showed that Diadema grazing caused the mysterious halos of bare sand found around Caribbean patch reefs (Ogden et al. 1973). Removal of Diadema on reefs at St. Croix and Jamaica resulted in shifts in dominance from algal turfs to filamentous or foliose macroalgae such as Dictyota, Padina, and Turbinaria (Sammarco et al. 1974; Sammarco 1982; Carpenter 1986). Since Diadema is also a reef bioeroder (e.g. Ogden 1977), and can influence coral settlement (Sammarco 1980), it shapes the entire reef community. Recent and apparently ongoing recovery of Diadema populations in Jamaica has been accompanied by reductions in macroalgal cover and increased coral abundance (Aronson and Precht 2000; Edmunds and Carpenter 2001).
Diadema mass mortality began in St. Croix in mid-January 1984, generally spreading west to east and offshore to onshore. In about 3 weeks the disease traveled approximately 18 km along the north (leeward) shore from Salt River to Tague Bay, (N.B.O., unpublished data; Fig. 1)—opposite to the direction of prevailing currents (Lugo-Fernández et al. 1998). A similar pattern was documented for the spread of the disease in Jamaica, where the mortality took about 2 weeks to travel from Negril to Discovery Bay, moving from west to east, about 25 km a week, against prevailing currents (Hughes et al. 1985). On a larger inter-island scale, the mortality did follow prevailing surface currents, as documented by Lessios et al. (1984). With the exception of Barbados, where the population recovered to 57% of pre-dieoff numbers by 1985 (Hunte and Younglao 1988), recovery has not occurred [reviewed in Lessios (1995)]. Stricken Diadema populations have experienced many years of low recruitment, failing to produce enough larvae to overcome planktonic mortality (Lessios 1995). Limited fertilization success of widely spaced individuals [Allee effect, e.g. Levitan (1991)] or lack of grazed substrate for settlement (Bak 1985) may have acted to keep populations low (Lessios 1995).
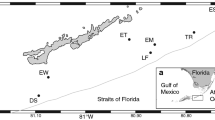
Map of St. Croix, showing study sites and progression of Diadema die-off in 1984. Back-reef sites are: SOL Solitude; POW Pow Point; YC Yellowcliff; TAG Tague Bay; TH Turner Hole; RB Rod Bay. The two patch reef sites, PR-2 and PR-3, are labeled with their ranks in progression of the die-off, as follows. Approximate dates of beginning of die-off: 1) Salt River, 1/18/84; 2) Buck Island, 1/30/84; 3) PR-2, 2/2/84; 4) Grassy Pt., 2/3/84; 5) PR-3, 2/6/84; 6) Romney Pt., 2/13/84; 7) Pull Pt., 2/25/84; 8) West Indies Laboratory flow-through aquaria, 3/3/84
This report documents the possible beginnings of recovery of Diadema populations in St. Croix, comparing spatial patterns of recovery with that of the mass mortality, evaluating effects on macroalgae, and comparing recent data (collected by R.J.M., A.J.A., and J.P.E.) with historical data (collected by N.B.O., J.C.O., and R.C. Carpenter). If such a recovery is persistent and widespread in the Caribbean, it may induce a phase shift on coral reefs, back to coral dominance from the present macroalgal dominance (Edmunds and Carpenter 2001).
Methods
Diadema antillarum was counted four times (in June 2000, October 2000, February 2001, and June 2001) at each of eight locations on St. Croix, USVI: two south-shore back-reef sites, four north-shore back-reef sites, and two north-shore patch reefs (Fig. 1). At each back-reef site at each sampling time, SCUBA divers counted Diadema in 14 randomly located 50×2-m transects. Densities from Tague Bay's back-reef from 1983–86 were obtained from Carpenter (1990); these data were collected in March 1986, and in December of all other years. Diadema were also counted on two patch reefs in Tague Bay, PR-2 (area ~800 m2) and PR-3 (area ~1,500 m2). On the two patch reefs, SCUBA divers counted all visible Diadema in 2000/2001, and complete counts of Diadema were made on a yearly basis during the years 1973 and 1982–84 (Ogden et al. 1973; N.B.O., unpublished data).
