Abstract
Human society is dependent on nature1,2, but whether our ecological foundations are at risk remains unknown in the absence of systematic monitoring of species’ populations3. Knowledge of species fluctuations is particularly inadequate in the marine realm4. Here we assess the population trends of 1,057 common shallow reef species from multiple phyla at 1,636 sites around Australia over the past decade. Most populations decreased over this period, including many tropical fishes, temperate invertebrates (particularly echinoderms) and southwestern Australian macroalgae, whereas coral populations remained relatively stable. Population declines typically followed heatwave years, when local water temperatures were more than 0.5 °C above temperatures in 2008. Following heatwaves5,6, species abundances generally tended to decline near warm range edges, and increase near cool range edges. More than 30% of shallow invertebrate species in cool latitudes exhibited high extinction risk, with rapidly declining populations trapped by deep ocean barriers, preventing poleward retreat as temperatures rise. Greater conservation effort is needed to safeguard temperate marine ecosystems, which are disproportionately threatened and include species with deep evolutionary roots. Fundamental among such efforts, and broader societal needs to efficiently adapt to interacting anthropogenic and natural pressures, is greatly expanded monitoring of species’ population trends7,8.
Similar content being viewed by others
Main
Although billions of dollars are spent annually on weather observations and prediction (for example, the US National Oceanic and Atmospheric Administration had a budget of US $5.4 billion in 2021), no countries are systematically monitoring broad-scale changes in biodiversity, as needed to directly inform human wellbeing outcomes. Global population trends can be inferred for only about 14,000 species9, largely through life-history studies of economically important and charismatic animals or terrestrial citizen science observations. Available reports indicate that the populations of many species are rapidly declining across broad scales, including insects10, plants11 and all vertebrate classes3,12, to the point of local extirpation of some species9,12,13,14. Indeed, on the basis of the approximately 290 documented global extinctions over the past century, extinction rates are now at least 100 times the background rate over the Earth’s history3, a conservative estimate given the small proportion of life on Earth whose extinction risk has been assessed. Management efforts directed towards maintaining stable populations has produced mixed results, as evident for many wild fisheries, with global catches falling since the 1990s15.
Nevertheless, not all populations across species’ geographic ranges are decreasing, but few data are available to systematically assess whether populations with increasing trends are relatively rare or go largely unreported. The poor general state of knowledge on global species population trends is highlighted by less than 10% of the more than 1 million known species on Earth having been assessed for the International Union for Conservation of Nature (IUCN) Red List of Threatened Species16. Among Red List-assessed species, 14% have insufficient information for threat characterization and are categorized as ‘data deficient’, and many of the 51% of species categorized as ‘least concern’ show declining population trends3.
Knowledge deficiencies are exacerbated in the marine realm, where population changes are hidden below the sea surface. Relative to terrestrial systems, much less information is available to assess the state of marine biodiversity, reflected in the heavy reliance on data from commercial fisheries for the Living Planet Index17 (LPI)—the widely known assessment of aggregated trends in populations. Long-term fisheries catch statistics are, however, confounded by changing effort and fishing down of populations18, whereas other large observational datasets such as plankton surveys rarely encompass species’ full distributional ranges19. Quantitative long-term reef monitoring programmes cover systems with exceptional richness and are likely to prove crucial for understanding the magnitude of broad-scale changes in marine ecosystems20. Coral reefs alone support around 25% of marine species21, and temperate reefs host many more, including phylogenetically unique animals and plants.
Here we undertake the most comprehensive assessment of marine species population trends to date across multiple phyla at a continental scale, through integration of three of the largest long-term reef monitoring programmes worldwide (Reef Life Survey22, Australian Temperate Reef Collaboration23 and the Australian Institute for Marine Sciences’ Long Term Monitoring Program24). These time series collectively extend from 1992 to the present, and use standardized scuba diver counts of species densities in 50-m-long transect blocks20,25, totalling 26.4 million observations of individuals belonging to 1,991 marine fish, 1,234 mobile invertebrate, 321 coral and 463 macroalgal taxa distributed around Australia to 2021. We focus on the 2008–2021 period with circum-continental coverage following commencement of Reef Life Survey, and on the 1,636 sites with at least 2 years of survey data (a map is shown in Extended Data Fig. 1 and site sampling frequency is shown in Extended Data Fig. 2), and 1,057 species with high survey frequency (a positive record in 60 or more year × site combinations). Our analysis includes extreme heatwaves reported in 2011 off southwestern Australia5, in 2016, 2017 and 2020 off northern Australia26,27,28, and ongoing ocean warming off southeastern Australia and Tasmania29.
Populations of 57% of reef species decreased in the decade to 2021, a percentage that was consistent whether all species (617 out of 1,057, 58%) or only species with significant trends (P < 0.05; 97 out of 172, 57%) were considered. Populations of 28% of species declined by more than 30% between 2011 and 2021 (9 corals, 36 invertebrates, 24 macroalgae and 227 vertebrates), thus passing the threshold that qualifies species as threatened for the IUCN Red List when generation length is unknown30. One notable example is the weedy seadragon (Phyllopteryx taeniolatus), an iconic southern Australian endemic fish that significantly declined by 59% from 2011 to 2021 (that is, to 0.41 times initial numbers). A total of 28 species potentially qualify as ‘critically endangered’ (more than 80% population decline), 110 species potentially qualify as ‘endangered’ (more than 50% decline) and 158 species potentially qualify as ‘vulnerable’ (more than 30% decline). For global Red List consideration, this national analysis requires further information on generation length and, for non-endemic species, population trends outside Australia.
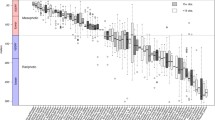
Population trends showed great variation among species within major taxonomic groupings, resulting in wide confidence intervals (Fig. 1); nevertheless, on average, a significant negative decadal trend (9.8% decline) was found across all species (as indicated in Fig. 1 by the 95% confidence limits not overlapping 1.0×). The identified patterns were robust to removal from analysis of sites with few years surveyed (Extended Data Fig. 3). Cool-temperate mobile invertebrates and vertebrates (18% and 19% decline, respectively), and tropical vertebrates (15% decline), showed significant mean decadal population changes (Fig. 1). The significant decline among cool-temperate invertebrates was largely driven by echinoderms (20% decline; 95% confidence interval: 7–31% decline; 21 species). Among warm-temperate invertebrates, echinoid echinoderms (sea urchins) also declined significantly (40% decline; 95% confidence interval: 16–57% decline; 11 species).
Population change between 2011 and 2021 in mean abundance (±95% confidence interval where n > 2) around the Australian continent for species belonging to major taxonomic and biogeographic groupings (assigned to species using mean sea surface temperature of all sites where that species had been recorded across Australia; tropical, T > 23 °C; warm-temperate (warm), 17.5 °C < T < 23 °C; cool-temperate (cool), T < 17.5 °C). The shaded region below 1.0× indicates decline. The number of species considered (n) and the two-tailed probability (P) that the mean value differs significantly from 1.0× using a t-test, are indicated. A significant effect is indicated by P < 0.05.
The 55 species of corals investigated did not change significantly as a group (1.6% mean increase) over the past decade (but see discussion below about longer-term trends). Populations of only one coral species (Astreopora myriophthalma) decreased significantly (18.6% decline; t-test P < 0.05, n = 107 non-zero observations), and four species (Acropora austera, Acropora loripes, Acropora valida and Echinopora lamellosa) increased significantly (51%, 13%, 204% and 108%, respectively; t-test P < 0.05, n = 56, 77, 73 and 110 non-zero observations, respectively). Macroalgae also showed no significant net decadal change across the continent (3.5% decrease) when assessed by t-test, but with contrary trends at the margins of significance in warm- and cool-temperate regions (P < 0.1; Fig. 1). The populations of 14 out of 134 macroalgal species significantly declined and 11 increased (P < 0.05).
