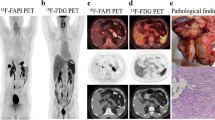
Abstract
Objective
To assess and compare the diagnostic performance of gallium-68-labelled fibroblast activation protein inhibitor ([68Ga]FAPI-04) and fluorine-18 fluorodeoxyglucose ([18F]FDG) positron emission tomography/computed tomography (PET/CT) in gastrointestinal cancer.
Methods
Fifty-one patients who underwent both [18F]FDG and [68Ga]FAPI-04 PET/CT for initial staging or restaging were enrolled. Histopathological findings, typical radiological appearances, and clinical imaging follow-up were used as the reference standard. The diagnostic performance of the two tracers was calculated and compared. The maximum standardised uptake value (SUVmax), mean SUV (SUVmean), tumour-to-mediastinal blood pool ratio (TBR), and tumour-to-liver ratio (TLR) of primary and metastatic lesions were measured and compared between two imaging modalities.
Results
In patient-based analysis, [68Ga]FAPI-04 showed much better diagnostic sensitivity than [18F]FDG in detecting primary tumour (94.44% [17/18] vs. 61.11% [11/18]), postoperative recurrence and metastases (95.65% [22/23] vs. 69.57% [16/23]), and peritoneal carcinomatosis (100% [28/28] vs. 60.71% [17/28]) (all p < 0.05). In lesion-based analysis, [68Ga]FAPI-04 showed higher sensitivity than [18F]FDG for detecting lymph node metastases. In peritoneal carcinomatosis, the median SUVmax (12.12 vs. 7.18) and SUVmean (6.84 vs. 4.11) with [68Ga]FAPI-04 were significantly higher than those with [18F]FDG (all p < 0.005). The TBR and TLR of [68Ga]FAPI-04 were significantly higher than those of [18F]FDG for detecting primary tumour, lymph node, liver, and peritoneal metastases (all p < 0.005). Therapeutic management changed in 13 patients according to [68Ga]FAPI-04 PET/CT compared with conventional imaging.
Conclusions
[68Ga]FAPI-04 is superior to [18F]FDG PET/CT for detecting primary tumour, postoperative recurrence and metastasis, and peritoneal carcinomatosis in gastrointestinal cancer.
Key Points
• [68Ga]FAPI-04 PET/CT showed significantly higher sensitivity than [18F]FDG PET/CT in the detection of primary tumour and postoperative recurrence and metastasis in patients with gastrointestinal carcinoma.
• [68Ga]FAPI-04 PET/CT had obvious advantages over [18F]FDG PET/CT in the detection of peritoneal carcinomatosis from gastrointestinal carcinoma with a much higher FAPI uptake value, TBR, and TLR.
• Although the median SUVmax and SUVmean of [68Ga]FAPI-04 were similar to those of [18F]FDG for the primary tumour, lymph node metastases, and liver metastases in gastrointestinal carcinoma, the TBR and TLR of the SUVmax and SUVmean were significantly higher on [68Ga]FAPI-04 PET/CT, causing the lesions to be displayed more clearly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gastric and colorectal cancers are two of the most common cancer types worldwide, being the fourth and second leading causes of cancer-related mortality, respectively [1]. Early diagnosis, accurate staging, and restaging are important for the management and prognosis of patients with gastrointestinal carcinoma. Computed tomography (CT) and magnetic resonance imaging (MRI) are the most common diagnostic methods used for evaluating gastrointestinal carcinoma, but they present some limitations in the detection of small primary lesions, occult lymph nodes, and peritoneal metastases.
Fluorine-18 fluorodeoxyglucose ([18F]FDG) positron emission tomography/computed tomography (PET/CT) has become an essential imaging modality for gastrointestinal cancer evaluation. However, FDG uptake varies with the histological type of gastrointestinal carcinoma, and the detection sensitivity and tracer uptake in signet ring cell carcinoma (SRCC) are low [2, 3]. Additionally, the physiological uptake of the gastrointestinal tract and acute gastroenteritis may mask lesion detection, leading to missed diagnosis of abdominal and pelvic lesions. A novel molecular imaging tracer is therefore needed for accurate evaluation of gastrointestinal carcinoma.
