Abstract
A 250 cm long core from El Palmar, a swamp area located along the Rio Hondo river in the south of the Yucatan Peninsula, near the Belizean border, reveals the environmental history of the mangrove and tropical forest of the last 5000 years. The period between 5000 and 4600 b.p. shows sandy deposits, which form the early infill and development of the swamp. A medium-statured tropical forest covered the area and members of the Moraceae and Fabacaeae dominated this early forest. The period between 4600 and 4000 b.p. presents a clear change to a mangrove system with Conocarpus erecta and Rhizophora mangle as dominant trees. This vegetational change is due to flooding of the Rio Hondo river, which deposits sediments of high salinity due to higher sea-level. The medium-statured forest became established at some distance from the swamp area. After 4400 b.p. C. erecta appears as the dominant mangrove species and the R. mangle stands are less predominant in the area. The tropical forest was close to the swamp area and was mainly composed of members of the Moraceae, Arecaceae and Fabaceae as dominant taxa of this vegetational mosaic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Holocene vegetation and climate history of the Yucatan Peninsula has long been of interest to palaeoecologists and archaeologists due to the impact of the Mayan culture on this tropical environment. However, until now, only few palaeoecological studies are available from this complex biogeographical region. Most of the available records are of Late Holocene age and few extend back beyond the Mid Holocene. The Lake Coba sediment core is one of the very few cores from the central part of the Yucatan Peninsula covering from the Mid Holocene onwards (Leyden et al. 1998). During the Mid Holocene semi-deciduous forest dominated the Lake Coba area. Earlier, Leyden et al. (1996) provided data on the Late Holocene vegetation development and high climatic variability from the San José Chulchaca cenote, located in the western region of the Yucatan Peninsula. Curtis et al. (1996) presented results of climatic variability from Punta Laguna, indicating that the period between 3300 and 1800 b.p. was relatively wet, while the following periods were considerable drier. Whitmore et al. (1996) studied the palaeolimnology of three lakes in the Yucatan Peninsula which gave evidence of changing water level fluctuations during the late Holocene.
The high resolution analysis of sediments from Lake Chichancanab by Hodell et al. (1995, 2001) gave clues to the high climatic variability of the Yucatan Peninsula and the catastrophic impact of drought on the Mayan culture. Changing climatic conditions during the Terminal Classic Period (a.d. 250–900) have been recently reported from the Cariaco basin (Venezuela) by Haug et al. (2003). Regional dry periods were dated to approximately a.d. 810, 860 and 910 (Haug et al. 2003). From the coast of the northern part of the State of Quintana Roo, data from Islebe and Sánchez (2002) showed vegetational changes in a mangrove ecosystem and also abrupt environmental change during the Classic period of the Maya culture. From the northern department of El Peten (Guatemala) palynological records from Leyden (1984), Vaughan et al. (1985) and Islebe et al. (1996) indicate human impact on the late Holocene vegetation. Rejmankova et al. (1995) studied floristic composition and standing crop in relation to soil characteristics in order to analyse the present day distribution of marsh vegetation in northern Belize.
This study reconstructs a tropical forest area in southern Quintana Roo near the Belizean border since the Mid Holocene, as sedimentary records spanning most of the Holocene are still rare (Islebe and Sánchez 2001). Because of its short distance from the Caribbean Sea, the record could present new evidence on changing sea levels for the area. Toscano and Macintyre (2003) constructed a corrected western Atlantic sea-level curve, and we were able to compare our calibrated 14C dates with those data published.