Diadema test diameters were measured in October 2000 at the same six back-reef sites as above (Fig. 1). All Diadema in seven haphazardly located 30×2-m transects at each site were counted and measured to the nearest millimeter with long-jawed calipers. Tripneustes ventricosus and Echinometra spp. (lucunter and viridis) were also counted. Pre-mortality (1983) and 1984–86 size frequencies from Tague Bay were obtained from Carpenter (1990). Test size frequencies for 1983, 1984–86, and 2000 were divided into three classes: small [<40 mm; mean size of 1-year-old Diadema = 48.6 mm (Karlson and Levitan 1990)], medium (41–60 mm), and large (>60 mm) and compared between years with chi-square tests (Sokal and Rohlf 1995).
Algal percent cover was estimated from the same transects as above at five sites: Pow Point, Yellowcliff, Tague Bay, Turner Hole, and Rod Bay. A video camera held ~1 m above bottom was moved along the transect line at a rate of ~3 m/min, filming a strip ~45 cm wide. Each video frame (~50×45 cm) was overlain by an acetate sheet printed with 10 randomly located dots, and the substrate under each dot was categorized as macroalgae (foliose or filamentous algae, not turfs), live coral, sand, or other. Macroalgal percent cover was calculated as the percent of total dots per transect on hard substrate (not including sand) that fell on algae.
Macroalgal percent covers were arcsine transformed (\(Sin^{ - 1} \sqrt x \), Sokal and Rohlf 1995) and regressed against Diadema densities. Data were analyzed by transect (n=32).
Results
Abundance
Diadema density at five of six back-reef sites increased by an average 100% from June 2000 to February 2001, then leveled off or decreased slightly from February to June 2001 (Table 1, Fig. 2). The exception was Rod Bay, where densities remained very low. Although Tague Bay density increased 114% during this time, density there also was still quite low at 0.004/m2. Diadema density generally increased from west to east among the north-shore back-reef sites, following the pattern of spread of the mortality (Fig. 3). In 1983, before the die-off, Carpenter (1990) recorded mean Diadema densities at Tague Bay back-reef of 6.4/m2 (±81 SE); by 1985, Tague Bay densities were at 0. Though back-reef densities at Tague Bay, the easternmost site, are still low (0.004/m2 in June 2001) densities at other sites are higher (0.2–0.8/m2), though still an order of magnitude lower than pre-mortality densities (Fig. 2).
Log Diadema density at six sites on St. Croix from June 2000 to June 2001, and at Tague Bay from 1983 (pre-mortality) to 1986 [data from Carpenter (1990)]. Error bars are 1 standard error. For clarity, most lines between 1986 and 2000 are omitted, error bars are shown for only one direction, and error bars for TH and YC, which are comparable with other sites, are not shown. Note difference in x-axis scaling across break. See Fig. 1 for site abbreviations
Diadema density across the four north-shore sites from west to east. Distances are in kilometers from Salt River to the west (Fig. 1). The negative slope is significant (F=15.74, p=0.007). Y=63.105−3.431X, R 2=0.08. Data points represent individual 50×2 m transects from June 2000 (n=14/site)
Diadema densities on the patch reefs grew steadily during this study, increasing an average 350% from June 2000 to June 2001 (Fig. 4). Density of Diadema on PR-3, the more eastern patch reef, remained lower than that of PR-2 throughout the sampling. Pre-mortality densities of PR-2 and PR-3 from 1973–83 were >10 Diadema/m2; by 1985 densities had declined to near zero. June 2001 densities are 0.52/m2 for PR-2 and 0.24/m2 for PR-3 (Fig. 4).