Cancer-associated fibroblasts (CAFs) in the tumour stroma can specifically express fibroblast activation protein (FAP), which is highly expressed by CAFs in more than 90% of human epithelial cancers; by contrast, FAP expression is nearly absent in normal adult tissues. Research has shown that high FAP expression is closely related to tumour invasion, lymph node metastasis, and a poor prognosis in many human malignant tumours [4]. Therefore, FAP has become an attractive therapeutic and diagnostic target in most frequent solid tumours. FAP-targeted treatment approaches mainly include immunoconjugates, chimeric antigen receptor T cells, vaccines, peptide-drug complexes, FAP inhibitors, and antibodies, and FAP imaging has been researched based on antibodies and inhibitors [5, 6]. Among the many radiopharmaceuticals available, quinolone-based FAP-specific small molecule inhibitors (FAPIs) show advantages over [18F]FDG, including high tumour accumulation, low background tissue uptake, and rapid in vivo clearance. These favourable characteristics have led FAPIs to be introduced into clinical imaging for the detection of many malignant tumours during the last few years [7,8,9,10]. [68Ga]FAPI-04 is the most widely used imaging agent and has great potential for detection of tumours and metastases [7].
Several recent studies showed that [68Ga]FAPI-04 had better detection sensitivity (100%) than [18F]FDG (53–86.6%) for the primary tumour of gastrointestinal carcinoma [10,11,12,13]. However, in the assessment of distant metastasis (e.g. peritoneal carcinomatosis and liver metastasis), the results of these relatively small sample studies (20–38 patients) were inconsistent. Therefore, we conducted the present study to assess and compare the diagnostic performance of [68Ga]FAPI-04 and [18F]FDG PET/CT in the detection of primary and metastatic lesions of gastrointestinal cancer and to quantify and compare the uptake of the two tracers. We further evaluated the diagnostic efficiency of the two tracers for detecting SRCC and mucinous adenocarcinoma (MAC) and analysed their impacts on patient management.
Materials and methods
Patients
This was a post hoc retrospective study of patients recruited as part of a larger ongoing single-centre prospective study to assess the application utility of [68Ga]FAPI-04 PET/CT in malignant tumours, which was approved by the medical ethics committee of Zhongnan Hospital of Wuhan University and registered in ClinicalTrials.gov (NCT05034146). Written informed consent was obtained from all included patients. Patients with newly diagnosed or previously treated gastrointestinal cancer who underwent both [18F]FDG and [68Ga]FAPI-04 PET/CT for staging or restaging between February and October 2021 were retrospectively collected. Patients were excluded because of the following reasons: (a) lack of a definitive diagnosis; (b) a history of other malignant tumours. Finally, fifty-one patients (31 men; median age, 57 years) were enrolled (Fig. 1).
Preparation of [68Ga]FAPI-04
[68Ga]FAPI-04 was prepared according to a previously validated process with minor modification using the 68Ge/68Ga generator and iQS-TS automated synthesis module [14] (supplementary material).
PET/CT image acquisition
Patients were required to fast for at least 6 h before the [18F]FDG PET/CT, and their blood glucose concentration was checked to ensure that it was less than 11.0 mmol/L. PET/CT (Biograph mCT; Siemens Healthineers) was performed approximately 40–60 min after an i.v. injection of [18F]FDG (3.70–5.55 MBq/kg) and 30–60 min after an i.v. injection of [68Ga]FAPI-04 (1.85–3.70 MBq/kg). The median time interval between [18F]FDG and [68Ga]FAPI-04 PET/CT was 1 day (interquartile range [IQR], 1–1). Details of the image acquisition and processing are provided in the supplementary material.
Image analysis
All PET/CT images were independently reviewed by two board-certified nuclear medicine physicians (C.L. and Y.T.) who were blinded to the conventional imaging (CT/MRI) findings and pathological information. In the qualitative analysis, the lesion was considered positive when its tracer uptake was obviously higher than that of the adjacent background tissue on visual evaluation (excluding physiological tracer uptake and definitive benign diseases). In the quantitative analysis, the maximum standardised uptake value (SUVmax) and mean SUV (SUVmean) of the lesions, mediastinal blood pool, and normal liver parenchyma were measured using Syngo Multimodality Workplace software (details in supplementary material). The tumour-to-blood pool ratio (TBR) and tumour-to-liver ratio (TLR) were calculated. Any differences in PET/CT findings between the two reviewers were resolved by consensus.
Reference standards
All primary tumours were confirmed by biopsy or surgical pathology. The reference standards for the final diagnoses of recurrence and/or metastatic lesions were histopathologic findings, typical CT and/or MRI findings, or clinical imaging follow-up of at least 3 months [14,15,16,17,18,19] (details in supplementary material).
