Abstract
Pollen analyses of sediment cores from two small lakes within the boreal forest in the central Scandes Mountains help to elucidate the Holocene forest dynamics of the region. Analyses of pore/pollen grain diameter ratios of Alnus grains indicate the early Holocene presence of Alnus glutinosa in the study area. The results are discussed in conjunction with available pollen records to evaluate the importance of thermophilous trees during the early Holocene and to deduce the regional spread of Picea abies. Corylus avellana, Alnus glutinosa and Ulmus glabra were probably common constituents of the early Holocene forest. Tilia cordata may have occurred there as a rare tree. Pollen stratigraphies from the region do not indicate the occurrence of Quercus robur. The regional spread of Picea abies can be separated into two phases: a mid-Holocene establishment or first expansion of small outpost populations and a late-Holocene population expansion. The mid-Holocene shift in vegetation composition may have been caused by changes in the westerly airflow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Holocene development of the Fennoscandian flora has been the focus of numerous publications and summaries at a subcontinental scale (for example, Berglund 1968; Huntley and Birks 1983; Vasari 1986; Lang 1994). Although there is no shortage of environmental archives in Fennoscandia, the spatial distribution of Holocene vegetation reconstructions is uneven. They are concentrated in areas where the action of humans has substantially altered the vegetation composition, or in ecotones where climate change is thought to have a strong effect on vegetation dynamics. Therefore knowledge of the vegetation development of vast areas of boreal forest is often based on few or rather old investigations. Recent finds of macro- and megafossils from the Scandes Mountains have led Kullman (1995, 1996, 1998a, b, 2000, 2001, 2002) to suggest that thermophilous trees extended much further into the boreal forest than previously assumed, and that Picea abies existed locally on the Scandinavian peninsula long before its presence is detectable in pollen diagrams. Modern palaeoecological investigations from the boreal forest zone are therefore necessary to assess whether these finds signify a general pattern that was previously overlooked or represent remote single outposts of only local importance. Furthermore, additional pollen stratigraphies from the boreal forest zone help to close the gaps in the spatial coverage of palaeoecological investigations and improve analyses of tree spread or biome response to climate change on a sub-continental scale (Brewer et al. 2002; Giesecke and Bennett 2004).
This study aims to: (1) elucidate the Holocene forest dynamics within the central Scandes Mountains on mineral soils and below the subalpine forest belt; (2) evaluate the significance of early Holocene mega- and macrofossil finds of Picea abies and thermophilous trees; and (3) investigate the effect of more oceanic versus more continental climate on Holocene vegetation development.
Vascular plant nomenclature follows Tutin et al. (1964–80). Ages are given as calendar years before present (cal B.P.) where ‘present’ is defined as A.D. 1950.
Regional settings and previous investigations
The central Scandes Mountains comprise a region of relatively low elevations within the Scandinavian mountain chain that runs between Norway and Sweden, separated into isolated ranges by wide valleys (Lundqvist 1969). Low parts of the mountain chain permit a stronger influence of north Atlantic air to penetrate there, resulting in areas with a more oceanic climate. The forest below the tree line is dominated by Pinus sylvestris and Picea abies and belongs to the middle and northern boreal forest zones (Sjörs 1999). Outpost populations of Ulmus glabra occur locally throughout the region (Fig. 1). Alnus glutinosa has outpost populations west of the mountains, while outposts do not reach much higher than 300 m a.s.l. in valleys to the east (Westman 1985). Corylus avellana occurs frequently along the Norwegian coast up to about 65°30’ north. Tilia cordata and Quercus robur are not present in the region (Hultén 1971).
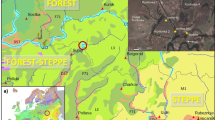
a–d Location and characteristics of the study sites. a Elevation image of the central Scandes Mountains with circles representing the occurrence of Ulmus glabra and diamonds marking occurrences of Alnus glutinosa and the broken white line marking the northern limit of more continuous occurrence of Alnus glutinosa (Hultén 1971). White stars mark the study sites, the black star indicates the area with finds of thermophilous trees (Kullman 1998a, b) and the position of the Storsnasen sites (Segerström and von Stedingk 2003). b Topographical map around Abborrtjärnen. c Topographical map around Styggtjärnen. d Bathymetric maps of the lakes, isobaths at 1 m intervals. The broken lines show an interval of 0.5 m
Macro remains of Quercus robur, Alnus glutinosa, Ulmus glabra and Corylus avellana are reported from the mountain Getryggen (63°10′ N, 12°22′ E) at an altitude of 740 m a.s.l. and a Tilia cordata inflorescence was recovered at Klockamyr not far from this site (Fig. 1). Radiocarbon analyses of these finds indicate early Holocene ages (Kullman 1998a, b).
Finds of Picea abies megafossils with dates spanning the time from about 9800 cal B.P. to 400 years ago are reported from different locations from the central Scandes Mountains (Kullman 2000, 2001). Two finds with late glacial ages originate from the mountain Åreskutan (63°53′ N, 15°43′ E) (Kullman 2002). Palynological investigations near Åreskutan (Ek 2004) and at Storsnasen (63°14′ N, 12°25′ E) (Segerström and von Stedingk 2003), from where many old P. abies macro- and megafossil finds are reported, do not show sufficient proportions of P. abies pollen to indicate the early Holocene presence of the tree, on palynological evidence. The pollen stratigraphy at Tönningfloarna (Lundqvist 1969) shows a discontinuous P. abies curve from the beginning of the sequence at about 9000 cal B.P. However, low pollen sums and the possibility of contamination during coring make it difficult to assess these small percentage values.
Selection and description of sites
Site selection was based on a survey of topographical maps and subsequent field evaluation of possible lakes. The survey was constrained by basin size of 100 to 300 m diameter, absence of incoming streams, proximity to present day Picea abies dominated forest, and accessibility. Thus the pollen collected in the lakes should to a large extent mirror the forest composition within a few kilometres around the lakes (Pennington 1979; Jacobson and Bradshaw 1981; Sugita 1993). An additional aim was to find a pair of sites from differing climate regions within the Scandes Mountains and to select one site in the same area as the Tönningfloarna site (Lundqvist 1969).
The two selected lakes, Abborrtjärnen and Styggtjärnen, are situated approximately 170 km apart in climatically differing areas of the Scandes Mountains (Table 1). Both are clear-water lakes with only a little peat growing at the lakeshore. Abborrtjärnen (63° 53′ N, 14° 27′ E), 6.5 m deep, is a bedrock basin at 387 m a.s.l. on a south facing slope that rises up to 640 m a.s.l. (Fig. 1). The forest around the lake is dominated by Picea abies with Pinus sylvestris, Betula spp., Populus tremula, Sorbus aucuparia, Salix spp. and Alnus incana. Dry Pinus sylvestris forests are frequent in the wider surroundings of the lake and on shallow soils within the lake catchment. Small bogs occur within the catchment of the lake (Fig. 1b). Forestry is the main land use in the area, and there is little farming.
Styggtjärnen (62°19′ N, 13°34′ E), 2 m deep, is located at an altitude of 715 m a.s.l. on the northeastern slope of the mountain Sånfjellet that reaches a maximum height of 1278 m a.s.l. (Fig. 1). The area is relatively sheltered from westerly cyclones by a large westward extension of the Scandes Mountains and thus belongs to the more continental regions in Scandinavia (Vedin 1995). The wide valleys are dominated by lichen-rich Pinus sylvestris forest, while Picea abies is often found along streams and rivers. With increasing altitude, P. sylvestris is gradually replaced by Picea abies, which dominates on the slopes of Sånfjellet between about 600 and 800 m a.s.l. Dry Pinus sylvestris forest grows on the hill northeast of the lake (Fig. 1) while Betula pubescens and Sorbus aucuparia are frequent in the Picea abies forest south of the lake. During summer the forest is used for cattle grazing. Trapping pits used for hunting elk and reindeer are mapped in the vicinity of both lakes. This hunting technique was used for at least the last 4000 years until it was banned in 1864 (Selinge 1974; Melander 1989) and the presence of the pits thus gives some information on the history of human impact around the lakes.