Diadema density at patch reefs PR-2 and PR-3. [1973–84 data from Ogden (Ogden et al. 1973, N.B.O. and J.C.O., unpublished data).] Despite higher historical densities at PR-3, PR-2, whose population crashed ~1 week prior to that of PR-3, now has higher densities
Size distributions
Mean test diameter of D. antillarum on St. Croix back-reef habitats in October 2000 was 48.6 mm (SD 20.8, n=362). The two southern sites (RB, TH) had larger urchins than the northern sites (Table 2). Mean test diameter was significantly different between sites (ANOVA, p<0.05) but test diameter was not correlated with population density (p>0.05). Size frequency of Diadema pooled across all sites is shown in Fig. 5, along with historical data. The October 2000 distribution is dominated by 31- to 40-mm urchins (31.6%). The pre-mortality size distribution [from Carpenter (1990)] is dominated by small to mid-sizes, particularly the 21- to 30-mm size class (25.5%), whereas the 1984–86 distribution (shortly after mass mortality) is dominated by very large sizes, with 32.8% >80 mm. The distribution of Diadema's small (<40 mm), medium (41–60 mm), and large (>60 mm) size classes in 2000 is not significantly different from the 1983 pre-mortality distribution (chi-square, p=0.07). The 1984–86 distribution, however, is very different from both the 2000 distribution (chi-square, p<0.0001) and the 1983 distribution (chi-square, p<0.0001).
Frequencies of Diadema antillarum test diameters for 1983 (pre-mortality, Tague Bay, n=400), 1984–86 (immediately post-mortality, Tague Bay, n=149), and October 2000 (Solitude, Pow Pt., Yellowcliff, Tague Bay, Rod Bay, and Turner Hole, n=364). [1983–86 data from Carpenter (1990)]
Grazing
Macroalgal cover showed a significant negative relationship with Diadema density (ANOVA, p=0.03, Fig. 6). Algal cover appears to be dramatically higher when urchin abundance is below about 0.1/m2. No significant trend was seen in coral cover (p>0.05), although our sampling method did not have the resolution to detect coral recruits, as Edmunds and Carpenter (2001) did.
Tripneustes abundances were very low, and Echinometra abundances were not correlated with either Diadema abundance or macroalgal cover (p>0.05, Table 1).
Discussion
Diadema densities on St. Croix are still far below pre-mortality numbers, but substantially higher than the near-zero densities that immediately followed the mass mortality. Recent rapid population growth (>100% increase on back-reefs, >350% on patch reefs) contrasts sharply with the very slow to nonexistent increases of the past 17 years. Recent size distributions indicate successful recruitment, suggesting that this could be the beginning of a full population recovery, potentially returning Diadema to its role as an important grazer on Caribbean reefs.
Small Diadema are now abundant in St. Croix, and the population is approaching the pre-dieoff size frequency distribution (Fig. 5). Urchins from 20–40 mm have become particularly common; this size corresponds with urchins less than 1 year old (Karlson and Levitan 1990). Mean test diameter is not correlated with population density across sites; this suggests that population densities are well below trophic carrying capacity, with no intraspecific competition (Levitan 1988).
The recently elevated Diadema densities in St. Croix are evidently affecting macroalgal cover (Fig. 6), as they did before the mass mortality (Ogden et al. 1973). We noticed that the rugose micro-sites that tend to harbor Diadema were often visible from a considerable distance due to the bright bare patches resulting from urchin grazing (R.J.M., A.J.A., J.P.E., J.C.O., personal observations). Of course, anecdotes and correlations do not prove causality, but decades of experimental and correlative studies on the effect of Diadema grazing [reviewed in Lessios (1988)] support this interpretation.
The 1984 mortality spread from west to east and offshore to onshore at St. Croix, as was particularly noted on the north shore (Fig. 1). Bank-barrier reef populations died off first, followed by populations on lagoonal patch reefs, and, finally, by urchins in the flow-through aquaria at the West Indies Laboratory, where the seawater intakes were located close to shore (N.B.O., unpublished data). This pattern recurred on an even smaller scale on patch reefs PR-2 and PR-3, which are less than 100 m apart: the more western PR-2 experienced the population crash ~1 week prior to PR-3, and urchins began dying on the seaward edge of both reefs, reflecting the offshore to onshore pattern. The beginnings of recovery documented here (June 2000) show a similar pattern to that of the mortality, with highest north-shore densities in the west on back reefs, and higher densities on PR-2, the more western patch reef, compared to PR-3 (Figs. 3 and 4).