Multiple linear regression models indicated considerable variation in potential drivers of population trends among taxa and biogeographic groupings (Fig. 2). Decadal population trends among tropical vertebrate species (465 fish and 2 reptile species) were significantly negative overall (intercept, Fig. 2; P < 0.001, n = 467), and were positively related to the frequency of occurrence (P = 0.046, n = 467) and negatively related to body size (P = 0.004, n = 467). Thus, populations of widespread tropical fishes were less likely to decrease in population numbers than highly localized species. Populations of large-bodied fishes tended to decline more rapidly than those of small fishes, consistent with the effects of fishing pressure compounding temperature effects18. Coral and tropical mobile invertebrate species that were most closely associated with high-chlorophyll locations showed less population increase than invertebrates associated with oligotrophic sites, which predominate in clear offshore waters.
Multiple linear regression model coefficient estimates (±95% confidence interval, assuming normal distribution, scaled and centred) for population trends predicted by six species traits, partitioned by major taxa and biogeographic grouping. a, Tropical (T > 23 °C) vertebrates. b, Tropical invertebrates. c, Tropical corals. d, Warm-temperate (17.5 °C< T < 23 °C) vertebrates. e, Warm-temperate invertebrates. f, Warm-temperate macroalgae. g, Cool-temperate (T < 17.5 °C) vertebrates. h, Cool-temperate invertebrates. i, Cool-temperate macroalgae. Filled dots indicate P < 0.05. Higher values of the traits are associated with population increases. Values that fall within the grey shaded areas are negative. The intercept term represents the mean population trend when traits are set to zero. Max., maximum.
Contrary to patterns for vertebrates, populations of tropical mobile invertebrates generally increased through time (intercept, Fig. 2; P = 0.005, n = 39), perhaps because of a reduction in fish predation or opportunities afforded to scavengers and herbivores when corals affected by heatwaves die and are overgrown by turfs31. Population increases were greatest among mobile invertebrate species living in cooler tropical regions (that is, the subtropics). Tropical coral and mobile invertebrate species that were primarily associated with high-chlorophyll inshore locations also showed disproportionate declines.
As was the case for tropical vertebrates, large-bodied warm-temperate vertebrates showed more pronounced population declines than small species (P = 0.02, n = 202). An affinity for sites with high chlorophyll levels was significantly related to population decline for warm-temperate vertebrates (P = 0.007, n = 202). Warm-temperate invertebrates showed contrary trends to the tropics for body size (P = 0.009, n = 59); warm-temperate invertebrate species with declining populations tended to be smaller, and to extend down to greater maximum depths (P = 0.01, n = 59). The declines of some species possibly resulted from migration below the depth range censused by divers as sea temperatures have warmed; 48% of all species examined and 44% of warm-temperate invertebrates, are known to occur below a depth of 30 m. For warm-temperate macroalgae, species with low frequency of occurrence (P = 0.02, n = 40) and species with affinity for cooler temperatures (P = 0.03, n = 40) showed significant declines relative to common species and species frequenting warmer temperatures.
Cool-temperate vertebrates (P = 0.02, n = 57) and invertebrates (P < 0.001, n = 44) exhibited net population declines, and macroalgae density also trended downwards but not significantly so (P = 0.066, n = 94). Populations of cool-temperate vertebrates with large body size declined less rapidly than small vertebrate species (P < 0.01). Cool-temperate invertebrates with distributions centred in the warmest water or with large body size exhibited less rapid population declines than species with cool affinity or small body size (P < 0.01 and P < 0.001, respectively). In contrast to results for animals living in warm-temperate waters, populations of cool-temperate invertebrate species with large depth ranges tended to decrease less rapidly than shallow water species. A conclusive understanding of this difference between warm-temperate and cool-temperate species requires further investigation, including through analysis of species that may change their depth distributions during a heatwave cycle.
Geographically, the proportion of species showing significant population declines was particularly pronounced for mobile invertebrates off southern Australia, particularly Tasmania (Fig. 3). Vertebrate population declines were also most pronounced across the same region, as well as along the Great Barrier Reef off northeastern Australia (Fig. 3). The proportion of species with significantly increasing populations was relatively low and consistent around the continent.
Variation in the percentage of reef species showing significant (P < 0.05) national declines (shown as percentage decrease, left) or increases (percentage increase, right) between 2011 and 2021 in 1° × 1° grid cells around Australia. Colours depict the percentage of species occurring within grid cells that are declining or increasing significantly across the continent rather than population trends within individual cells. Data for tropical corals and warm- and cool-temperate macroalgae are plotted on the same map with latitudinal subdivision; southern corals and northern macroalgae were excluded owing to small sample sizes.
Aggregation of interannual trends by latitude revealed that species’ populations generally increased Australia-wide from 2008 to 2015, then declined from 2015 to 2021 (Fig. 4). For each of the three main biogeographic groups of species, populations increased near the cool range edge and decreased near the warm range edge—consistent with a poleward shift in abundance. Thus, although warm-temperate species showed relatively little net change in population numbers across their range (Figs. 1 and 2), abundances have generally declined in northern sites in recent years, but with matching increases in the south. The steep overall decline among cool-temperate species (Figs. 1 and 2) presumably resulted from an absence of shallow reef habitat south of 45° S, where compensatory population increases would otherwise have occurred.
Trends were calculated relative to 2008 as a mean trend among all species recorded within 10° latitudinal bands for three biogeographic groupings (tropical, warm-temperate and cool-temperate). Patterns shown were calculated using population trend assessment method 1 (Methods), as the annual observed density of species across all sites where present with interpolation for unsampled years, averaged for sites within 1° × 1° grid cells; grid cells then averaged for each species, log-standardized relative to 2008 values, and finally averaged across species for the period 2011–2021 and exponentially back-transformed. The shaded area indicates one standard error.
Parsing of the latitudinal patterns by region reveals correspondence with changing sea surface temperatures. Extreme heating, which differed in timing between regions, was followed by population decline of cooler affinity species in all cases (Fig. 5). The 2011 southwestern heatwave9—the most pronounced around Australia during the 2008–2021 period—was followed by rapidly declining populations of cool-temperate species that have not subsequently recovered. These declines were accompanied by a massive spike in densities of tropical species (Figs. 5b and 6b). Southwestern macroalgal populations showed particularly strong population declines (Fig. 6b), with greater falls than among macroalgae living further south in the southern (Fig. 6c) and Tasmanian regions (Fig. 6f).
Species trends relative to 2008 were categorized within three biogeographic groupings (tropical, warm-temperate and cool-temperate) for six regions around Australia. a, Northwest: Northern Territory and west coast north of 27° S. b, Southwest: west coast south of 27° S. c, South: south coast east to 148° S. d, Northeast: Queensland and Coral Sea. e, Southeast: New South Wales and Victoria west to 148° S. f, Tasmania. Patterns reflect total species population trends as calculated using population trend assessment method 1 (Methods). Population change over a decade is plotted as x-fold change. The deviation in mean temperature from 2008 values is shown as a dashed line. The shaded area indicates one standard error.