Statistical analysis
All statistical analyses were performed using SPSS statistics (version 19.0; IBM). Categorical variables are expressed as frequency and percentage. Continuous variables are expressed as mean ± standard deviation or median (IQR). Interobserver agreement was assessed using Cohen’s kappa (κ). Diagnostic performance characteristics including sensitivity, specificity, positive predictive value, and negative predictive value were calculated. Continuous variables were compared using the Mann-Whitney U test. McNemar’s test was used to compare the diagnostic sensitivity. A two-sided p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
Fifty-one patients with gastrointestinal cancer were retrospectively enrolled, and their specific diagnoses included 28 gastric, 21 colorectal, and 2 appendiceal cancers (Table 1, supplementary Table S1). In total, 18 patients had a primary tumour; thirty-two patients had suspected postoperative recurrence and metastasis undergoing restaging; one patient’s primary tumour disappeared after chemotherapy and targeted therapy, but the patient showed extensive peritoneal carcinomatosis.
Interobserver agreement assessment
On [68Ga]FAPI-04 PET/CT, the two observers showed substantial consistency in assessment of the primary tumour (κ = 0.771, p = 0.001) and perfect agreement in evaluation of postoperative overall recurrence and metastasis (κ = 1.000, p < 0.001). On [18F]FDG PET/CT, the two readers also showed substantial agreement with a corresponding κ value of 0.776 (p = 0.001) and 0.747 (p < 0.001), respectively.
Primary tumour detection
The diagnostic performances of [68Ga]FAPI-04 and [18F]FDG for primary tumour are summarised in Table 2. [68Ga]FAPI-04 had much higher sensitivity for detection of gastrointestinal primary tumour than did [18F]FDG PET/CT. Of the 18 patients, [68Ga]FAPI-04 detected 17 of them giving a sensitivity of 94.44% (17/18), and [18F]FDG detected 11 giving a sensitivity of 61.11% (11/18) (p = 0.031). One cystic low-grade appendiceal MAC was not FAPI-avid. Five primary gastric cancers and two appendiceal MACs were false-negative on [18F]FDG PET/CT.
Comparisons of the primary tumour uptake of [68Ga]FAPI-04 and [18F]FDG are summarised in Table 3, Fig. 2, and supplementary Fig. S1. The median SUVmax and SUVmean of primary tumours were slightly lower with [68Ga]FAPI-04 than with [18F]FDG (11.60 vs. 12.11 and 6.62 vs. 7.13), but the differences were not significant (all p > 0.05). However, [68Ga]FAPI-04 showed significantly higher TBR and TLR than did [18F]FDG. The median TLR of the [68Ga]FAPI-04 SUVmax was approximately 3.34 times that of [18F]FDG (11.05 vs. 3.31, p < 0.001), and the median TLR of the [68Ga]FAPI-04 SUVmean was 4.48 times that of [18F]FDG (10.71 vs. 2.39, p < 0.001).
Comparison of primary tumour and metastatic lesions uptake activity between [68Ga]FAPI-04 and [18F]FDG. SUVmax, TBR, and TLR of primary tumour (a–c), lymph node involvement (d–f), liver metastases (g–i), and peritoneal carcinomatosis (j–l). Lines in plot represent median with interquartile range. TBR, tumour-to-mediastinal blood pool uptake ratio based on SUVmax; TLR, tumour-to-normal liver parenchyma uptake ratio based on SUVmax
Postoperative recurrence and metastasis detection
Thirty-two patients with suspected postoperative recurrence and metastasis of gastrointestinal carcinoma were evaluated. Of these, 23 patients were diagnosed with recurrence and/or metastasis. Eight patients had loco-regional relapse. Metastatic locations included lymph nodes, liver, peritoneum, ovary, lung, pancreas, muscle, and rectum. The sensitivity of [68Ga]FAPI-04 for detection of postoperative overall recurrence and metastasis was significantly higher than that of [18F]FDG (95.65% [22/23] vs. 69.57% [16/23], p = 0.031), but the specificities of [68Ga]FAPI-04 and [18F]FDG were the same (Table 2). A representative case is shown in Fig. 3.