Material and methods
Sampling and sediment analyses
Cores were collected in winter from beneath the frozen lake surface. Two sets of overlapping cores were obtained from the centres of the lakes using a modified Livingstone piston corer (Wright 1967). Core segments were extruded in the field, wrapped in plastic film and aluminium foil and secured in supportive liners. The sediment-water interface and the loose uppermost part of the sediment sequences were collected using a Willner gravity sampler, immediately cut into 1 cm thick slices and packed in plastic bags. Care was taken that the cores would not freeze during fieldwork and all samples were stored in a cold room at 4°C until processing.
Magnetic susceptibility was measured on the cleaned and flattened core surfaces using a Bartington Instruments meter with a MS2E1 surface-scanning sensor (Bartington Ltd., Oxford, UK) together with the TAMISCAN-TS1 core logging system (http://www.geol.lu.se/PERSONAL/PRS/Tamiscan%20homepage.htm). Measurements were made at 2 mm intervals so that the 3.8 mm wide sensor sampled overlapping intervals to obtain a continuous record. A three-term running mean was applied to reduce noise.
Before subsampling the cores were described visually. Volumetric subsamples of 0.5 cm3 were taken for pollen analyses, the determination of water content and loss on ignition at 500°C (LOI). Samples were taken at even intervals using a modified syringe with a 0.5 cm diameter. Water content and loss on ignition were analysed immediately after subsampling.
Pollen analyses
Sample preparation for pollen analyses followed Bennett and Willis (2001). A known number of Lycopodium tablets (Stockmarr 1971, 1972) was added before preparation to enable the calculation of pollen concentrations and accumulation rates. Tablets were dissolved in warm water in the same test tubes used for the treatment of the samples. Samples were not sieved to allow for a maximum detection of stomata in the pollen slides. The residues were suspended in silicon oil. A minimum number of 1000 pollen grains and spores was counted in nearly all samples to reduce statistical uncertainty of less abundant pollen types (Maher 1972; Birks and Birks 1980). Pollen taxonomy follows Bennett (2004), modified for Sweden using the checklist by Karlsson (1997). Pollen percentage calculations were based on the sum of terrestrial pollen and spores, excluding aquatics and Sphagnum spores. Microscopic charcoal and stomata were tallied during standard counts. The diameters of well-preserved Alnus grains and their pores were measured in two samples from Abbortjärnen following Donner (1954) using a 100× oil-immersion objective.
Sediment age determination
Where possible, plant macrofossils were retrieved from predetermined sections of each core for age determination. About 2 cm3 of bulk sediment was collected from levels for radiocarbon dating if the core segment did not yield sufficient terrestrial plant remains. Age determination of the material was carried out by accelerator mass spectrometry (AMS). Results were calibrated against the IntCal98 calibration curve (Stuiver et al. 1998) using the BCal online system (http://bcal.shef.ac.uk) to improve probability estimates for samples with overlapping probability intervals and to obtain numerical descriptions of the confidence intervals. Age-depth models for both lakes were constructed using PSIMPOLL 4.10 (Bennett 2003). A polynomial was fitted to the age determinations by singular value decomposition to the weighted averages of the probability distributions. Sediment surfaces are assumed to have an age of −50±50 B.P. Confidence intervals for the age-depth plots were obtained by simulation, drawing random ages from the probability distributions of the calibrated ages (Bennett 2003).
Numerical analyses
Both percentage pollen diagrams were zoned statistically using the technique of optimal splitting by information content (Bennett 2003) independently on each of the datasets. The broken stick model (Bennett 1996) was used to assess the number of statistically significant zones. The calculation of confidence intervals on the pollen accumulation values follows Maher (1981), with additional use of propagation of errors methods (Parratt 1961). Principal component analysis (PCA) was employed to display the main trends in both datasets in two dimensions. The analysis was carried out on the covariance matrix of the taxon-combined percentage data from Abborrtjärnen and Styggtjärnen after square root transformation. All analyses and calculations were run on taxa that exceeded 1% total terrestrial pollen using PSIMPOLL 4.10 (Bennett 2003).
Results
Chronology and sediment accumulation
Both sets of radiocarbon age determinations from the two lakes are internally consistent (Table 2). However the dates for both lakes deviate from a near linear relationship so that different age-depth models used for the interpolation between the age determinations result in significantly different ages for the samples between the radiocarbon dates. For this reason a four-term polynomial, which only reflects the main trends, has been fitted to the data. Dates obtained from the age-depth models are rounded to the nearest 100 years without indicating errors.
The age-depth model for Abborrtjärnen (Fig. 2a) suggests even sediment accumulation with a change in sedimentation rate at around 3500 cal B.P. and an age estimate of 10240±140 cal B.P. for the basal sample. However the model is poorly constrained in the lowermost 15 cm. A linear extrapolation using the two lowermost dates gives a basal age of 9800±140 cal B.P., which may be a better estimate for the beginning of the sequence.
The uppermost radiocarbon date from the Abborrtjärnen sequence was obtained from bulk sediment and may therefore be influenced by a reservoir effect of the lake. Although this effect may be in the order of several decades it has only little influence on the age depth model shown in Fig. 2a. The radiocarbon age determinations on bulk sediment from Styggtjärnen may also be influenced by a reservoir effect of the lake. However, this effect may be smaller than at Abborrtjärnen as the lake is shallow and well aerated.
The age-depth model for Styggtjärnen (Fig. 2b) indicates a basal age of 9950±160 cal B.P. for the sequence. Linear extrapolation however yields a later date of 9450±150 cal B.P., which seems more likely as the material for dating was extracted from the lowermost highly organic sediment and the basal sample was derived from minerogenic sediments that may have been deposited more rapidly. Two age determinations give an age difference of only 10 years and each date lies within the error interval of the other. This suggests a rapid sedimentation in the lake centre during the time from about 4400 to 3800 cal B.P., which is also supported by the adjacent dates (Fig. 2b). Pollen concentration changes little in samples from this time period, while pollen accumulation rate calculations result in major peaks of all taxa during this period. The pollen spectra of samples from this time period are very similar and do not show signs of the incorporation of older material. It is therefore likely that during the time interval from about 4400 to 3800 cal B.P. more sediment was focused in the middle of the lake.
Although the age-depth models (Fig. 2) indicate changes in the rate of sediment accumulation in both lakes, the LOI and magnetic susceptibility records (Fig. 3) reveal that there were no major disturbance events in the catchments of the lakes. Changes in the sediment composition of both lakes are displayed in Fig. 3 and described in Table 3. In general, Abborrtjärnen collects more inorganic material than Styggtjärnen as a result of the much larger catchment area.