Mean species trends relative to 2008 were aggregated among species categorized within major taxa for six regions around Australia. a, Northwest: Northern Territory, west coast north of 27° S. b, Southwest: west coast south of 27° S. c, South: south coast east to 148° S. d, Northeast: Queensland and Coral Sea. e, Southeast: New South Wales and Victoria west to 148° S; f, Tasmania. Patterns reflect total species population trends as calculated using population trend assessment method 1 (Methods). Population change over a decade is plotted as x-fold change. The deviation in mean temperature from 2008 values is shown as a dashed line. The shaded area indicates one standard error.
Patterns depicted in Figs. 3–6 were calculated as the average of population trends with weighting of sites by abundance and with interpolation of unsampled years (Methods, ‘Population trend assessment method 1’). An alternative depiction of population trends that weights sites equally regardless of abundance, without interpolation, showed a sharper peak associated with immigration of tropical species in 2012 in southwestern Australia (Extended Data Fig. 4b) and reduced magnitude of change, including for cool-temperate species in Tasmania (Methods, ‘Population trend assessment method 2’). The prolonged presence of tropical species in southwestern Australia for assessments based on total population size (Fig. 5b) relative to assessments based on average rate of decline per site (Extended Data Fig. 4b) suggests greater persistence of tropical species at favourable sites where present at high densities, and curve smoothing resulting from interpolation of missing years (discussed in Methods). The flattening of curves with equal weighting of high and low abundance sites (Fig. 5b versus Extended Data Fig. 4b) suggests that species populations generally decline more rapidly at sites where they are initially present in high abundance than at marginal sites where they are present in low numbers.
Sea surface temperatures peaked in both the northeast (Fig. 5d) and northwest (Fig. 5a) in 2016, with consistent mean interannual fluctuations in these two regions, presumably because broad-scale climate oscillations such as El Niño operated across tropical Australia. Averaged sea temperature patterns may, however, conceal considerable within-region variability, particularly across the large northwestern region (Fig. 6a) where geographically divergent drivers of ecological change have previously been described32. Reefs north of 18° S were reported as most impacted by heat stress and coral bleaching during strong El Niño events, whereas coral communities further south were found to be more affected by extreme temperatures during La Niña phases32.
Extensive coral mortality was reported across the northern Great Barrier Reef and Coral Sea following the 2016 heatwave, with additional coral bleaching in 2017 and 202026,27,28. Populations of vertebrate and coral species declined steeply following a two-year lag (Fig. 6d). Nevertheless, contrary to expectations, our data indicate that tropical coral populations have trended upwards off northeastern Australia since 2008 (Fig. 6d,e), while declining in the northwest. The slight northeastern coral increase was primarily facilitated by increased densities of some coral species towards their cooler range margins (that is, southern Great Barrier Reef and Coral Sea, Fig. 3). These results are consistent with the detailed investigation of trends across the Great Barrier Reef by the Australian Institute of Marine Science’s (AIMS) Long Term Monitoring programme. AIMS data indicate a long-term downward trend in live coral cover from commencement of monitoring in 1985, with a period of substantial variation from 2011 to the present, and with higher mean cover in 2020 than 2008 in the central and southern Great Barrier Reef regions33. Thus, although our data demonstrate an increase in corals over the past decade, this should be considered within the context of general decline over the past 45 years.
The strong northern Australian heatwave in 2016 (Fig. 5d) also propagated into the southeastern region (Fig. 5e). Warm-temperate fish species with ranges extending into the tropics declined precipitously in northeastern Australia following the 2016 heatwave (Fig. 5d). Only 6 of 24 warm-temperate species initially observed at subtropical sites in the southern Great Barrier Reef and Coral Sea region have been recorded across the same set of sites since 2017. The stronger apparent decline among vertebrate than coral species may reflect the changing physical structure of sites as coral habitat transforms with heatwaves despite little change in total per cent coral cover, and as particular coral taxa attractive to fishes are disproportionately lost. Specialist fishes that depend on coral habitat were over-represented among the 98 species with significantly declining populations. Notable in this regard were the butterflyfishes (Chaetodontidae), a family that includes mostly corallivores and other coral-dependent species. A total of 11 butterflyfish species showed significant population declines, compared with only one significant population increase (Chaetodon citrinellus).
Rather than intense short-lived heatwaves, as in the tropical regions of this study, mean water temperatures off eastern Tasmania have progressively increased by 0.5 °C over the past decade (Fig. 5f), in addition to an increase of around 1 °C over the preceding 50 years29. The earlier period saw substantial population declines in many commercial fishery species and the habitat-forming giant kelp Macrocystis pyrifera29. The cumulative long-term effect of warming has probably driven the large decline in cool-temperate invertebrates through the past decade (Fig. 6f), whereas approximately equal numbers of vertebrate and macroalgal species experienced positive and negative trends. In the southeastern region (Fig. 5e), reef populations of vertebrates, macroalgae and corals tended to rise with increasing water temperature from 2008 to 2016, after which populations declined. Populations in the southern region changed relatively little over the past decade (Fig. 5c).
Across Australia, a temperature effect threshold at approximately 0.5 °C above 2008 values is indicated. Most biotas increased in years with lower temperatures and declined in abundance once this threshold was exceeded. Further investigation is needed to determine which signals of temperature variability affects population trends the most, particularly for heatwaves where the maximum temperature and degree heating week thresholds probably have greater relevance than the mean annual values plotted here34.
Increasing ocean temperature associated with changing climate clearly presents an existential threat for many Australian reef species, with indirect effects to fisheries and other ecosystem services. Commercial fishery catches around Australia have declined rapidly over the past two decades, with particularly acute downward trends in cool-temperate species (64% decline in Tasmanian catch since 1992) relative to tropical species18 (24% rise in catch in Northern Territory and 7% decline in Queensland). Fishery stock models can no longer be relied upon if they are calibrated using historical data collected under environmental regimes that greatly differ from the present35.
To date, overfishing has been regarded as the greatest global threat to marine life—as concluded by the 2020 LPI report, which attributed most of the marine population declines worldwide to over-exploitation including fishing. However, marine species included in the marine LPI are dominated by exploited species because commercial fisheries represent the primary data source. Such species contributed relatively little to total tallies of species trends in our assessment (8.1% of species). Our study indicates that a warming climate has become a significant—and probably overriding—driver of change, with probable acceleration into the future. Synergistic interactions between climate, fishing pressure and loss of habitat-forming species are also likely. Exploited fish species showed more rapid decreases on average (29% decline; 95% confidence interval: 15–41% decline; n = 85) than unexploited fishes (12% decline; 95% confidence interval: 7–16% decline; n = 660). Moreover, loss of large exploited species can profoundly influence population numbers of other species, through predation and associated trophic impacts36,37.
Continental patterns described here using site observations are not equivalent to changing total numbers of species across the continent. No reef species assessed here has become extinct. However, total species numbers are likely to ultimately decline across the continent if rapid temperature-related ecosystem change continues because of the mismatch in time between the decades to centuries required for extinction, versus millions of years for evolution of new species. Some gains could occur through immigration from regions warmer than Australia, although such movement is constrained by an equatorial limit on species distributions, where species richness drops for many taxa38,39, and the recent decline for populations living in the warmest most northern Australian latitudes (Fig. 4).