Study area
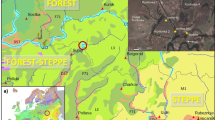
The study area is located near the small village of El Palmar (18°26′48″N, 88°31′47″W, 10 m elevation) about 50 km southeast of Chetumal, the capital of the state of Quintana Roo (Fig. 1). The coring site is a swampy area along the Rio Hondo, which forms the border between Mexico and Belize. The distance from the Caribbean Sea is about 23 km. The following climate data from Subteniente Lopéz are considered to be representative: the mean annual temperature is about 26°C, with a mean annual precipitation of 1300 mm (CETENAL 1975). Most of the precipitation falls between May and November and the area is under considerable threat of hurricane impact during those months (Sánchez and Islebe 1999). Vegetation types in the area include mangroves with stands of Rhizophora mangle, Avicennia germinans, Conocarpus erecta and Laguncularia racemosa. R. mangle forms the coastline and A. germinans, C. erecta and L. racemosa are found behind the R. mangle fringe. Canopy heights of the mangroves reach 15 m and generally mangrove forests contain few species of plants compared to the surrounding tropical forests or savanna types (Miranda 1958; Islebe 1998). The dominant vegetation type in the area is the medium-statured semi-evergreen tropical forest, which is also the dominant vegetation of the Yucatan Peninsula (Rzedowski 1978; Sánchez and Islebe 2002). This forest type has canopy heights up to 30 m with well defined strata (Sánchez 2000). Characteristic families of those forests are Moraceae, Sapotaceae, Meliaceae, Fabaceae, Burseraceae, Anacardiaceae, and Bignoniaceae among others. Dominant species of trees of these vegetation types are Manilkara zapota, Bursera simaruba, Brosimum alicastrum, Piscida piscipula, Metopium brownei and Sideroxylon spp.
Materials and methods
A 250 cm long core was collected in May 2000; extracted in 20 cm lengths using a Dachnovsky corer. The sampling interval of the El Palmar record was 10 cm. We used standard acetolysis techniques and heavy liquid separation with bromoform to extract the fossil pollen grains (Faegri and Iversen 1975) from subsamples of 1–2 cm3 of sediment. The residue was mounted in a glycerine gelatine medium. We aimed at a minimum pollen sum of 200 grains, but this was not achieved in all samples. Fern and fungal spores were not included in the pollen sum, because they are local elements. The pollen zones were established using TWINSPAN (Hill 1979), a divisive classification technique, which has proved to be very useful in defining indicator species and groups in paleoecology (Van't Veer et al. 1995). The order of the taxa in the pollen diagram was arranged according to ecological groups which form the present day vegetation (Sánchez and Islebe 2002). Those ecological groups include mangroves, medium-statured tropical forest and herb taxa of the medium-statured forest type. The pollen diagram was plotted with Palaeo Data Plotter (Juggins 2002).
The identification of the fossil pollen was made with help of the recent pollen collection of the ECOSUR Chetumal Herbarium, which hosts some 400 taxa from the Yucatan Peninsula. Published pollen atlases of Palacios et al. (1991), Hooghiemstra (1984) and Roubik and Moreno (1991) were used. AMS radiocarbon dating of unidentified organic material and fossil wood (Table 1) was done at INSTAAR, University of Colorado. The radiocarbon dates were calibrated with the CALIB 4.4.2 program (Stuiver and Reimer 1993; Stuiver and Braziunas 1993; Stuiver et al. 1998a, b) to compare them with data presented by Toscano and Macintyre (2003).
Results
Stratigraphy of core El Palmar
Sandy material was found at the bottom of the record between 220–80 cm. Organic rich material was found between 80 cm and the upper part of the core.
Description of the pollen diagram
Four pollen zones were identified (Fig. 2).
Pollen zone I (220–190 cm, about 5080–4800 b.p.) is characterised by high percentages of Moraceae (>50%), and lower percentages of taxa of the medium-statured forest, with taxa like Rubiaceae, Fabaceae types, Boraginaceae and Euphorbiaceae. Brosimum alicastrum is well represented in this zone. The mangrove vegetation is represented by increasing values of Conocarpus and Rhizophora (up to 30%). At the transition to pollen zone II Sapotaceae are present, indicating a well-developed forest (Islebe et al. 2001).
Pollen zone II (190–165 cm, about 4800–4600 b.p.) is dominated by high percentages of Conocarpus and Rhizophora (40%). Moraceae pollen decrease considerably compared to the previous zone. Percentages of Acacia (to 15%), Euphorbiaceae and Solanaceae increase.
Pollen zone IIa (165–140 cm, about 4600–4400 b.p.) is characterised by a steady decrease of Rhizophora and Conocarpus, increase of Moraceae, and higher percentages of the tropical forest families like Rubiaceae and Leguminosae-Acacia, Solanaceae is conspicuous.
In pollen zone III (140–80 cm, about 4400–3900 b.p.) Conocarpus dominates, while Rhizophora is less abundant (20%). Moraceae percentages decrease compared to zone IIa. A typical member of Conocarpus mangroves is Bravaisia tubiflora, which appears in low percentages at the top.