Vegetation history
The vegetation history reflected in the pollen records from Abborrtjärnen and Styggtjärnen follows a similar trend at both sites (Fig. 4, Table 4). Statistical splitting of the records yielded 5 significant zones for Styggtjärnen and 6 for Abborrtjärnen. However the lowermost zone in the pollen stratigraphy from Abborrtjärnen consisted of only one sample and this split was therefore not used. This lowermost sample from Abborrtjärnen contains a high proportion of Hippophaë rhamnoides pollen. The steep slopes north of the lake may have provided a suitable habitat for the shrub. This initial pioneer stage is not seen in the Styggtjärnen record. The further development of the vegetation around both lakes is described in Table 4 and although it follows the same general pattern at both sites, changes are more pronounced in the Abborrtjärnen record.
a–b Percentage pollen diagrams showing selected taxa. a Abborrtjärnen; b Styggtjärnen. A ten-fold exaggeration is used to depict rare taxa. Microscopic charcoal and stomata are expressed as percentage of the pollen sum. Note that charcoal was not estimated for the lowermost samples in Abborrtjärnen below 9000 cal B.P
Measurements of the pore/pollen grain diameter ratio of four-pored Alnus pollen from two samples from Abborrtärnen (Fig. 5) show different distributions. The older sample contains a higher proportion of grains with low ratios from 0.07 to 0.09, while 70% of the grains in the younger sample have ratios of 0.1 and 0.11. The younger sample did not contain sufficient numbers of five-pored Alnus pollen, but measurements on the five-pored Alnus grains in the older sample reveal a similar distribution as for the four-pored grains. Both distribution curves show two peaks with ratios of 0.08 and 0.10–0.11 respectively. These values correspond well to the average values for the ratios of about 0.08 for Alnus glutinosa and 0.1 for A. incana obtained by Donner (1954). Thus, the sample dating to 8800 cal B.P. probably contains a mixture of A. glutinosa and A. incana pollen while the 400-year-old sample has probably only A. incana pollen.
a–b Frequency distribution of the pore/diameter ratio of Alnus pollen at Abborrtjärnen. a Measurements on four-pored grains; solid line with diamonds = sample dated to 8800 cal B.P., n=82; broken line with circles = sample dated to 400 cal B.P., n=49. b Measurements on five-pored grains from the sample dated to 8800 cal B.P., n=78
Principal component analyses
The combined PCA of the two datasets (Fig. 6) separates the samples into the same groups as the stratigraphically constrained zonation of the records. The first axis explaining the largest variance splits the samples according to their proportion of Picea abies versus Alnus and Betula pollen. The separation of samples according to their proportion of Pinus sylvestris versus Alnus and Betula pollen takes place on the second axis. Samples pre-dating the arrival of Picea abies pollen are clustered in a narrow band stretching between the vectors for the deciduous trees on one side and Pinus sylvestris on the other. The sequence begins with samples dominated by P. sylvestris (zone A1, S1), which then plot at the far end of the band as a consequence of higher proportions of pollen from deciduous trees (zone A2, S2). The third cluster (zone A3, S3) falls in between the above two groups, corresponding to the decline of Alnus pollen and an increase in P. sylvestris pollen percentages. Samples containing Picea abies pollen plot in a trajectory oriented at right angles to the alignment of the former groups. They are spread according to their amount of P. abies pollen so that samples from zones A4, S4 and A5, S5 form distinct groups.
a–b Principal component analyses of the taxon combined pollen percentage data from Abborrtjärnen and Styggtjärnen. The first two axes are significant and explain the variance to 62% and 19% respectively. a Plot of eigenvectors, broken line marks the space of the sample plots. b Plot of samples from Abborrtjärnen. c Plot of samples from Styggtjärnen. Affiliation of samples to pollen zones (Fig. 4) are marked with symbols (rectangle = zone 1, ex = zone 2, circle = zone 3, triangle = zone 4, diamond = zone 5)
Pollen accumulation rates at Abborrtjärnen
Pollen accumulation rates of Abborrtjärnen (Fig. 7), which give a better insight into the direction of vegetation change around Abborrtjärnen than pollen percentages, have been calculated from the pollen concentrations and the age-depth relationship (Fig. 2). The sediment history at Styggtjärnen is complex, probably due to sediment focussing, and the pattern of pollen accumulation rates (not shown) reflects sedimentation history and not vegetation history.
Pollen accumulation rates from Abborrtjärnen illustrate how Picea abies displaces not only Betula but also Pinus sylvestris, the percentages of which increase (Fig. 4a). The increase and decline of Alnus pollen on the other hand occurs independently of changes in the accumulation rates of the other tree taxa. It is interesting to note that the pollen accumulation rates of most tree taxa are rising across the A-1/A-2 zone boundary, but P. sylvestris accumulation rates do not decline until the following sample. It becomes apparent that the decline of Alnus and Ulmus at the A-2/A-3 boundary is accompanied by a decline in Betula without an increase in P. sylvestris pollen accumulation unlike its percentages, which occurs later in this zone.
The loss on ignition (LOI) record (Fig. 7) may indicate changes in catchment hydrology and lake productivity and allows examination of parallels in vegetation composition. The rise in LOI at 7100 cal B.P. coincides with a drop in Alnus accumulation rates. The drop in LOI before 3300 cal. B.P. precedes the initial rise of the Picea abies curve and coincides with a peak in Juniperus communis as well as a decline in fern spores. Also noticeable is the significant drop in Betula and Alnus pollen accumulation rates at 8200 cal B.P., which has no reflection in the LOI record.
Discussion
Deglaciation and early forest establishment
The pattern of deglaciation in the southern Swedish mountains is well described from geomorphological evidence (Borgström 1989), although the timing of the different stages remains uncertain (Lundqvist 2002). Borgström (1989) suggests an ice retreat from the west, uncovering first the high mountains along the border with Norway and later single mountain tops to the east, which formed islands between the receding glaciers. Finds of wood remains on top of the mountain Åreskutan (Kullman 2002) suggest that such nunataks occurred as early as 16700 cal B.P. The growth of boreal trees on late glacial nunataks is controversial, but the existence of ice-free islands during the late glacial seems likely (McCarroll and Nesje 1993; Dahl et al. 1997; Rye et al. 1997).
The Dovre Mountains in central Norway were already ice-free at the onset of the Holocene and lake sedimentation there started as early as 11600±1000 cal B.P. (Eide 2003). The earliest vegetation cover on Dovre consisted of pioneer taxa including Hippophaë rhamnoides, Salix, Dryas octopetala and Saxifraga oppositifolia. Tree birch reached the site (at 1169 m a.s.l.) only at 10500 cal B.P. (Eide 2003). Lake sediments or peat predating the onset of the Holocene have not been recovered from the study region. The oldest estimate for the onset of the lake sedimentation in the study area is reported from Lake Spåime at 887 m a.s.l. with a basal age of 10700 cal B.P. (Hammarlund et al. 2004). The macrofossil record from this site starts with Salix leaves and fruits, while Betula pubescens fruits are recorded from 9700 cal B.P. onwards.
During deglaciation, the Hotagen valley, where Abborrtjärnen is situated, harboured a system of ice-dammed lakes separated by stagnating ice (Lundqvist 1969). The bottom sediments of Abborrtjärnen do not show laminated clays, but contain fine gravel, which may indicate that the basin was filled with stagnant ice until the onset of organic sedimentation. The meltdown of the ice probably left unstable slopes especially north of the lake, which were immediately occupied by Hippophaë rhamnoides. The occurrence of this shrub generally signifies the first Holocene stage of plant succession on unstable ground within the Scandes Mountains (Moe et al. 1996; Engelmark 1996). Pinus sylvestris and Betula may have been well established around Abborrtjärnen at the time that lake sedimentation started. The find of P. sylvestris stomata and the drop of Poaceae pollen in the second sample indicate a rapid forest establishment in the lake catchment.