Whereas media and public interest focus on photogenic coral reefs, species associated with temperate reefs appear to be in greater jeopardy of extinction than tropical species for four reasons. First, little attention has historically been directed towards the conservation of temperate rocky reefs. This is particularly evident in Australia, a country with near equal extent of tropical and temperate coastlines, but where human activity is concentrated in the temperate coastal strip (67% of the population lives within 50 km of temperate coasts40). High human population density has led to numerous human activities and associated cumulative stressors (urban effluent, nutrient runoff, wild catch fisheries, aquaculture, introduced species, foreshore development, marine debris, modified freshwater flows and catchment land clearance) that interact with climate change and each other29,41,42. Marked biome differences in management also extend to the distribution of no-fishing marine protected areas—a key conservation tool. Internationally, ‘no-fishing’ marine protected areas are nearly absent (less than 1%) from the temperate coasts of Europe, Asia and North America, whereas very large (more than 100,000 km2) tropical no-fishing reserves are located in European overseas and US Pacific territories43. In Australia, ‘no-fishing’ marine reserves are also predominantly located in the tropics, covering only 1% of cool-temperate coastal waters around Tasmania compared with 14% of tropical waters off Queensland44.
Second, cool-temperate invertebrate, fish and macroalgal species appear to be extremely sensitive to recent warming, leading to numerous species with diminishing populations (Fig. 1). Such declines are presumably partly direct, through temperature affecting growth and survival, but also indirect, through increased densities of tropical fish predators and herbivores increasing consumption rates on small prey and some habitat-forming macroalgal species37,45,46. Adding to past impacts, climate predictions indicate that southeastern Australia is also a global hotspot for future warming, so the effects of climate change are ongoing and expected to intensify across the region47.
Third, many temperate species—including in Australia—inhabit a climate trap, with no reef habitat available for poleward retreat for shallow water species as conditions warm48. Additional climate traps exist on isolated islands such as Galapagos49, and for inshore species along east–west continental margins of southern Africa, South America, Antarctica and the Arctic.
Fourth, our study indicates that temperate species have a much higher degree of endemism than tropical species, and generally lack distant refuges in other countries from which to re-establish; 339 out of the 487 temperate species in our dataset are endemic to Australia (70%), compared with only 19 out of the 570 tropical species (3%). Temperate species also possess much deeper phylogenetic roots. The 331 temperate fish and invertebrate species assessed here are classified within 105 families (mean 3.2 species per family), whereas nearly twice as many tropical fish and invertebrate species (538) belong to many fewer (60) families (mean 9.0 species per family). Substantial loss of temperate marine species would represent a major decline in the deep phylogenetic diversity of all life on Earth.
Although our investigation was focused on Australian reef-dwelling species, populations are probably also declining in other rapidly warming temperate seas. Among the sparse data available, a multi-decadal investigation of Californian inshore and pelagic fish communities found marked population declines since the 1970s for most studied species, with disproportionately large decreases among colder water taxa50. In the north Atlantic, shifts in fish and plankton community composition closely match heating anomalies51. Rapid declines of seastar populations due to wasting disease have been reported along the west North American coast, including the critically endangered habitat-engineer seastar Pycnopodia helianthoides52. In addition to climate, the global concentration of human population density53 between 20° N and 40° N will probably increase the extinction risk on Northern Hemisphere temperate reefs54.
Unfortunately, few broad-scale ecological monitoring datasets are available for tracking species population trends on temperate reefs; consequently, extinctions of species will generally occur undocumented. Broad-scale monitoring of echinoderms is particularly needed, as this phylum included the highest proportion of species tentatively identified as threatened in our investigation. Echinoderms may be disproportionately sensitive to increasing temperatures owing to disease outbreaks that are facilitated by their open vascular systems, and to changes in oceanic conditions that affect the ‘boom and bust’ life histories of many species with complex planktonic stages.
Additional management focus, investment and research are all needed to safeguard worldwide biodiversity values associated with temperate inshore habitats. Marine monitoring programmes urgently need expansion to allow assessment of extinction risk for macroalgae and invertebrates29,55,56, and for understanding the roles of direct, indirect and cumulative stressors in driving loss of reef biota. An important initial step is the recognition that human activity and associated stressors are concentrated on the continental shelf and upper slope in temperate latitudes, and this is also where much of the world’s deep phylogenetic diversity is currently under threat.
Methods
Reef monitoring data
No statistical methods were used to predetermine sample size.
Density data were obtained through three long-term reef monitoring initiatives progressed by the author team: Reef Life Survey (RLS; 2007–2021)22, Australian Temperate Reef Collaboration surveys (ATRC; 1992–2021)23,58, and the Australian Institute for Marine Science Long Term Monitoring programme (AIMS; 1992–2021)24. The ATRC programme involves application of standardized underwater census methods by management agencies in Tasmania (Department of Natural Resources and Environment), New South Wales (Department of Primary Industries), South Australia (Department of Environment, Water and Natural Resources) and Western Australia (Department of Biodiversity, Conservation and Attractions), with additional data from a Parks Victoria ecological monitoring programme that used the same methodology.
All initiatives apply comparable underwater visual surveys along 50-m-long transect lines20, with diver searches extending out from the transect line within a 5-m-wide block for large fishes and a 1-m-wide block for small or cryptic fishes. Large (>1 cm) mobile invertebrates are counted in 50 m × 1 m blocks by RLS and ATRC divers, and 5 quadrats (each 0.25 m2 with 50 points) per transect scored in situ by ATRC divers for macroalgae. A total of 20 photoquadrats (~0.2 m2) were photographed by RLS divers along each 50 m transect line. Photoquadrats were scored for corals by a single expert (E.T.) to highest taxonomic level (generally species) at a subset of 481 sites distributed across all major reef systems, with 385 sites providing data in multiple years. Using the annotation program Squidle+59, a quincunx grid of 5 points was overlaid on each image and corals under each point were recorded; thus, taxa under 100 points per transect were enumerated. A total of 480 coral taxa were recorded in images, of which 321 were identifiable species.
The geographic scope of the three monitoring programmes differed. RLS extended Australia-wide; AIMS was restricted to the Great Barrier Reef (northeastern Australia); ATRC encompassed temperate locations from southwestern Western Australia to southeastern Australia including Tasmania. Consequently, no macroalgal data were available for coral-dominated northern Australia. AIMS surveys were confined to 210 fish species from 10 families. ATRC and RLS considered all vertebrate and large mobile invertebrate species sighted (plus giant clams for RLS). A total of 2,046, 750 and 279 sites were censused in at least one year through RLS, ATRC and AIMS programmes, respectively, where each AIMS site included three survey locations a few hundred metres apart on a single reef.
Transect block data were averaged per site. The number of transect blocks assessed in survey years within a site varied between methods: five 50 × 5 m2 large fish blocks and five 50 × 1 m2 small fish blocks for AIMS; eight (occasionally 16) 50 × 5 m2 and four (occasionally eight) 50 × 1 m2 blocks for ATRC; and generally four 50 × 5 m2 blocks and four 50 × 1 m2 blocks for RLS. Most transects (>95%) were laid between 3 m and 10 m depth (range 0.1 m below low water mark to 42 m).
Data from all 3,075 investigated sites were used to map the distribution of species showing significant declines (Fig. 3). The full dataset included 1,991 marine fish, 1,234 mobile invertebrate, 321 coral and 463 macroalgal species. Trend analyses were based on 1,636 sites surveyed at the same GPS position with at least two separate year values between 2008 and 2021 (1,105 RLS sites, 356 ATRC sites, 279 AIMS sites, including 104 sites surveyed by both RLS and ATRC divers). The >4,000 species recorded across all programmes were reduced for analysis to 1,057 species observed with a positive record in 60 or more year × site combinations. The majority were bony fishes (705 species), with sharks (19), reptiles (2), corals (55), asteroids (27), echinoids (20), crinoids (7), holothurioids (11), gastropods (55), bivalves (giant clams, 5), cephalopods (4), crustaceans (13), brown algae (48), red algae (71) and green algae (15) also represented.