Pollen zone IV (80–0 cm, about 3800–3200 b.p.) is characterised by Moraceae around 20%, high percentages of Conocarpus (20–40%) and relatively low presence of Rhizophora. A peak in Arecaceae pollen is conspicuous, probably belonging to the species Acoelorraphe wrightii, characteristic of tasistal vegetation, a vegetation type present between mangroves and medium-statured forest. Borreria and Malphigiaceae are relatively well represented.
Discussion
The core shows the last 5000 years of vegetation change from a tropical forest type to a mangrove dominated forest and later a transition to a mixture of tropical forest and mangroves due to sea-level changes. During the Mid Holocene, taxa of the high and medium-statured forest dominated in the area until approximately 4800 b.p. Trees of the Moraceae, Rubiaceae and Leguminosae dominated during that period. However, only a few pollen grains of the Sapotaceae or Bignoniaceae were recorded, indicating more primary forest conditions as corroborated by modern pollen rain studies in the area (Islebe et al. 2001). In pollen zone I, a first peak of Moraceae appears with higher percentages of Brosimum alicastrum and Fabaceae types. This indicates moister conditions than at present. After 4800 b.p., environmental conditions changed drastically and the vegetation became a mangrove ecosystem, dominated by Rhizophora and Conocarpus.
Sea level changes
Changes in sea level changed the environment into a saline one. The changing sea-level coincides with corrected data presented by Toscano and Macintyre (2003). Comparing our calibrated data with mangrove peat data from Florida, Belize and Jamaica, the sea-level rose ca. 2–3 m between 6000 and 4000 cal b.p. Rise in water levels was earlier interpreted as rise in sea level by High (1975).
Between ca. 4600 and 3800 b.p. the soils had higher saline concentrations than most medium-statured forest trees can tolerate. The change from the closed forest to a more open mangrove system is related to changes of the Rio Hondo river, which inundated the area with saline sediments continuously from 4600 b.p. onwards. After 4300 b.p. the mangrove Rhizophora mangle increased up to 20%, however it only covers one sample from our core. Percentages of Rhizophora between 10 and 20% indicate an increase of this mangrove type close to the coring site near the coastline. Rhizophora and Conocarpus also form the present coastline vegetation, and the dominance of both mangrove species around 3800 b.p. could indicate the definite establishment of the present coastline in this area. Siemens (1978) found that present day hydrology is mainly controlled by intra-annual changes in the water table of ca. 1 m.
Vegetation change
About 4800 b.p., the nearby forest had a more secondary character as indicated by the presence of taxa like Burseraceae, Acacia and the appearance of Malvaceae. This first important development of mangrove vegetation lasted until ca. 4600 b.p., when for a brief period the tropical forest dominated in the area, as shown by the increase in Moraceae and Rubiaceae. The local swamp vegetation was characterised by Poaceae and Chenopodiaceae, nowadays typical of transition zones between mangrove areas and tropical forest. Species of Poaceae and Chenopodiaceae in the region can resist a wide range of ecological conditions, from occasional flooding to dry conditions. These conditions of altered Conocarpus mangrove are very common along the coasts of Quintana Roo, as these taxa tolerate changing hydrological conditions very well (Islebe and Sánchez 2002).
After ca. 4400 b.p. (pollen zone III) a strong increase in Conocarpus is observed and all relevant tropical forest taxa decrease considerably with exception of Euphorbiaceae. The remaining tropical forest was relatively distant from the coring site. The peak in trilete spores is probably from Acrostichum (Tryon and Tryon 1982), which is common in the area in brackish or salt water, and is also characteristic of mangrove communities. This is related to another slight increase in sea-level. However, we know from recent modern pollen rain studies in the area that the forest is not necessarily far away, the distance possibly being from 1 to 5 km (Islebe et al. 2001; Torrescano and Islebe unpubl.). The type of mangrove forest which dominated during this period is very similar in composition to present day mangrove stands distributed along the coast of Quintana Roo and has low species diversity (Islebe and Sánchez 2002). Such a species-poor environment suggests seasonal flooding, but Conocarpus erecta can also tolerate longer periods of drier conditions than R. mangle stands.