The late start of the Styggtjärnen record, situated at the northern slope of Sånfjället, may be explained by its proximity to an ice divide and thus late deglaciation (Borgström 1989). The lack of Hippophaë rhamnoides pollen in the basal samples suggests that mountain slopes above the lake must have been stabilised by vegetation cover at the onset of the lake sedimentation. Moreover, low proportions of herb and heath pollen and the presence of Pinus sylvestris stomata in the lowermost minerogenic sample indicate that a woodland consisting of Betula and Pinus was already established around the lake. It can be presumed that the small and shallow lake basin might not have been blocked by dead ice long after deglaciation of its surroundings. Therefore the presence of woodland from the beginning of the lake sedimentation may indicate that woodland was established on the slopes of Sånfjället before final wasting of the glacier in the valley.
The spread of Alnus
The expansion of Alnus populations in southern and south-central Sweden occurred almost simultaneously at about 9500 cal B.P. (for example, Digerfeldt 1972, 1977; Göransson 1977; Almquist-Jacobson 1994). The rise of the Alnus curve at Abbortjärnen is centred at about 9000 cal B.P. and slightly later at Styggtjärnen. However both records show the presence of the pollen type before that time.
Even though separation of the pollen of Alnus glutinosa and A. incana is possible (Erdtman 1953; Donner 1954), it is not regularly carried out in pollen investigations in Fennoscandia, and the Alnus curve in many pollen diagrams probably represents both A. glutinosa and A. incana. Only a few studies address the different vegetation histories of the two trees (for example, Andersson 1893; Wenner 1968; Tallantire 1973, 1974). In southern Sweden, where A. incana does not occur naturally today, it has been shown that the rise of Alnus pollen frequencies coincides well with frequent finds of A. glutinosa fruits, while fruits of A. incana are absent (Gaillard 1984; Thelaus 1989). The establishment of A. incana in Troms, northern Norway, may have occurred as early as 9400 cal B.P. (Jensen et al. 2002). However the identity of the species of Alnus which arrived first and caused the steep rise in the Alnus curve in central Sweden is unclear (Almquist-Jacobson 1994). Wenner (1968) suggested that A. incana was the first to arrive in central Sweden, according to morphological differentiation of Alnus pollen at the beginning of the continuous curve. This succession is also supported by Tallantire (1974) based on macrofossil investigations from central Norway (Tallantire 1973) and published finds of Alnus fruits (Tallantire 1974).
Measurements on Alnus pollen from a sample from Abborrtjärnen dated to 8800 cal B.P., just before the Alnus maximum indicate that both Alnus species grew around the lake during that time. Samples from the beginning of the continuous Alnus curve yield too little Alnus pollen to be able to analyse the distribution of the pore/pollen grain diameter ratios. It is not unlikely that A. incana was the first to arrive, possibly from the east; however the early Holocene Alnus maximum may reflect a high abundance of both Alnus species. At present the limit of the continuous distribution of A. glutinosa is situated far to the southeast of both sites. It is therefore likely that the decline in Alnus pollen was caused by a retreat of A. glutinosa due to changing climate conditions. Declining Alnus accumulation rates are not matched by increases in pollen accumulation rates of other pollen types, suggesting that the habitat that was formerly occupied by A. glutinosa may have been left open. This could represent a transition from alder carrs to Sphagnum dominated bogs that bear vegetation with low pollen production. Thus the decline of A. glutinosa may also be connected to habitat loss due to paludification.
It seems likely that the rise of the Alnus curve in central Sweden represents at least partly the expansion of A. glutinosa, which indicates an almost synchronous expansion of the tree over the vast area of southern and central Sweden (cf. Wenner 1968). Furthermore the Holocene history of A. glutinosa in central Sweden seems to compare well to that of Corylus avellana, with an early Holocene maximum extension of the distribution limits and a later decline. However this pattern differs from that on the other side of the Scandes Mountains, where the rise of the Alnus and C. avellana-type curves occurs at about 7800 cal B.P. (Tallantire 1973).
The importance of thermophilous trees during the early Holocene
Both pollen records show the occurrence of Corylus avellana-type pollen from the first sample onwards. The Abborrtjärnen record shows a C. avellana-type maximum of 1.4% at the time of the rise of the Alnus curve at 9000 cal B.P. At Styggtjärnen, the curve starts with 1.6% and reaches a maximum value of 2.7% at the same pollen stratigraphic position. However, the diagram from Storsnasen (Segerström and von Stedingk 2003), which is close to a site with finds of C. avellana macrofossils (Kullman 1998a), shows C. avellana-type pollen percentage values of less than 0.5% during the same time period. While the pollen percentages at Storsnasen are too low to indicate the presence of C. avellana, the values at Abborrtjärnen and Styggtjärnen may indicate the presence of local outpost populations within the region (Huntley and Birks 1983).
Ulmus was more abundant around Abborrtjärnen with a maximum of 4% at 6700 cal B.P., while the pollen type reaches a maximum of only 1.2% at 6300 cal B.P. in the Styggtjärnen record. However, Styggtjärnen shows a continuous curve from the beginning of the sequence about 9500 cal B.P., and at Abborrtjärnen the continuous curve begins at about 9500 cal B.P. Stornasen I shows a continuous Ulmus curve from about 7500 cal B.P. onwards with a maximum of over 1% at about 5500 cal B.P., however the Stornasen II record shows 1% Ulmus pollen already at about 9700 cal B.P. (Segerström and von Stedingk 2003). Alnus glutinosa was probably more widespread in the central Scandinavian peninsula than at present, as discussed above.
It is difficult to reconstruct the past altitudinal range limit of a taxon in a mountainous area by means of pollen analyses. However, the pollen records from Abborrtjärnen and Styggtjärnen suggest that Ulmus and Alnus glutinosa were frequent constituents of the early- to mid-Holocene forest of the region and that Corylus avellana may have been present locally during the early Holocene. The occurrence of these tree taxa at Getryggen (Kullman 1998a), at an altitude similar to that of Styggtjärnen, might have been possible. The radiocarbon dates for the macrofossils of A. glutinosa and C. avellana (Kullman 1998a) are in agreement with the pollen record. An Ulmus glabra leaf dates to about 9500 cal B.P. (Kullman 1998a), which is earlier than the maximum occurrence of the pollen type and may reflect an early outpost of the tree.
At Abborrtjärnen sparse finds of Tilia cordata pollen start at 7300 cal B.P., while the Styggtjärnen record has frequent finds starting at 7000 cal B.P. that form a discontinuous curve. The pollen stratigraphy from Storsnasen (Segerström and von Stedingk 2003) shows single finds of Tilia pollen back to about 8000 cal B.P. The low pollen productivity of T. cordata (Sugita et al. 1999) makes it difficult to decide whether single finds of T. cordata pollen originated from small populations within the region or if they were transported from larger populations to the south. Before the expansion of Picea abies, T. cordata was frequent in the present day southern boreal forest (Giesecke in press). It seems possible that its distribution had extended up Ljusnan valley near Styggtjärnen and perhaps small populations grew on favourable sites even further north, but it was not a common tree in the region. However if single finds of the pollen type in the Storsnasen record (Segerström and von Stedingk 2003) are taken as an indication for the scattered occurrence of the tree at lower elevations then Kullman’s (1998b) radiocarbon date of a T. cordata inflorescence is consistent with the pollen record.
In contrast to Tilia, Quercus pollen production is relatively high (Sugita et al. 1999) and the pollen type is well dispersed. The Abborrtjärnen record shows only a discontinuous curve of Quercus pollen for the early Holocene and the continuous curve at Styggtjärnen consists of values that are as low as in the surface sample. These records show that it is unlikely that the tree occurred regularly this far north in the Scandes Mountains, and the early Holocene macrofossil find of Q. robur (Kullman 1998a) at a high latitude and altitude may represent an odd early Holocene outpost of the tree.