Sites surveyed in multiple years, and thus used in temporal analyses, included 1104, 356 and 279 sites for RLS, ATRC, and AIMS programmes, respectively. A total of 36% of sites were surveyed in 2 years only, and 26% of sites in at least 7 different years (Extended Data Fig. 2).
Population trend analyses
Field survey data included numerous zero records, greatly complicating analysis of change, which is a multiplicative process. Decisions on how best to deal with zeros (that is, whether replaced by a small or large number relative to positive records during log transformation) affected analytical outcomes, as described below in ‘Issues considered in methods for quantifying population trends’.
We analysed population change in two complementary ways, as: (1) the best estimate of continental population trend using the mean density of each species within surveyed 1° latitude × 1° longitude grid cells (‘Population trend assessment method 1’), and (2) as proportional change in density of a species each year relative to mean density across all years for each site (‘Population trend assessment method 2’). Population trend assessment method 1 used untransformed data and thus provided the best estimate of continental trends in the total population, but required considerable interpolation of data for years not surveyed; consequently, year-by-year data were not independent. When interpreting trends, we have prioritized conclusions based on method 1 given that trends should show a 1:1 relationship with total population size as generally recognized (that is, the total number of individuals across subpopulations; for example, in IUCN Red List9). Population trend assessment method 2 assessed change across all sites where a species was present without interpolation and without consideration of abundance differences between sites (that is, change at two sites with means of 1 and 100 individuals per transect were equally weighted); data thus remained independent. This approach was applied when assessing the statistical significance of population changes using the Spearman correlation coefficient.
Population trend assessment method 1
Mean site data were initially compiled as a site × species × year matrix for the period 1992–2021 after excluding sites surveyed in a single year only. Zero counts were added when a particular species had been observed at the same site in another year. We interpolated values for non-surveyed years by assuming the site density of a species changed linearly in missing years between surveyed years. For years prior to the first survey at a site, or subsequent to the final survey, we assumed density was the same as in the closest year of survey.
Species densities were averaged within each 1° × 1° latitude × longitude grid cell to prevent large spatially aggregated sets of sites biasing continental-scale patterns. The magnitude of change in abundance over the 2011–2021 decade was assessed by log transforming the continental mean of grid cell averages for each species in each year, then calculating the slope of the linear regression between log population density and year. Aggregated statistics (for example, for different biogeographic groupings) were calculated as the mean of slopes among species, with exponential back-transformation to proportional change over a 10-year period. The process of log transformation, calculation of slope, then back-transformation, resulted in similar values to the ratio of mean data from 2010–2011 divided by mean data from 2020–2021 (R2 = 0.90 using log data). In plots, data for each year were standardized relative to the 2008 baseline year or the earliest year with data. Confidence intervals were calculated by assuming that species estimates within groups follow a normal distribution (1.96 × s.e.).
Population trend assessment method 2
Data for each site, species and year were standardized by calculating the log ratio of year density to site mean (that is, the difference between log density in any year and mean log density for that species and site across all years). Zero records were replaced by the lowest value for that species at the site across all years (that is, low detection limit) divided by 2 (see ‘Dealing with multiplicative effects’ below). To remove bias associated with highly clumped sites, we calculated mean values from all sites within each 1° × 1° latitude × longitude grid cell, then we determined mean continental values for each species across all cells (132 cells in total).
Significant continental population trends for each species were inferred using Spearman rank correlation relating year of survey to log ratio for each year relative to site mean. Significant results were based on α = 0.05, two-tailed test. Approximately 28 species could be expected to be incorrectly assigned significant negative trends due to statistical Type 1 commission errors associated with the 0.05 significance cut-off applied to 1,057 species. On the other hand, strong downward trends for many other species are masked by site-to-site and year-to-year variability (type 2 omission errors), meaning the number of biologically meaningful trends is likely to be substantially underestimated.
Issues considered in methods for quantifying population trends
Continental-scale population trends were initially investigated using the best estimate of mean density of each species at each site in each year (population trend assessment method 1), and thus involved interpolation of data in years when sites were not surveyed. As interpolation led to non-independence of data between successive years, site density data in each survey year were standardized relative to the mean across all years to assess the significance of trends (population trend assessment method 2). Hypothesis testing in this case related to the consistency of change across all sites continent-wide where a species had been recorded, with equal weighting of sites regardless of population density (that is, whether contributing much or little to the total population size).
Dealing with multiplicative effects
Data describing population density across sites or times typically possess a log normal distribution and are appropriately treated as proportional change rather than additive change. For example, population change between density counts of 1 and 2 (1 density unit, 2× change) is intuitively much closer to change between density counts of 100 and 200 (100 units, 2× change) than the trivial difference between density counts of 100 and 101 (1 unit, 1.01× change). Log data transformation is thus appropriate.
Nevertheless, log transformation cannot be applied to the many zero records in our dataset. Treating these as missing values is inappropriate given that the zero possesses high information content, particularly for threatened species. However, for our dataset where only a small proportion of the seabed (<5%) is covered by transects during each site survey, zero records rarely indicate that the species is absent if previously recorded at the site (that is, extirpation), rather, the species is probably present but below survey detection limits. Thus, a zero record suggests a density between 0 and the minimum density recorded among other species present at the site.
Zero-inflated data are generally treated by either adding a constant n to all records (that is, log(x + n), often with n = 1) or to the zero records only. We chose the latter transformation to avoid distorting annual data for sites without zero records. log transformation after adding a constant affects individual density counts in different ways (small densities are affected more than large densities), hence geometric means do not then directly convert back to an unlogged number for precise description of per cent change through time. Survey detection limits varied by up to an order of magnitude from site-to-site and time-to-time, depending on the number of transects conducted within a site. For example, mean density values down to 0.1 were possible at sites with 10 transects, whereas densities could not extend below 1 at sites with a single transect. Consequently, application of a constant value of n in the log(0 + n) transformation added pronounced bias towards small or large density estimates, depending on the subjective choice of n. For an example time series [0, 0.1, 1], replacement of 0 with 1 results in a quite different temporal pattern [1, 0.1, 1] to replacement of 0 by 0.05 [0.05, 0.1, 1]. We chose to place n at the midpoint between zero and the site detection limit; that is, n = min(x)/2, where min(x) is the minimum density recorded for that species in other years, a value that declines as the number of transects conducted at the site within a year increases. After log transformation, replacement of 0 by min(x)/2 results in a step between zero and min(x) that is equal to the step between min(x) and the second lowest possible value (2 × min(x)).
Data interpolation
Our survey data were patchy, with large gaps in the data matrix for years when sites were not resurveyed. Consequently, annual data averaged across large subsets of sites were noisy because regions with highest densities of a species may be surveyed in one year but not again until several years later, causing erratic fluctuations in continental density estimates between years. As an example, most species encountered in the Coral Sea were either much more or much less abundant than on the Great Barrier Reef, but Coral Sea surveys only commenced through the RLS programme in 2011 and were repeated several years apart. Without correction, the addition of Coral Sea data in a year when surveyed would cause major rises or declines in overall species’ averages. Thus, without interpolation or standardization, considerable noise obscured population trends.
We interpolated missing values for species at each site and year by assuming density changed linearly between the years when sites were surveyed. For example, if densities of a species were observed to be 0.5 and 2 in 2010 and 2013, then densities were assumed to be 1 and 1.5, in 2011 and 2012, respectively. For years prior to the first survey at a site, or subsequent to the final survey, we extrapolated using the same ‘best estimate’ process, by assuming density was the same as in the closest year of survey.