Images of a 68-year-old man with a history of postoperative chemotherapy for rectal mucinous adenocarcinoma who underwent PET/CT for restaging. (a–c) Delayed abdominal and pelvic [68Ga]FAPI-04 PET/CT images show a markedly thickened posterior bladder wall with intense FAPI uptake (SUVmax = 15.12) and a FAPI-avid lesion in the prostate and presacral soft tissue (SUVmax = 14.06) (a, maximum intensity projection, MIP; b and c, axial fused images). In addition, a FAPI-avid benign lesion in the left 10th posterior rib (SUVmax = 13.56) and degenerative disease with intense FAPI uptake (SUVmax = 8.06) in the L2 vertebra were also revealed on the delayed [68Ga]FAPI-04 MIP image (a). (d–f) Delayed [18F]FDG PET/CT images show negative uptake in these lesions (d and e, axial fused images; f, MIP). In addition, a dilated left ureter is visible on both the [68Ga]FAPI-04 (a) and [18F]FDG (f) MIP images, and a slightly dilated right ureter is also visible on [18F]FDG MIP image (f). The patient underwent biopsies of the bladder and prostate, and histopathological findings confirmed the lesions to be postoperative loco-regional relapse with bladder and prostate infiltration
Metastatic lymph node detection
Among the 51 included patients, 11 had lymph node involvement, with a total of 45 metastatic lymph nodes (38 in abdomen and pelvis, 5 in supraclavicular region, and 2 in mediastinum). The diagnostic sensitivity of [68Ga]FAPI-04 and [18F]FDG was 81.82% (9/11) and 54.55% (6/11), respectively, in patient-based analysis (p = 0.250) (Table 2) and 88.89% (32/36) and 55.56% (20/36) in lesion-based analysis (p = 0.002) (supplementary Table S3). The median SUVmax of [68Ga]FAPI-04 and [18F]FDG was 9.14 and 10.65 (p = 0.880), respectively, and the SUVmean was 5.85 and 6.39 (p = 0.888). The TBR and TLR of the SUVmax and SUVmean of [68Ga]FAPI-04 were significantly higher than those of [18F]FDG (all p < 0.005) (Table 3, Fig. 2, supplementary Fig. S1).
Liver metastasis detection
Among all 51 included patients, 10 showed liver metastases and a total of 25 hepatic lesions with a median largest diameter of 2.05 cm (IQR, 1.36–3.32 cm) were assessed. Nine and eight patients were diagnosed on [68Ga]FAPI-04 and [18F]FDG, respectively, with a sensitivity of 90% (9/10) and 80% (8/10) (p = 1.000) (Table 2). Twenty-one and 24 lesions were detected on [18F]FDG and [68Ga]FAPI-04 PET/CT, respectively, with a sensitivity of 84% (21/25) and 96% (24/25) (p = 0.25) (supplementary Table S3). Three hepatic metastatic lesions were missed on [18F]FDG in a patient with gastric cancer, but these lesions showed intense uptake on [68Ga]FAPI-04 (Fig. 4). One small liver metastasis (9 × 7 mm) in a patient with colon MAC was missed on both [18F]FDG and [68Ga]FAPI-04. In hepatic metastases, the median SUVmax (9.68 vs. 11.35, p = 0.211) and SUVmean (6.02 vs. 6.61, p = 0.207) were similar between [18F]FDG and [68Ga]FAPI-04. However, the TLR of SUVmax and SUVmean was 2.26 and 3.41 times higher on [68Ga]FAPI-04 than on [18F]FDG, indicating that liver metastases could be clearly displayed on [68Ga]FAPI-04 PET/CT (Table 3, Fig. 2, supplementary Fig. S1).
Images of a 61-year-old man with a history of postoperative chemotherapy for gastric antrum cancer (moderately differentiated adenocarcinoma, pT4bN0M0) over 2 years who underwent PET/CT for restaging. (a–e) Three FAPI-avid hepatic lesions (SUVmax = 8.48–14.99; a, maximum intensity projection, MIP; b–d, axial fused images; arrows) and one pancreatic head lesion with intense FAPI uptake (SUVmax = 26.03; e, axial fused image; arrow) are observable on [68Ga]FAPI-04 PET/CT images, suggesting liver metastases and loco-regional relapse (involvement of the pancreas). (f–j) [18F]FDG PET/CT reveals false-negative FDG uptake in the liver lesions and mild FDG uptake in the pancreatic head lesion (SUVmax = 4.89; f–i, axial fused images; j, MIP)
Peritoneal carcinomatosis detection
Among all 51 included patients, 31 had suspected peritoneal carcinomatosis and 28 were finally diagnosed, including 16 with nodular-type and 12 with diffuse-type lesions. [68Ga]FAPI-04 revealed 28 true-positive patients with significantly higher sensitivity (100%) than [18F]FDG (60.71% [17/28]) (p = 0.001) (Table 2); Fig. 5 shows a representative case. Eleven patients had false-negative diagnoses on [18F]FDG (ten gastric cancers and one colonic MAC). One patient with reactive nodular fibrous hyperplasia mimicking peritoneal carcinomatosis showed false-positive finding on [68Ga]FAPI-04 (Fig. 6). Two patients with peritoneal inflammation had false-positive diagnoses on [18F]FDG and [68Ga]FAPI-04 PET/CT, and a case is shown in supplementary Fig. S2. Compared with [18F]FDG PET/CT, [68Ga]FAPI-04 yielded a significantly higher median SUVmax (12.12 vs. 7.18, p = 0.004) and SUVmean (6.84 vs. 4.11, p = 0.003). The median TLR of the SUVmax on [68Ga]FAPI-04 was nearly 5.36 times higher than that on [18F]FDG (10.78 vs. 2.01, p < 0.001) (Table 3, Fig. 2, supplementary Fig. S1).