Mid Holocene vegetation change
Both pollen records reveal a significant change in the composition of the pollen spectra between 5000 and 6000 cal B.P. but there was no major forest tree expansion in the region during the mid-Holocene. The change in pollen composition at the A2/A3 and S2/S3 boundary is not sharp, but rather a trend from high Alnus and Betula proportions to increasing Pinus sylvestris pollen percentages (Fig. 6). The decline of Ulmus marks the boundary in both records, while an increase in Ericaceae pollen occurs at the boundary only in the Styggtjärnen pollen stratigraphy. This increase in Ericaceae pollen coincides with the more frequent occurrence of Rubus chamaemorus pollen and a marked rise in Sphagnum spores, indicating paludification near the lake edge. A major shift in the composition of peat forming plants also occurred at Storsnasen about 130 km to the north (Segerström and von Stedingk 2003), however about 800 years earlier. Although these changes in local hydrology might have been triggered by a regional increase in effective moisture (Korhola 1995; Bauer et al. 2003), changes in pollen composition (Fig. 6) rather indicate a trend to drier conditions.
The time interval between 5000 and 6000 cal B.P. is characterised by a fragmentation and decline of the tree-limit ecotone in the southern and central Scandes Mountains (Kullman 1995; Gunnarsdóttir 1996; Barnett et al. 2001; Eide 2003). Furthermore reconstructions of sea-surface temperatures of the Norwegian Sea show a progressive cooling from 5500 cal B.P. onwards (Calvo et al. 2002). Chironomid-based temperature reconstructions from Lake Spåime (Hammarlund et al. 2004) however, indicate higher temperatures between 5000 and 6000 cal B.P. and a subsequent cooling to persistently lower temperatures. A slightly different temperature trend emerges from chironomid analyses from south-central Norway (Velle et al. 2005) and northern Finland (Seppä et al. 2002), both showing a short-term drop in temperatures at about 5500 cal B.P. and a final decline at about 4000 cal B.P.
At Abborrtjärnen the A2/A3 boundary dates to 5620±50 cal B.P., which agrees well with the dates for the corresponding zone boundaries in the records of two small lakes (5680±70 and 5660±50 cal B.P.) about 360 km to the south and 250 km to the southeast respectively (Giesecke in press). The shift in vegetation composition around those lakes is interpreted to reflect a shift to a more continental climate with somewhat shorter growing seasons and possibly a prolonged snow cover. A shift of climate parameters in the above manner would be consistent with the lowering of the tree-line (Hammarlund et al. 2004) as well as with the reduction of broad-leaved trees at lower altitudes. A late snowmelt at higher elevations may also locally increase the effective moisture and enhance paludification. Styggtjärnen is situated at a higher altitude and in an already more continental situation, which may explain the somewhat delayed shift.
The spread of Picea abies
The most significant change in Holocene forest composition around both sites is the spread and expansion of Picea abies (Fig. 6). While the sub-continental population expansion of P. abies can be reconstructed using pollen analyses (Giesecke and Bennett 2004), the past presence of isolated trees in the surroundings of a coring site is difficult to deduce using pollen analyses only (Bennett 1988). Although P. abies pollen grains have a higher fall speed than those of Pinus sylvestris (Eisenhut 1961), grains may still be transported over long distances (Hicks 2001). The production of Picea abies pollen in Fennoscandia is low compared to that of Pinus sylvestris and Betula (Sugita et al. 1999), so the beginning of the continuous curve may be taken as an indication of regional if not local presence (cf. Birks 1989). A continuous pollen curve implies that a population of trees became established and at least maintained its niche and flowered continuously through time. The establishment of a small population in the vicinity of the coring site that subsequently declined would remain unnoticed. Thus the onset of the continuous curve, for a heavy and underrepresented pollen grain, marks the earlier phase of a gradually expanding population subsequently reaching great local abundance. A discontinuous curve on the other hand may represent small local populations affected by occasional disturbance events or long distance transport.
The onset of the continuous curve dates to about 4000 cal B.P. at Styggtjärnen, and to 3500 cal B.P. at Abborrtjärnen. At Storsnasen the continuous curve starts at about 5900 cal B.P., while small peaks preceding the continuous curve are interpreted as indications of early Holocene presence of the tree (Segerström and von Stedingk 2003). The pollen record from Tönningfloarna, 30 km east of Styggtjärnen, shows a continuous Picea abies pollen curve starting at about 5600 cal B.P. but with a peak in P. abies pollen as early as about 7500 cal B.P. (Lundqvist 1969). A pollen stratigraphy from Lidsjömyren, 60 km northeast of Abborrtjärnen, has a continuous P. abies curve dating back to 5300 cal B.P., but the abrupt rise of the curve occurs only at about 2500 cal B.P. (Lundqvist 1969). The pattern of an initial P. abies curve with low values and a later rise of the curve at about 2500 cal B.P. is also shown in the record from Abborrtjärnen. Likewise the P. abies curve at Styggtjärnen is divided into a slow rising part before, and maximum values after about 2500 cal B.P., which corresponds to the pattern in the neighbouring site Tönningfloarna (Lundqvist 1969). However other diagrams from the region (Lundqvist 1969; Königsson 1986) indicate a large variation in the timing of the onset of the continuous curve and to a smaller degree also in the timing of the rise of the curve. The records published by Lundqvist (1969) (including Lidsjömyren and Tönningfloarna) were partly obtained with the use of a Hiller corer and possible contamination of samples for pollen analyses and radiocarbon dating may be a factor.
The establishment and/or expansion of Picea abies in Fennoscandia often coincides with forest disturbance by fire or humans (Huttunen 1980; Bradshaw and Hannon 1992; Björkman 1996; Segerström 1997; Hörnberg et al. 1999). Compared to pollen records to the southeast (Giesecke 2004), microscopic charcoal is rare in the pollen slides from both Abborrtjärnen and Styggtjärnen, indicating that disturbance through fire was of little importance throughout the Holocene. However both records show an increased sedimentation rate over the time of the onset of the continuous curve of P. abies. Although the rapid sedimentation in Styggtjärnen from about 4400 to 3800 cal B.P. was probably caused by internal lake dynamics, it may have been induced by changes in the lake catchment. At Abborrtjärnen, the start of the continuous P. abies curve coincides with a drop in the LOI curve, a decline in Alnus pollen influx and a peak in Juniperus communis pollen. The first decline in LOI at Styggtjärnen predates the beginning of the continuous curve while the second coincides with the S3/S4 boundary that marks the first minor rise of the P. abies curve and an increase in Poaceae pollen proportions. Human activity is established around both lakes by the presence of trapping pits. However, the timing of the construction and use of these pits is uncertain. Human impact on the vegetation is difficult to detect in the pollen records from both lakes. Pollen taxa that may indicate human activity in the boreal forest (Reynaud and Hjelmroos 1980; Hicks 1985) might also become more frequent due to paludification, changes in forest structure or a lowering of the tree line. However at Styggtjärnen Rumex pollen is found only from 4200 cal B.P. onwards just as the P. abies curve becomes continuous, and the first Plantago lanceolata grain was found at the S3/S4 boundary. Apart from J. communis pollen, taxa indicating human impact are not consistently present in the Abborrtjärnen pollen record before about 1000 cal B.P.