Interpolation of missing values is dealt with differently in the LPI60, which includes calculation of a generalized additive model (GAM) for each species’ population time series rather than assuming linear change between time points. A GAM smooths out year-to-year variation and is the better approach for describing long-term trends. Nevertheless, GAMs were not used for our analyses because many sites had insufficient year points for plotting GAMs. Of equal importance, GAMs smooth out extreme values and abrupt changes, whereas a particular focus of our study was the magnitude of variation accompanying extreme heatwaves.
The linear data interpolation method used here resulted in considerable smoothing of peaks and troughs in our data, albeit less so than with GAMs. If a site was not surveyed in a year of major change, then interpolated data will be closer to the mean than the unobserved extreme value. Peaks depicted in plotted time series (Figs. 4–6) should thus be interpreted as minimum estimates. Also, gradual ramps leading up to and away from an annual abundance peak may not reflect the rapidity of population flux. This smoothing is evident when population trends associated with the strong 2011 southwestern heatwave are compared between population trend assessment method 1 (interpolation and weighting of sites by abundance; Fig. 5b) and population trend assessment method 2 (no interpolation and equal weighting of all sites; Extended Data Fig. 4b).
The most pronounced smoothing of curves will occur for sites surveyed in only two years, where interannual peaks and troughs in abundance will generally be missed. This is particularly the case for corals, which were less frequently sampled than other taxa (Extended Data Fig. 1). Regardless, infrequently surveyed sites provided important information on long-term trends and helped to fill regional gaps, so contributed usefully to study outcomes.
Standardization by mean, maximum value or initial value
Calculation of effect size by standardization of densities within sites provides a mechanism for assessing trends without interpolation, while maintaining independence of data values. Standardization can occur relative to the maximum, long-term mean, or first year of the time series. We applied standardization by the mean, for the following reasons. Standardization by the maximum (that is, dividing density values by the maximum recorded at the site in any year) generated substantial biases associated with number of years a site was surveyed. Each additional year of survey included the possibility that a new maximum would be encountered, which then reduced the density estimates for all other years, and thus the contribution of those sites to the overall continental-scale mean. As an example, a site with only 2 years of surveys has a minimum possible mean across years of 0.5 when scaled to 1 (values of 1 and 0), whereas a site with 10 years of surveys has a minimum possible mean of 0.1 (values of 1 and 9 × 0). A site with only 2 years of surveys would thus typically contribute more to mean continental patterns than a more thoroughly investigated site with 10 years of survey. Short time series were also more frequent late in the study period as new sites were added to the RLS monitoring programme, inflating continental averages scaled to the maximum value through time because new sites with higher mean values were included in calculations of averages.
Standardization relative to the initial value was also problematic, particularly when the initial value was low and calculated ratios thus very high. In the extreme case of initial value = 0, then ratios could not be calculated.
Standardization by the mean (that is, dividing density values in each year by the site mean across all years, with log transformation to equally scale rises and falls) avoided this problem, hence was applied here.
Dealing with spatially aggregated site data
Sites have a highly clumped continental distribution because divers surveyed multiple sites in close proximity each day. This high spatial autocorrelation was removed by averaging site data within 1° × 1° (latitude × longitude) grid cells. Continental patterns were thus assessed by averaging density estimates for all grid cells where a species presence had been recorded. To reduce stochasticity, species with few (<60) positive abundance records were excluded from our study.
Trait and environmental covariates
Species’ population trends were related to site level variation in sea surface temperature and to six species traits. Daily values of sea surface temperature in each year from 1992 to 2021 were extracted for each site surveyed using down-scaled Coral Reef Watch temperature data61 (available through https://coralreefwatch.noaa.gov/product/5km/), where the maximum distance between a site and nearest SST value is 3.7 km. Site temperature data were averaged by year for each of six regions distributed around Australia (Northwest: Northern Territory and west coast north of 27° S; Southwest: west coast south of 27° S; South: south coast east to 148° S; Tasmania; Southeast: New South Wales and Victoria west to 148° S; Northeast: Queensland and Coral Sea).
Six species-level traits were investigated as predictors of population trends, namely:
Temperature affinity
Temperature affinity was calculated using Coral Reef Watch data for each species by firstly estimating the mean sea surface temperature at each site with positive sighting record, then calculating the mean of those site temperature values. On the basis of two minima (23 °C and 17.5 °C) in a plot of number of species versus temperature affinity, species were categorized by temperature affinity into 3 biogeographic groupings (tropical >23 °C; warm-temperate >17.5 °C, <23 °C; cool-temperate <17.5 °C).
Chlorophyll affinity
Chlorophyll affinity was calculated in a similar way to temperature affinity, but using mean chlorophyll values for individual sites extracted from Bio-Oracle62.
Maximum depth
The maximum depth recorded for the species (in m). Most records were from refs. 63,64; others were from Fishbase65 and Sealifebase (https://www.sealifebase.org/).
Maximum length
The maximum length recorded for the species (in cm). Most records were from refs. 63,64; others were from Fishbase65 and Sealifebase (https://www.sealifebase.org/).
Latitude span
The difference in latitude between northernmost and southernmost Australian records of the species in the combined RLS, ATRC and AIMS dataset.
Frequency of occurrence
Number of latitude × longitude (1° × 1°) grid cells surveyed around Australia in which the species had been recorded.
Statistical modelling
To test for differences in population trends for species we applied a multiple linear regression modelling approach using generalized least squares (package nmle66) in R67 with the six different species traits as predictor variables and log decadal slope (population trend assessment method 1) as the response variable. To account for heteroskedastic residuals, we used a variance structure modelled for each taxonomic class separately. Independent models were run for each taxonomic group (vertebrate, invertebrate, coral and macroalgae) and biogeographic grouping. Maximum depth, maximum body length, chlorophyll, and frequency of occurrence were log transformed; all variables were scaled and centred prior to analyses.
Base maps for Australia shown in figures were produced using the R package mapdata68.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Raw data from the Reef Life Survey and Australian Temperate Reef Collaboration programmes are accessible through a live data portal accessed via either the Reef Life Survey (https://www.reeflifesurvey.com/) or Australian Ocean Data Network (https://portal.aodn.org.au/) websites using the keywords ‘National Reef Monitoring Network’. Sea surface temperature data were obtained from Coral Reef Watch (available through https://coralreefwatch.noaa.gov/product/5km/), chlorophyll data were obtained from Bio-ORACLE (https://www.bio-oracle.org/), fish trait data were obtained in part from Fishbase (https://www.fishbase.org/), and invertebrate trait data from Sealifebase (https://www.sealifebase.ca/), as described in Methods.
Code availability
Analytical computations were undertaken in R version 4.2.0 (using libraries tidyverse. janitor, zoo, magick, cowplot, scales, patchwork, ggplot, rag, gt, gtable, grid, nlme, here and kableExtra)57, as described in R markdown script available at https://github.com/FreddieJH/continental_reef_trends.
References
Whitmee, S. et al. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet 386, 1973–2028 (2015).
Cardinale, B. J. et al. Biodiversity loss and its impact on humanity. Nature 486, 59–67 (2012).
Ceballos, G., Ehrlich, P. R. & Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl Acad. Sci. USA 114, E6089–E6096 (2017).
Duffy, J. E. et al. Toward a coordinated global observing system for seagrasses and marine macroalgae. Front. Mar. Sci. 6, https://doi.org/10.3389/fmars.2019.00317 (2019).