In the upper part of our sedimentary record there is a hiatus and the last 2500 years b.p. are not represented. At the transition from pollen zone III to IV, there is a noteworthy increase in percentages of pollen grains from Arecaceae. The most likely palm species based on the fossil pollen identifications are Acoelorraphe wrightii and Thrinax radiata, as both species are characteristic of transition zones between low-statured forests and mangroves, and are abundant at mangrove borders (Quero 1982; Sánchez and Islebe 2002). In addition, both palm species are relatively good pollen producers. The increase in Poaceae pollen supports the evidence of a development of a local palm swamp called tasistal. The recovery of the nearby forest is conspicuous with an increased representation of Moraceae, Malpighiaceae and Fabaceae.
Conclusions
Vegetation change from the Mexican-Belizean border area is inferred from the pollen record of El Palmar swamp area. The Mid Holocene climate in the region was humid, probably more humid than today. The change to a mangrove dominated vegetation cover is due to sea-level rise. The vegetation changed into a Conocarpus dominated mangrove forest and this vegetation type covered the swamp area until the top of the profile. The record shows that C. erecta replaced R. mangle as the dominant mangrove tree in the area. The present study elucidates the highly dynamic character of the vegetation types distributed within the southern part of the Yucatan Peninsula. However, more fossil pollen data and radiocarbon dates are needed to understand the palaeoecology of the region in detail.
References
CETENAL (1975) Estudio de gran visión para la colonización de la zona de Ucum, estado de Quintana Roo. Subsecretaria de la Presidencia. Departamento de estudios interdisciplinarios, México, D.F.
Curtis JH, Hodell DA, Brenner M (1996) Climate variability on the Yucatan Peninsula (Mexico) during the past 3500 years, and implications for Maya Cultural Evolution. Quatern Res 46:37–47
Faegri K, Iversen J (1975) Textbook of pollen analysis, 3rd edn. Blackwell, Oxford
Haug GA, Guenther D, Peterson LC, Sigman DM, Hughen KA, Aeschlimann B (2003) Climate and the collapse of Maya Civilization. Science 299:1731–1735
High LR (1975) Geomorphology and sedimentology of Holocene coastal deposits, Belize. In: Grace JB, Pussey III WC (eds) Belize shield carbonate sediments, clastic sediments, and ecology. American Association of Petroleum Geologists, Tulsa, pp 53–96
Hill MO (1979) TWINSPAN-A Fortran program for arranging multivariate data in an ordered two-way table by classification of the individuals and attributes. Cornell University, Ithaca
Hodell DA, Curtis JH, Brenner M (1995) Possible role of climate in the collapse of Classic Maya civilization. Nature 375:391–394
Hodell DA, Brenner M, Curtis JH, Guilderson T (2001) Solar forcing of drought frequency in the Maya lowlands. Science 292:1367–1370
Hooghiemstra H (1984) Vegetational and climate history of the high plain of Bogotá, Colombia: a continuous record of the last 3.5 million years. Dissertationes Botanicae 79. Cramer, Vaduz
Islebe GA (1998) Vegetación de Quintana Roo. In: Xacur J (ed) Enciclopedia de Quintana Roo, vol 8, pp 337–343
Islebe GA, Sánchez O (2001) Pasado y presente de la vegetación de Quintana Roo. Foresta Veracruzana 3:47–52
Islebe GA, Sánchez O (2002) History of Late Holocene vegetation at Quintana Roo, Caribbean coast of Mexico. Plant Ecol 160:187–192
Islebe GA, Hooghiemstra H, Brenner M, Curtis JH, Hodell DA (1996) A Holocene vegetation history from lowland Guatemala. The Holocene 6:265–271
Islebe GA, Villanueva R, Sánchez O (2001) Relación lluvia de polen—vegetación en selvas de Quintana Roo. Boletín de la Sociedad Botánica de México 69:31–38
Juggins S (2002) Palaeo Data Plotter. Version 1.0
Leyden BW (1984) Guatemalan forest synthesis after Pleistocene aridity. Proc Nat Acad Sci USA 81:4856–4859