There is therefore slight evidence that the successful establishment of P. abies coincides with increased forest disturbance. However, no indication of disturbance coincides with the later expansion of P. abies populations. The first expansion of the population to sizes that are palynologically detectable occurred locally after 6000 cal B.P. This may represent an expansion of small outpost populations of P. abies that were regionally present from the early Holocene onwards (Kullman 2000, 2001, 2002; Segerström and von Stedingk 2003). However in the absence of strong biostratigraphic evidence for the presence of the tree during the early Holocene it might also be hypothesised that P. abies spread in the Scandes Mountains during the mid-Holocene at low population densities. Local establishment might have been affected by site dynamics and disturbance at different times. When comparing the timing of the onset of the continuous curve between the available records with different site characteristics there seems to be no clear association between site characteristics and early P. abies establishment.
Differences between sites
The comparison of pollen abundances between two sites is not straightforward, as differences in regional vegetation composition affect the representation of the vegetation surrounding the basin (Jackson and Wong 1994; Sugita 1994). However, Pinus sylvestris and Betula are the dominant pollen types at both sites and their different proportions have only a small effect on the proportions of the less abundant taxa, although the construction of difference diagrams is not feasible due to the coarse time resolution of the lower part of the Styggtjärnen record. Additionally, pollen concentrations and accumulation estimates from the lower part of the Styggtjärnen core (not shown) can be used for comparison with estimates from Abborrtjärnen.
Most prominent is the difference in the amplitude of change (Fig. 6) between both sites. While major changes in vegetation composition during the Holocene are mirrored in the Abborrtjärnen sediment, the vegetation composition around Styggtjärnen was more stable. The present day differences in climate between the two sites (Table 1) are caused by their positions in the Scandes Mountains (Fig. 1a), which have not changed during the course of the Holocene. Changes in westerly airflow should therefore have had a stronger influence on the vegetation around Abborrtjärnen than at Styggtjärnen. The forests surrounding Styggtjärnen were probably always growing under a more continental and dryer climate, which finds its expression in the continuously high values of Pinus sylvestris and, relative to Abborrtjärnen, lower early- to mid-Holocene abundances of Alnus and Ulmus pollen. The still relatively high Betula pollen proportions at Styggtjärnen probably originate to a large extent from the subalpine Betula belt on the slopes of Sånfjellet. Thus, the differences in vegetation composition between the sites during the Holocene support the hypothesis that the mid-Holocene vegetation change was caused by a reduction in westerly oceanic airflow (Giesecke in press).
The low winter precipitation (Table 1) and frequent late frosts (Vedin 1995) in the valleys around Sånfjellet may cause desiccation of Picea abies seedlings (Frey 1983). This may limit the occurrence of the tree to watercourses and higher elevations with more persistent snow cover. Due to the stronger influence of Atlantic air of the area around Abborrtjärnen, P. abies reaches a higher regional dominance here. The much slower expansion of P. abies populations around Styggtjärnen may be explained by infrequent years with successful regeneration at this altitude (Hofgaard 1993).
Conclusions
-
(1)
Early Holocene vegetation succession and the establishment of Pinus sylvestris, Hippophaë rhamnoides and Betula species in the central Scandes Mountains closely followed the decay of valley glaciers and stagnant ice or the drainage of ice-dammed lakes. The earliest woods may have occurred on the slopes of mountain ranges at times when the valleys were still occupied by glaciers, stagnant ice or ice-dammed lakes. Scattered occurrences of Corylus avellana might have been present in the central Scandes before the expansion of Alnus at about 9000 cal B.P. The rise of the Alnus pollen curve represents the expansion of both A. glutinosa and A. incana at Abborrtjärnen. Ulmus became regionally frequent in the lowlands at about 7300 cal B.P. and its decline between 5000 and 6000 cal B.P. marks a period of changing forest composition. The spread of Picea abies in the central Scandes is divided into two phases. Early P. abies outpost populations are palynologically detectable from 6000 cal B.P. onwards, while the final expansion of populations frequently occurs around 2500 cal B.P. Human activity has probably had little impact on the forest composition in the central Scandes Mountains.
-
(2)
Early Holocene macrofossil finds of Corylus avellana, Alnus glutinosa and Ulmus glabra (Kullman 1998a) show some correspondence with pollen records from the region. Tilia cordata was never a common constituent of the forests within the region, but may have had scattered occurrences. Pollen stratigraphies from the area show no indication of the occurrence of Quercus robur and the find of its macrofossils (Kullman 1998b) may only indicate a rare occurrence.
-
(3)
At present there is no palynological evidence from the Scandinavian mountains to support the indications of late glacial tree growth on Åreskutan (Kullman 2002). Finds of early Holocene Picea abies megafossils (Kullman 2000, 2001) remain without parallel in palynological records. However, they may signify the presence of P. abies populations that are too small to be detected palynologically.
-
(4)
The vegetation composition at Styggtjärnen with a more continental climate setting within the Scandes Mountains has changed little during the time of the Holocene. Abborrtjärnen, a comparable site in a more oceanic area on the other hand shows pronounced changes in vegetation composition. These differences may be partly caused by changes in westerly airflow that had a smaller influence on the area around Styggtjärnen because the large westward extension of the Scandes Mountains reduced the influence of mild and moist Atlantic air throughout the Holocene.
References
Alexandersson, H., Karlström, C., Larsson-McCann, S. (1991). Temperaturen och nederbörden i Sverige 1961–90. Referensnormaler [Temperature and precipitation in Sweden, 1961–90. Reference normals]. Sveriges Meteorologiska och Hydrologiska Institut (SMHI), Meteorologi, 81, 1–87
Almquist-Jacobson, H. (1994). Interaction of the Holocene climate, water balance, vegetation, fire, and cultural land-use in Swedish Borderland. Lundqua Thesis, 30, 1–82
Andersson, G. (1893). Studier öfver svenska växtarters utbredning och invandringsvägar. I Alnus glutinosa (L) Gaertn. och Alnus incana (L) Willd [Studies on the spread and origin of Swedish plant species. I Alnus glutinosa (L) Gaertn. and Alnus incana (L) Willd]. Botaniska Notiser, 217–239
Barnett, C., Dumayne, P.L., Matthews, J.A. (2001). Holocene climatic change and tree-line response in Leirdalen, central Jotunheimen, south central Norway. Review of Palaeobotany and Palynology, 117, 119–137