Wernberg, T. et al. Climate-driven regime shift of a temperate marine ecosystem. Science 353, 169–172 (2016).
Hughes, T. P., Kerry, J. T. & Simpson, T. Large-scale bleaching of corals on the Great Barrier Reef. Ecology 99, 501 (2017).
Jetz, W. et al. Essential biodiversity variables for mapping and monitoring species populations. Nat. Ecol. Evol. 3, 539–551 (2019).
Day, J. The need and practice of monitoring, evaluating and adapting marine planning and management—lessons from the Great Barrier Reef. Mar. Policy 32, 823–831 (2008).
Loh, J. et al. The Living Planet Index: using species population time series to track trends in biodiversity. Phil. Trans. R. Soc. B 360, 289–295 (2005).
Vogel, G. Where have all the insects gone? Science 356, 576–579 (2017).
Vieilledent, G. et al. Combining global tree cover loss data with historical national forest cover maps to look at six decades of deforestation and forest fragmentation in Madagascar. Biol. Conserv. 222, 189–197 (2018).
Dirzo, R. et al. Defaunation in the Anthropocene. Science 345, 401–406 (2014).
Regan, E. C. et al. Global trends in the status of bird and mammal pollinators. Conserv. Lett. 8, 397–403 (2015).
Bergstrom, D. M. et al. Combating ecosystem collapse from the tropics to the Antarctic. Glob. Change Biol. 27, 1692–1703 (2021).
Watson, R. A. & Tidd, A. Mapping nearly a century and a half of global marine fishing: 1869–2015. Mar. Policy 93, 171–177 (2018).
Species Survival Commission. 2006 IUCN Red List of Threatened Species (IUCN, 2006).
Living Planet Report 2020—Bending The Curve of Biodiversity Loss (World Wildlife Fund, 2020).
Edgar, G. J., Ward, T. J. & Stuart-Smith, R. D. Rapid declines across Australian fishery stocks indicate global sustainability targets will not be achieved without expanded network of ‘no-fishing’ reserves. Aquat. Conserv. 28, 1337–1350 (2018).
Pitois, S. G., Lynam, C. P., Jansen, T., Halliday, N. & Edwards, M. Bottom-up effects of climate on fish populations: data from the continuous plankton recorder. Mar. Ecol. Progr. Ser. 456, 169–186 (2012).
Stuart-Smith, R. D. et al. Assessing national biodiversity trends for rocky and coral reefs through the integration of citizen science and scientific monitoring programs. BioScience 67, 134–146 (2017).
Knowlton, N. et al. in Life in the World’s Oceans: Diversity, Distribution and Abundance (ed. McIntyre, A. D.) 65–77 (Wiley–Blackwell, 2010).
Edgar, G. J. & Stuart-Smith, R. D. Systematic global assessment of reef fish communities by the Reef Life Survey program. Sci. Data 1, 140007 (2014).
Edgar, G. J. & Barrett, N. S. An assessment of population responses of common inshore fishes and invertebrates following declaration of five Australian marine protected areas. Environ. Conserv. 39, 271–281 (2012).
Emslie, M. J. et al. Decades of monitoring have informed the stewardship and ecological understanding of Australia’s Great Barrier Reef. Biol. Conserv. 252, 108854 (2020).
Edgar, G. J. et al. Establishing the ecological basis for conservation of shallow marine life using Reef Life Survey. Biol. Conserv. 252, 108855 (2020).
Hughes, T. P. et al. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377 (2017).
Stuart-Smith, R. D., Brown, C. J., Ceccarelli, D. M. & Edgar, G. J. Ecosystem restructuring along the Great Barrier Reef following mass coral bleaching. Nature 560, 92–96 (2018).
Hughes, T. P. et al. Emergent properties in the responses of tropical corals to recurrent climate extremes. Curr. Biol. 31, 5393–5399.e5393 (2021).
Johnson, C. R. et al. Climate change cascades: shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. J. Exp. Mar. Biol. Ecol. 400, 17–32 (2011).
IUCN Standards and Petitions Working Group. Guidelines for Using the IUCN Red List Categories and Criteria, Version 7.0. http://intranet.iucn.org/webfiles/doc/SSC/RedList/RedListGuidelines.pdf (2008).
Fraser, K. M., Stuart-Smith, R. D., Ling, S. D. & Edgar, G. J. High biomass and productivity of epifaunal invertebrates living amongst dead coral. Mar. Biol. 168, 102 (2021).
Gilmour, J. P. et al. The state of Western Australia’s coral reefs. Coral Reefs 38, 651–667 (2019).
Long-Term Monitoring Program Annual Summary Report of Coral Reef Condition 2020/2021 (Australian Institute of Marine Science, 2021).
Eakin, C. M. et al. Caribbean corals in crisis: record thermal stress, bleaching, and mortality in 2005. PLoS ONE 5, e13969 (2010).
Edgar, G. J., Ward, T. J. & Stuart-Smith, R. D. Weaknesses in stock assessment modelling and management practices affect fisheries sustainability. Aquat. Conserv. 29, 2010–2016 (2019).
Babcock, R. C. et al. Decadal trends in marine reserves reveal differential rates of change in direct and indirect effects. Proc. Natl Acad. Sci. USA 107, 18256–18261 (2010).
Ling, S. D., Johnson, C. R., Frusher, S. D. & Ridgway, K. R. Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proc. Natl Acad. Sci. USA 106, 22341–22345 (2009).
Stuart-Smith, R. D., Edgar, G. J. & Bates, A. E. Thermal limits to the geographic distributions of shallow-water marine species. Nat. Ecol. Evol. 1, 1846–1852 (2017).
Chaudhary, C., Richardson, A. J., Schoeman, D. S. & Costello, M. J. Global warming is causing a more pronounced dip in marine species richness around the equator. Proc. Natl Acad. Sci. USA 118, e2015094118 (2021).
Bennett, S. et al. The ‘Great Southern Reef’: social, ecological and economic value of Australia’s neglected kelp forests. Mar. Freshwater Res. 67, 47–56 (2016).
Stuart-Smith, R. D. et al. Loss of native rocky reef biodiversity in Australian metropolitan embayments. Mar. Pollut. Bull. 95, 324–332 (2015).
Ling, S. D. et al. Pollution signature for temperate reef biodiversity is short and simple. Mar. Pollut. Bull. 130, 159–169 (2018).
Davies, T. E., Maxwell, S. M., Kaschner, K., Garilao, C. & Ban, N. C. Large marine protected areas represent biodiversity now and under climate change. Sci. Rep. 7, 9569 (2017).
Grech, A., Edgar, G. J., Fairweather, P., Pressey, R. L. & Ward, T. J. in Austral Ark (eds Stow, A., Maclean, N. & Holwell, G. I.) 582–599 (Cambridge Univ. Press, 2014).
Bates, A. E., Stuart-Smith, R. D., Barrett, N. S. & Edgar, G. J. Biological interactions both facilitate and resist climate-related functional change in temperate reef communities. Proc. R. Soc. B 284, 20170484 (2017).
Verges, A. et al. Tropicalisation of temperate reefs: Implications for ecosystem functions and management actions. Funct. Ecol. 33, 1000–1013 (2019).
Popova, E. et al. From global to regional and back again: common climate stressors of marine ecosystems relevant for adaptation across five ocean warming hotspots. Glob. Change Biol. 22, 2038–2053 (2016).
Edgar, G., Jones, G., Kaly, U., Hammond, L. & Wilson, B. Endangered Species: Threats and Threatening Processes in the Marine Environment. Issues Paper (Australian Marine Science Association, 1991).