Leyden BW, Brenner M, Whitmore T, Curtis JH, Piperno D, Dahlin B (1996) A record of long and short-term climatic variation from northwest Yucatan: Cenote San Jose Chulchacá. In: Fedick SL (ed) The managed mosaic: Ancient Maya agriculture and resource use. University of Utah Press, Utah, pp 30–50
Leyden BW, Brenner M, Dahlin B (1998) Cultural and climatic history of Coba, a lowland Maya City in Quintana Roo, Mexico. Quatern Res 49:111–122
Miranda F (1958) Estudios acerca de la vegetación. Instituto Mexicano de Recursos Renovables México
Palacios CP, Ludlow-Wiechers B, Villanueva-Gutierrez R (1991) Flora palinológica de la Reserva de Sian Ka'an, Quintana Roo, México. CIQRO, Quintana Roo, México
Quero E (1982) Las palmas silvestres de la península de Yucatán, UNAM, Instituto de Biología, México
Rejmankova E, Pope KE, Pohl MD, Rey-Benayas JM (1995) Freshwater wetland plant communities of northern Belize: Implications for paleoecological studies of Maya wetland agricultura. Biotropica 27:28–36
Roubik DW, Moreno JE (1991) Pollen and spores of Barro Colorado Island. Miss Bot Garden 36:1–270
Rzedowski J (1978) Vegetación de México. Editorial Limusa
Sánchez O (2000) Análisis estructural de la selva del jardín botánico. In: Sánchez O, Islebe GA (eds) El Jardín Botánico Dr. Alfredo Barrera Marín, fundamento y estudios particulares, El Colegio de la Frontera Sur and Consejo Nacional de la Biodiversidad, pp 59–74
Sánchez O, Islebe GA (1999) Hurricane Gilbert and structural changes in a tropical forest in southeastern Mexico. Glob Ecol Biogeogr 8:29–37
Sánchez O, Islebe GA (2002) Tropical forest communities in southeastern Mexico. Plant Ecol 158:183–200
Siemens AH (1978) Karst and pre-hispanic Maya in the Southern Lowlands. In: Harrison PD, Turner BL (eds) Pre-Hispanic Maya agriculture. University of New Mexico Press, Albuquerque, pp 117–143
Stuiver M, Braziunas TF (1993) Modelling atmospheric 14C influences and 14C ages of marine samples back to 10000 bc. Radiocarbon 35:137–189
Stuiver M, Reimer PJ (1993) Extended14C database and revised CALIB radiocarbon calibration pogram. Radiocarbon 35:215–230
Stuiver M, Reimer PJ, Bard E, Beck JW, Burr GS, Hughen KA, Kromer B, McComarc FG, Plicht J van der, Spurk M (1998a) INTCAL98 radiocarbon age calibration 24000-0 cal b.p. Radiocarbon 40:1041–1083
Stuiver M, Reimer PJ, Brazuinas TF (1998b) High-precision radiocarbon age calibration for terrestrial and marine samples. Radiocarbon 40:1127–1151
Toscano MA, Macintyre IG (2003) Corrected western Atlantic sea-level curve for the last 11, 000 years based on calibrated14C dates from Acropora palmata framework and intertidial mangrove peat. Coral Reefs 22:257–270. DOI 10:1007/s00338-003-0315-4
Tryon RA, Tryon AF (1982) Ferns and Allied Plants with special reference to tropical America. Springer, New York
Van't Veer R, Ran ETH, Mommersteeg JPM, Hooghiemstra H (1995) Multivariate analysis of the Middle and Late Pleistocene Funza pollen records of Colombia. Mededelingen Rijks Geologische Dienst 52:195–212
Vaughan HH, Deevey ES, Garrett-Jones SE (1985) Pollen stratigraphy of two cores from the Petén Lake District. In: Pohl M (ed) Prehistoric lowland Maya environment and subsistence economy. Harvard University Press, Cambridge, pp 73–89
Whitmore TJ, Brenner M, Curtis JH, Dahlin B, Leyden BW (1996) Holocene climatic and human influence on lakes of the Yucatan Peninsula, Mexico: an interdisciplinary, paleolimnological approach. The Holocene 6:273–287
Acknowledgements
CONACYT is acknowledged for financial support to the Project (N25035) “Sinopsis actual and paleoecológica de la selva de Quintana Roo”. We thank O. Sanchez, H. Weissenberger, H. Behling and one anonymous reviewer for comments on the manuscript
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Felix Bittmann
Rights and permissions
About this article
Cite this article
Torrescano, N., Islebe, G.A. Tropical forest and mangrove history from southeastern Mexico: a 5000 yr pollen record and implications for sea level rise. Veget Hist Archaeobot 15, 191–195 (2006). https://doi.org/10.1007/s00334-005-0007-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00334-005-0007-9