Bauer, I.E., Gignac, L.D., Vitt, D.H. (2003). Development of a peatland complex in boreal western Canada: Lateral site expansion and local variability in vegetation succession and long-term peat accumulation. Canadian Journal of Botany, 81, 833–847
Bennett, K.D. (1988). Holocene geographic spread and population expansion of Fagus grandifolia in Ontario, Canada. Journal of Ecology, 76, 547–557
Bennett, K.D. (1996). Determination of the number of zones in a biostratigraphical sequence. New Phytologist, 132, 155–170
Bennett, K.D. (2003). ‘Psimpoll’ and ‘pscomb’: C programs for analysing pollen data and plotting pollen diagrams. Available online from Uppsala University Palaeobiology program at URL http://www.kv.geo.uu.se/psimpoll.html
Bennett, K.D. (2004). Pollen catalogue of the British Isles. Available online from Uppsala University Palaeobiology program at URL http://www.kv.geo.uu.se/pc-intro.html
Bennett, K.D., Willis, K.J. (2001). Pollen. In: Smol, J.P., Birks, H.J.B., Last, W.M. (eds) Tracking environmental change using lake sediments. Terrestrial, algal, and siliceous indicators, vol 3. Kluwer, Dordrecht, pp 5–32
Berglund, B.E. (1968). Vegetationsutvecklingen i Norden efter istiden. [Vegetation history in Norden since the last ice age] Sveriges Natur, Årsbok, Geologiska institutionen, Göteborg, 31–52
Birks, H.J.B. (1989). Holocene isochrone maps and patterns of tree-spreading in the British Isles. Journal of Biogeography, 16, 503–540
Birks, H.J.B., Birks, H.H. (1980). Quaternary Palaeoecology. Arnold, London
Björkman, L. (1996). The Late Holocene history of beech Fagus sylvatica and Norway spruce Picea abies at stand-scale in southern Sweden. Lundqua Thesis, 39, 1–4
Borgström, I. (1989). Terrängformerna och den glaciala utvecklingen I södra fjällen [Geomorpology and glacial history of the middle Swedish mountains]. Meddelanden från Narurgeografiska Institutionen vid Stockholms Universitet, Älvsbytryck
Bradshaw, R.H.W., Hannon, G. (1992). Climatic change in the control of vegetation dynamics within Fiby Forest, Sweden. Journal of Ecology, 80, 625–32
Brewer, S., Cheddadi, R., de Beaulieu, J.L., Reille, M. (2002). The spread of deciduous Quercus throughout Europe since the last glacial period. Forest Ecology and Management, 156, 27–8
Calvo, E., Grimalt, J., Jansen, E. (2002). High resolution U37K sea surface temperature reconstruction in the Norwegian Sea during the Holocene. Quaternary Science Reviews, 21, 1385–394
Dahl, S.O., Nesje, A., Øvstedal, J, (1997). Cirque glaciers as morphological evidence for a thin Younger Dryas ice sheet in east-central southern Norway. Boreas, 26, 161–80
Digerfeldt, G. (1972). The Post-Glacial development of Lake Trummen. Folia Limnologica Scandinavica, 16, 1–6
Digerfeldt, G. (1977). The Flandrian development of Lake Flarken. Regional vegetation history and palaeolimnology. University of Lund Department of Quaternary Geology. Report, 13, 1–101
Donner, J. (1954). Measurements of pollen of Alnus glutinosa and A. incana. Bulletin de la Commission Geologique de Finlande, 166, 49–55
Eide, W. (2003). Plant macrofossils as a terrestrial climate archive for the last 11000 years in south and central Norway. Doctoral thesis, University of Bergen, 1–134
Eisenhut, G. (1961). Untersuchungen über die Morphologie und Ökologie der Pollenkörner heimischer und fremdländischer Waldbäume. Forstwissenschaftliche Forschungen, Beihefte zum Forstwissenschaftlichen Centralblatt, 15, 1–68
Ek, L.-G. (2004). The establishment of Norway spruce (Picea abies (L.) Karst.) on two mountains in the Åre area – a follow-up of the macrofossil finds on Mount Åreskutan. Institutionen för skoglig vegetationsekologi SLU, Examensarbeten, 1, 1–17
Engelmark, R. (1996). North Sweden. In: Berglund, B.E., Birks, H.J.B., Ralska-Jasiewiczowa, M. (eds) Palaeoecological events during the last 15000 years: regional syntheses of palaeoecological studies of lakes and mires in Europe.Wiley, Chichester, pp 266–276
Erdtman, G. (1953). On the difference between the pollen grains in Alnus glutinosa and those in Alnus incana. Svensk Botanisk Tidskrift, 47, 449–450
Frey, W. (1983). The influence of snow on growth and survival of planted trees. Arctic and Alpine Research, 15, 241–251
Gaillard, M.-J. (1984). A palaeohydrological study of Krageholmssjön (Scania, South Sweden): regional vegetation history and water-level changes. Lundqua report, 25, 1–40
Giesecke, T. (2004). The Holocene spread of spruce in Scandinavia. Comprehensive summaries of Uppsala dissertations from the Faculty of Science and Technology, 1027
Giesecke, T. (in press). Holocene dynamics of the southern boreal forest in Sweden. The Holocene
Giesecke, T., Bennett, K.D. (2004). The Holocene spread of Picea abies (L.) Karst. in Fennoscandia and adjacent areas. Journal of Biogeography, 1523–1548
Göransson, H. (1977). The Flandrian vegetational history of Southern Östergötland. Lundqua thesis, 3, 1–147
Gunnarsdóttir, H. (1996). Holocene vegetation history and forest-limit fluctuations in Smådalen, eastern Jotunheimen, South Norway. European Palaeoclimate and Man, 13, 233–255
Hammarlund, D., Velle, G., Wolfe, B.B., Edwards, T.W.D., Barnekow, L., Bergman, J., Holmgren, S., Lamme, S., Snowball, I., Wohlfart, B., Possnert, G. (2004). Palaeolimnological and sedimentary responses to Holocene forest retreat in the Scandes Mountains, west-central Sweden. The Holocene, 14, 862-876
Hicks, S. (1985). Problems and possibilities in correlating historical/archaeological and pollen-analytical evidence in a northern boreal environment: an example from Kuusamo, Finland. Fennoscandia Archaeologica, 2, 51–84
Hicks, S. (2001). The use of annual arboreal pollen deposition values for delimiting tree-lines in the landscape and exploring models of pollen dispersal. Review of Palaeobotany and Palynology, 117, 1–29
Hofgaard, A. (1993). Seed rain quantity and quality, 1984–1992, in a high altitude old-growth spruce forest, Northern Sweden. New Phytologist, 125, 635–640
Hörnberg, G., Östlund, L., Zackrisson, O., Bergman, I. (1999). The genesis of two Picea-Cladina forests in Northern Sweden. Journal of Ecology, 87, 800–814
Hultén, E. (1971). Atlas över växternas utbredning i Norden. AB Kartografiska Institutet, Stockholm
Huntley, B., Birks, H.J.B. (1983). An atlas of past and present pollen maps for Europe: 0–13000 years ago. Cambridge University Press, Cambridge
Huttunen, P. (1980). Early land use, especially the slash-and-burn cultivation in the commune of Lammi, Southern Finland, interpreted mainly using pollen and charcoal analyses. Acta Botanica Fennica, 113, 1–45
Jackson, S.T., Wong, A. (1994). Using forest patchiness to determine pollen source areas of closed-canopy pollen assemblages. Journal of Ecology, 82, 89–99
Jacobson, G.L., Bradshaw, R.H.W. (1981). The selection of sites for paleovegetational studies. Quaternary Research, 16, 80–96
Jensen, C., Kuiper, G.J., Vorren, K.-D. (2002). First post-glacial establishment of forest trees: early Holocene vegetation, mollusc settlement and climate dynamics in central Troms, North Norway. Boreas, 31, 285–301
Karlsson, T. (1997). Förteckning over svenska kärlväxter [The vascular plants of Sweden – a checklist]. Svensk Botanisk Tidskrift, 91, 241–560
Königsson, L.-K. (1986). The Fjällnäs Project natural and cultural components in landscape formation. Striae, 24, 177–186