Edgar, G. J. et al. El Niño, fisheries and animal grazers interact to magnify extinction risk for marine species in Galapagos. Glob. Change Biol. 16, 2876–2890 (2010).
Koslow, J. A., Miller, E. F. & McGowan, J. A. Dramatic declines in coastal and oceanic fish communities off California. Mar. Ecol. Progr. Ser. 538, 221–227 (2015).
Burrows, M. T. et al. Ocean community warming responses explained by thermal affinities and temperature gradients. Nat. Clim. Change 9, 959–963 (2019).
Hamilton, S. L. et al. Disease-driven mass mortality event leads to widespread extirpation and variable recovery potential of a marine predator across the eastern Pacific. Proc. R. Soc. B 288, 20211195 (2021).
Kummu, M. & Varis, O. The world by latitudes: a global analysis of human population, development level and environment across the north–south axis over the past half century. Appl. Geogr. 31, 495–507 (2011).
Halpern, B. S. et al. A global map of human impact on marine ecosystems. Science 319, 948–951 (2008).
Edgar, G. J. et al. Abundance and local-scale processes contribute to multi-phyla gradients in global marine diversity. Sci. Adv. 3, e1700419 (2017).
Krumhansl, K. et al. Global patterns of kelp forest change over the past half-century. Proc. Natl Acad. Sci USA 113, 13785–13790 (2016).
R Core Team. R: A language and environment for statistical computing., (R Foundation for Statistical Computing, 2021).
Stuart-Smith, R. D., Barrett, N. S., Stevenson, D. G. & Edgar, G. J. Stability in temperate reef communities over a decadal time scale despite concurrent ocean warming. Glob. Change Biol. 16, 122–134 (2010).
James, L. C., Marzloff, M. P., Barrett, N., Friedman, A. & Johnson, C. R. Changes in deep reef benthic community composition across a latitudinal and environmental gradient in temperate Eastern Australia. Mar. Ecol. Progr. Ser. 565, 35–52 (2017).
Collen, B. et al. Monitoring change in vertebrate abundance: the Living Planet Index. Conserv. Biol. 23, 317–327 (2009).
Skirving, W. et al. CoralTemp and the Coral Reef Watch Coral Bleaching Heat Stress Product Suite Version 3.1. Remote Sens. 12, 3856 (2020).
Tyberghein, L. et al. Bio-ORACLE: a global environmental dataset for marine species distribution modelling. Global Ecol. Biogeogr. 21, 272–281 (2012).
Edgar, G. J. Australian Marine Life: The Plants and Animals of Temperate Waters 2nd edn (Reed New Holland, 2008).
Edgar, G. J. Tropical Marine Life of Australia: Plants and Animals of the Central Pacific (New Holland, 2019).
Froese, R. & Pauly, D. FishBase 2000: Concepts, Designs and Data Sources (ICLARM, 2000).
Pinheiro, J., Bates, D., DebRoy, S. & Sarkar, D. nlme: linear and nonlinear mixed effects models (version 3.1-155). http://CRAN.R-project.org/package=nlme (2015).
R Core Team. R: A Language and Environment for Statistical Computing (version 4.2.0). http://www.R-project.org/ (R Foundation for Statistical Computing, 2013).
Becker, R. A., Wilks, A. R. & Brownrigg, R. mapdata: Extra map databases. R package version 2.3.0 (2018).
Acknowledgements
Support for field surveys and analyses was provided by the Australian Research Council; the Australian Institute of Marine Science; the Institute for Marine and Antarctic Studies; Parks Australia; Department of Natural Resources and Environment Tasmania; New South Wales Department of Primary Industries; Parks Victoria; South Australia Department of Environment, Water and Natural Resources; Western Australia Department of Biodiversity, Conservation and Attractions; The Ian Potter Foundation; Minderoo Foundation; and the Marine Biodiversity Hub, a collaborative partnership supported through the Australian Government’s National Environmental Science Programme. We thank the many colleagues and Reef Life Survey (RLS) divers who participated in data collection. RLS and ATRC data management is supported by Australia’s Integrated Marine Observing System (IMOS)—IMOS is enabled by the National Collaborative Research Infrastructure Strategy (NCRIS).
Author information
Authors and Affiliations
Contributions
G.J.E. conceived the study. G.J.E., R.D.S.-S., N.S.B., E.T., H.S., M.J.E., D.J.B., J.H., B.F., S.C.B., S.A.H., A.J., N.A.K., P.M., A.T.C., E.S.O., G.A.S., C.M., S.D.L., J.W.T., P.B.D., M.F.L., Y.S., J.S.-S., E.C., T.R.D., J.S., D.S., O.J.J., Y.H.F., L.D.-R. and T.J. contributed to collection of field survey data. E.T. identified corals and digitized coral density data. J.C.D. led sea surface temperature analysis. G.J.E., A.E.B. and F.J.H. analysed data. All photographs were taken by G.J.E. G.J.E. wrote the initial manuscript draft and all authors contributed to the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Sergio Floeter and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Sites surveyed around Australia.
Sites visited on single occasions are shown in blue, and in at least two years in red. Most individual sites are overlapping.
Extended Data Fig. 2 Total number of sites surveyed with differing annual survey frequency.
Number of annual surveys undertaken at sites from 2008 to 2021.
Extended Data Fig. 3 Recent change in mean abundance around the Australian continent for different species groupings.
Major taxonomic and biogeographic groupings were assessed using data with different site sampling frequency. a. Full dataset, as shown in Fig. 1, that includes sites surveyed in at least two separate years. b. Reduced dataset that includes sites surveyed in at least 3 years. c. Reduced dataset that includes sites sampled in at least four years. Data relate to the 10-year period from 2011 to 2021. The number of species considered per grouping and 95% confidence intervals are shown. Images by GJE.
Extended Data Fig. 4 Mean annual population trends for reef species with different biogeographic affinity in different regions.
Species trends relative to 2008 were categorised within three biogeographic groupings (tropical >23 °C; warm-temperate >17.5 °C, <23 °C; cool temperate <17.5 °C) for six regions around Australia. a, Northwest: Northern Territory, Western Australian coast north of 27°S. b, Southwest: western coast south of 27°S. c, South: southern coast east to 148°S. d, Northeast: Queensland, Coral Sea. e, Southeast: New South Wales; Victoria west to 148°S; f, Tasmania. In contrast to data presented in Fig. 5, relative abundance for each species and site was calculated using Population Trend Assessment Method 2, as the log ratio relative to site mean across years, then averaged for sites within 1° x 1° grid cells. Shading indicates ±1 SE (assuming a normal distribution across species estimates).
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Edgar, G.J., Stuart-Smith, R.D., Heather, F.J. et al. Continent-wide declines in shallow reef life over a decade of ocean warming. Nature 615, 858–865 (2023). https://doi.org/10.1038/s41586-023-05833-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-05833-y
- Springer Nature Limited
This article is cited by
-
Marine fishes experiencing high-velocity range shifts may not be climate change winners
Nature Ecology & Evolution (2024)
-
Marine protected areas promote stability of reef fish communities under climate warming
Nature Communications (2024)
-
Limited net poleward movement of reef species over a decade of climate extremes
Nature Climate Change (2024)
-
Exploring benthic habitat assessments on coral reefs: a comparison of direct field measurements versus remote sensing
Coral Reefs (2024)
-
Endangered giant kelp forests support similar fish and macroinvertebrate communities to sympatric stipitate kelp forests
Biodiversity and Conservation (2024)