Larval filtering—higher settlement at upstream versus downstream sites, as larvae settle on suitable substrates and are depleted en route (Gaines et al. 1985)—might explain these spatio-temporal patterns of recovery. Though net flow along the north shore of St. Croix is westward, tide-driven current reversals occur frequently (Lugo-Fernández et al. 1998), and it is also conceivable that larval behavior could result in eastward transport (e.g. Pineda 1999). Caselle and Warner (1996) noted a similar pattern of highest recruitment on western upcurrent reefs of the north shore of St. Croix in the reef fish Thalassoma bifasciatum, and Swearer et al. (1999) inferred from trace elements in juvenile otoliths that larval retention on western reefs caused the high recruitment. A reversal of prevailing currents off St. Croix's north shore in 1997 was caused by a mesoscale anti-cyclonic eddy, which may be a regular event in the area (Harlan et al. 2002). This may facilitate larval retention on western reefs, or contribute larvae of distant origin to the reefs, and help explain the spatio-temporal pattern in our data.
Interestingly, the spread of mass mortality on Jamaica's north coast was also west to east in direction, and it moved with similar speed (Hughes et al. 1985). Unlike St. Croix, present densities at some of the same sites cited in Hughes et al. (1985) do not reflect a similar pattern of recovery (Edmunds and Carpenter 2001). However, the recovery in Jamaica is much more advanced than that in St. Croix [densities of ~5/m2 in 'urchin zones'; Edmunds and Carpenter (2001)], and early patterns may no longer be visible. In addition, facilitation by Tripneustes or Echinometra grazing does not seem to have played a role in the recovery in St. Croix, as it may have in Jamaica (Aronson and Precht 2000).
What caused the sudden increase in Diadema densities in St. Croix, after 15 years of negligible recovery? The population may have been seeded by larvae from some upstream source—a likely candidate is Barbados, where Diadema populations recovered relatively quickly after the die-off (Hunte and Younglao 1988), and reefs still enjoy relatively high densities of Diadema (1.94 /m2 at Bellairs Reef in 2000; CARICOMP data). However, we do not have data from sites between St. Croix and Barbados to support this conjecture, and this recolonization route does not by itself explain why it would take so long for significant numbers of larvae to reach St. Croix. Moreover, Hunte and Younglao (1988) concluded that Barbados is not a regional larval source, since they saw no downcurrent recruitment of Diadema at St. Lucia or Dominica coinciding with recruitment pulses at Barbados. Seeding from distant larval sources may be episodic, with recruitment in most years largely from resident populations, as has been suggested for some reef fish populations on St. Croix (Swearer et al. 1999). This would account for a very slow initial recovery, followed by more rapid population increases when local populations achieve critical densities. The high proportions of juvenile Diadema appearing now in St. Croix may indicate that these critical densities have been achieved.
This research, coupled with recent reports from Jamaica, and unpublished CARICOMP data, suggest that a large-scale recovery of Diadema populations may be underway. Immediate and continued monitoring of Diadema populations throughout the Caribbean is essential if we are to understand how this recovery proceeds.