Korhola, A. (1995). Holocene climatic variations in southern Finland reconstructed from peat-initiation data. The Holocene, 5, 43–58
Kullman, L. (1995). Holocene tree-limit and climate history from the Scandes Mountains, Sweden. Ecology, 76, 2490–2502
Kullman, L. (1996). Norway spruce present in the Scandes Mountains, Sweden at 8000 BP: New light on Holocene tree spread. Global Ecology and Biogeography Letters, 5, 94–101
Kullman, L. (1998a). Non-analogous tree flora in the Scandes Mountains, Sweden, during the early Holocene - macrofossil evidence of rapid geographic spread and response to palaeoclimate. Boreas, 27, 153–161
Kullman, L. (1998b). The occurrence of thermophilous trees in the Scandes Mountains during the early Holocene: evidence for a diverse tree flora from macroscopic remains. Journal of Ecology, 86, 421–428
Kullman, L. (2000). The geographical history of Picea abies in Northern Sweden and adjacent parts of Norway. A contrarian hypothesis of postglacial tree-immigration patterns. Geo-Öko, 21, 141–172
Kullman, L. (2001). Immigration of Picea abies into North-Central Sweden. New evidence of regional expansion and tree-limit evolution. Nordic Journal of Botany, 21, 39–54
Kullman, L. (2002). Boreal tree taxa in the central Scandes during the Late-Glacial: Implications for Late-Quaternary forest history. Journal of Biogeography, 29, 1117–1124
Lang, G. (1994). Quartäre Vegetationsgeschichte Europas—Methoden und Ergebnisse. Gustav Fischer, Jena
Lundqvist, J. (1969). Beskrivning till Jordartskarta över Jämtlands Län [Description of the soil map of Jämtlands county]. Sveriges Geologiska Undersökning, Ca, Stockholm, 45, 1–418
Lundqvist, J. (2002). Weichsel-istidens huvudfas. In: Wastenson, L., Fredén, C. (eds) Sveriges Nationalatlas, Berg och jord [The National Atlas of Sweden, Geology]. Sveriges nationalatlas (SNA), Vällingby, pp 124–135
Maher, L.J. Jr. (1972). Nomograms for computing 95% limits of pollen data. Review of Palaeobotany and Palynology, 13, 85–93
Maher, L.J. Jr. (1981). Statistics for microfossil concentration measurements employing samples spiked with marker grains. Review of Palaeobotany and Palynology, 32, 153–192
McCarroll, D., Nesje, A. (1993). The vertical extent of ice sheets in Nordfjord, western Norway: measuring degree of rock surface weathering. Boreas, 22, 255–265
Melander, J. (1989). Fångstgropar i Jämtland [Trapping pits in Jämtland]. Fornvårdaren, 23, 115–127
Moe, D., Vorren, K.D, Alm, T., Fimreite, S., Mørkved, B., Nilssen, E., Paus, A., Ramfjord, H., Selvik, S.F., Sørensen, R. (1996). Norway. In: Berglund, B.E., Birks, H.J.B., Ralska-Jasiewiczowa, M. (eds) Palaeoecological events during the last 15000 years: regional syntheses of palaeoecological studies of lakes and mires in Europe. Wiley, Chichester, pp 162–167
Parratt, L.G. (1961). Probability and experimental errors in science: an elementary survey. Wiley, New York
Pennington, W. (1979). The origin of pollen in lake sediments: an enclosed lake compared with one receiving inflow streams. New Phytologist, 83, 189–213
Reynaud, C., Hjelmroos, M. (1980). Pollen evidence and radiocarbon dating of human activity within the natural forest vegetation of the Pohjanmaa region (northern Finland). Candonella, 35, 257–304
Rye, N., Nesje, A., Lien, R., Blikra, L.H., Eikenaes, O., Hole, P.A., Torsnes, I. (1997). Glacial geology and deglaciation chronology of the area between inner Nordfjord and Jostedalsbreen—Strynefjellet, western Norway. Norsk Geologisk Tidsskrift, 77, 51–63
Segerström, U. (1997). Long-term dynamics of vegetation and disturbance of a southern boreal spruce swamp forest. Journal of Vegetation Science, 8, 295–306
Segerström, U., von Stedingk, H. (2003). Early-Holocene spruce Picea abies (L.) Karst, in west central Sweden as revealed by pollen analysis. The Holocene, 13, 897–906
Selinge, K.-G. (1974). Fångstgropar [Trapping pits]. Fornvårdaren, 12, 1–39
Seppä, H., Nyman, M., Korhola, A., Weckström, J. (2002). Changes of treelines and alpine vegetation in relation to post-glacial climate dynamics in northern Fennoscandia based on pollen and chironomid records. Journal of Quaternary Science, 17, 287–301
Sjörs, H. (1999). The background: Geology, climate and zonation. Acta Phytogeographica Suecica, 84, 5–15
Stockmarr, J. (1971). Tablets with spores used in absolute pollen analysis. Pollen et Spores, 13, 615–621
Stockmarr, J. (1972). Determination of spore concentration with an electronic particle counter. Danmarks Geologiske Undersøgelse, Årbog, 87–89
Stuiver, M., Reimer, P.J., Bard, E., Beck, J.W., Burr, G.S., Hughen, K.A., Kromer, B., McCormac, F.G., van der Plicht, J., Spurk, M. (1998). INTCAL98 radiocarbon age calibration, 24,000-0 cal BP. Radiocarbon 40, 1041–1083
Sugita, S. (1993). A model of pollen source area for an entire lake surface. Quaternary Research, 39, 239–244
Sugita, S. (1994). Pollen representation of vegetation in Quaternary sediments - Theory and method in patchy vegetation. Journal of Ecology, 82, 881–897
Sugita, S., Gaillard, M.-J., Broström, A. (1999). Landscape openness and pollen records: a simulation approach. The Holocene, 9, 409–421
Tallantire, P.A. (1973). Some data on the history of alder in Trondelag Norway. Grana, 13, 18–24
Tallantire, P.A. (1974). The palaeohistory of the grey alder (Alnus incana (L.) Moench.) and black alder (A. glutinosa (L.) Gaertn.) in Fennoscandia. New Phytologist, 73, 529–546
Thelaus, M. (1989). Late Quaternary vegetation history and palaeohydrology of the Sandsjön-Årshult area, southwestern Sweden. Lundqua thesis, 26, 1–77
Tutin, T.G., et al. (1964–80). Flora Europaea. Vol 1 (1964); Vol 2 (1968); Vol 3 (1972); Vol 4 (1976); Vol 5 (1980). Cambridge University Press, Cambridge
Vasari, Y. (1986). The Holocene development of the Nordic landscape. Striae, 24, 15–19
Vedin, H. (1995). Air temperature. In: Wastenson, L., Raab, B., Vedin, H. (eds) National Atlas of Sweden, Climate, Lakes and Rivers. Almqvist and Wiksell, Stockholm, pp 44–57
Velle, G., Larsen, J., Eide, W., Peglar, S.M., Birks, H.J.B. (2005). Holocene environmental history and climate of Råtåsjøen, a low-alpine lake in central Norway. Journal of Paleolimnology, 33, 129–153
Wenner, C.-G. (1968). Comparison of varve chronology, pollen analysis and radiocarbon including an investigation of A0 as a synchronous level in Sweden. Stockholm Contributions in Geology, 18, 75–97
Westman, G. (1985). Klibbal som relikt i mellersta Norrland [Black alder as a relict in middle Norrland]. Svensk Botanisk Tidskrift, 79, 51–64
Wright, H.E. (1967). A square-rod piston sampler for lake-sediments. Journal of Sedimentary Petrology, 37, 975–976
Acknowledgements
I thank S. Fontana for participation in all fieldwork and K. Bennett for comments on the manuscript and help with the English language. I also appreciated the comments and suggestions of H.H. Birks and B. Odgaard. Field trips and radiocarbon analyses were financed by grants from the Swedish Society for Anthropology and Geography
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giesecke, T. Holocene forest development in the central Scandes Mountains, Sweden. Veget Hist Archaeobot 14, 133–147 (2005). https://doi.org/10.1007/s00334-005-0070-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00334-005-0070-2