References
Aronson RB, Precht WF (2000) Herbivory and algal dynamics on the coral reef at Discovery Bay, Jamaica. Limnol Oceanogr 45(1):251–255
Bak RPM (1985) Recruitment patterns and mass mortalities in the sea urchin Diadema antillarum. Proc 5th Int Coral Reef Congr 5:267–272
Carpenter RC (1986) Partitioning herbivory and its effects on coral reef algal communities. Ecol Monogr 56:345–63
Carpenter RC (1990) Mass mortality of Diadema antillarum I. Long-term effects on sea urchin population-dynamics and coral reef algal communities. Mar Biol 104:67–77
Caselle JE, Warner RR (1996) Variability in recruitment of coral reef fishes: the importance of habitat at two spatial scales. Ecology 77:2488–2504
Edmunds PJ, Carpenter RC (2001) Recovery of Diadema antillarum reduces macroalgal cover and increases abundance of juvenile corals on a Caribbean reef. Proc Natl Acad Sci 98:5067
Gaines SD, Brown S, Roughgarden J (1985) Spatial variation in larval concentrations as a cause of spatial variation in settlement for the barnacle, Balanus glandula. Oecologia 67:267–272
Harlan JA, Swearer SE, Leben RR, Fox CA (2002) Surface circulation in a Caribbean island wake. Cont Shelf Res 22:417–434
Hughes TP, Keller BD, Jackson JBC, Boyle MJ (1985) Mass mortality of the echinoid Diadema antillarum Phillipi in Jamaica. Bull Mar Sci 36:377–384
Hunte W, Younglao D (1988) Recruitment and population recovery of Diadema antillarum (Echinodermata; Echinoidea) in Barbados. Mar Ecol Prog Ser 45:109–119
Karlson RH, Levitan DR (1990) Recruitment-limitation in open populations of Diadema antillarum: an evaluation. Oecologia 82:40–44
Lessios HA (1988) Mass mortality of Diadema antillarum in the Caribbean: what have we learned? Annu Rev Ecol Syst 19:371–393
Lessios HA (1995) Diadema antillarum 10 years after mass mortality: still rare, despite help from a competitor. Proc R Soc Lond B 259:331–337
Lessios HA, Robertson DR, Cubit JD (1984) Spread of Diadema mass mortality through the Caribbean. Science 226:335–337
Levitan DR (1988) Algal-urchin biomass responses following mass mortality of Diadema antillarum Phillipi at St. John, U.S. Virgin Islands. J Exp Mar Biol Ecol 119:167–178
Levitan DR (1991) Skeletal changes in the test and jaws of the sea urchin Diadema antillarum in response to food limitation. Mar Biol 111:431–435
Lugo-Fernández A, Roberts HH, Wiseman WJ Jr, Carter BL (1998) Water level and currents of tidal and infragravity periods at Tague Reef, St. Croix (USVI). Coral Reefs 17:343–349
Ogden JC (1977) Carbonate-sediment production by parrot fish and sea urchins on Caribbean reefs. In: Frost SH, Weiss MP, Saunders JB (eds) Reefs and related carbonates—ecology and sedimentology. American Association of Petroleum Geologists Studies in Geology no 4, Tulsa, Oklahoma, pp 281–288
Ogden JC, Brown RA, Salesky N (1973) Grazing by the echinoid Diadema antillarum. Formation of halos around West-Indian patch reefs. Science 182:715–717
Pineda J (1999) Circulation and larval distribution in internal tidal bore warm fronts. Limnol Oceanogr 44:1400–1414
Sammarco PW (1980) Diadema and its relationship to coral spat mortality: grazing, competition, and biological disturbance. J Exp Mar Biol Ecol 45:245–272
Sammarco PW (1982) Effects of grazing by Diadema antillarum Philippi (Echinodermata: Echinoidea) on algal diversity and community structure. J Exp Mar Biol Ecol 65:83–105
Sammarco PW, Levinton JS, Ogden JC (1974) Grazing and control of coral reef community structure by Diadema antillarum (Echinodermata:Echinoidea): a preliminary study. J Mar Res 32:47–53
Sokal RR, Rohlf FJ (1995) Biometry. WH Freeman, New York
Swearer SE, Caselle JE, Lea DW, Warner RR (1999) Larval retention dynamics drive recruitment patterns in an island population of a coral reef fish. Nature 402:799–802
Acknowledgements
This research was funded by a grant from the NOAA/NMFS/Saltonstall-Kennedy Program (NA97FD0070) to J.P.E., an NSF-GRT award to R.J.M. and A.J.A., and generous support from the Biology Department of the University of Massachusetts Boston. We thank K. Gloger for excellent work in the field, G. Skomal for lodging on St. Croix, D. Ward of Seaward Research for field support, NOAA/NOS for aerial photographs of benthic habitat, the St. Croix Yacht Club, and the staff at Anchor Dive Center for excellent care and service. R.C. Carpenter, D.R. Levitan, and D. Linton (CARICOMP) generously provided data for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miller, R.J., Adams, A.J., Ogden, N.B. et al. Diadema antillarum 17 years after mass mortality: is recovery beginning on St. Croix?. Coral Reefs 22, 181–187 (2003). https://doi.org/10.1007/s00338-003-0301-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-003-0301-x