Images of a 23-year-old woman with a history of gastric signet ring cancer and peritoneal carcinomatosis who received four cycles of chemotherapy. PET/CT was performed to assess treatment efficacy. (a–e) [68Ga]FAPI-04 PET/CT images show an intense FAPI-avid primary gastric tumour (SUVmax = 10.24) and diffuse peritoneal carcinomatosis with high FAPI uptake (SUVmax = 12.76) (a, maximum intensity projection, MIP; b–e, axial fused images; arrows). (f–j) [18F]FDG PET/CT images show negative FDG uptake in the primary tumour and peritoneal metastases (f–i, axial fused images; j, MIP)
A representative false-positive case mimicking peritoneal metastasis on [68Ga]FAPI-04 PET/CT. A 67-year-old woman presented with a 1-week history of intestinal obstruction. Seven years previously, she had undergone partial gastrectomy followed by two cycles of chemotherapy for gastric cancer. (a–d) [68Ga]FAPI-04 PET/CT images (a, maximum intensity projection, MIP; b–d, axial CT, PET, and fused slices) show a FAPI-avid nodular lesion in the omentum measuring 27 × 18 mm (SUVmax = 9.01; arrows) that was adherent to an abdominal incision and the adjacent ileum, suggesting the possibility of peritoneal metastasis. (e–h) [18F]FDG PET/CT reveals negative uptake in the peritoneal lesion (e–g, axial CT, PET, and fused images; h, MIP). The patient subsequently underwent surgery, and histopathological examination confirmed the peritoneal lesion to be a reactive nodular fibrous pseudotumour, not peritoneal metastasis
Local recurrence and other metastasis detection
As shown in Table 2 and supplementary Table S3, there was no statistical significance in the detection of local recurrence or ovarian and pulmonary metastases between [18F]FDG and [68Ga]FAPI-04 (all p > 0.05). Three patients had extensive bone metastases detected on both [18F]FDG and [68Ga]FAPI-04, with comparable diagnostic efficiency by visual analysis. In addition, a sacral lesion in a patient with a history of rectal cancer that was finally diagnosed as a sacral insufficiency fracture was given a false-positive diagnosis on [18F]FDG but showed no FAPI uptake.
SRCC and MAC detection
The patients in this study had 7 SRCCs of the stomach and 11 MACs (3 gastric, 6 colorectal, and 2 appendiceal cancers) (supplementary Table S4). The detection rate of [68Ga]FAPI-04 for primary tumour of SRCC and MAC was 85.71% (6/7), which was much higher than that of [18F]FDG (28.57%, 2/7) (p = 0.125). Ten patients had suspected postoperative recurrence and metastasis, and seven patients were finally diagnosed. The detection sensitivity was 85.71% (6/7) for [68Ga]FAPI-04 and 42.86% (3/7) for [18F]FDG PET/CT (p = 0.250). Of 18 SRCCs and MACs, 12 were associated with peritoneal metastases. The detection rates were 100% (12/12) for [68Ga]FAPI-04 and 66.67% (8/12) for [18F]FDG (p = 0.125) (Table 4), and the median SUVmax of peritoneal metastases on [68Ga]FAPI-04 was significantly higher than that on [18F]FDG (Table 3).
Changes in therapeutic management
Table 5 and supplementary Table S5 show changes in treatment management. Among the 51 patients, 4 had no available CT or MRI data. Of the remaining 47 patients, 34 (72.3%, 34/47) had no changes in their treatment. Finally, 13 (27.7%, 13/47) changed their treatment regimens because of the detection of new primary and/or metastatic lesions or a decreasing disease extent according to [68Ga]FAPI-04 PET/CT compared with conventional imaging. In the initial staging assessment, [68Ga]FAPI-04 did not change the patients’ clinical stage because most patients with advanced disease were included; however, three treatment-naïve patients’ primary tumour, which had been missed on conventional imaging, was clearly displayed on [68Ga]FAPI-04 PET/CT. Major changes occurred in eight patients, three of whom underwent surgery and adjuvant therapy. Two patients refused systematic therapy, and their actual regimens were therefore unavailable.
Discussion
In this study, we evaluated and compared diagnostic performance and tracer uptake between [68Ga]FAPI-04 and [18F]FDG PET/CT in primary tumour and metastatic lesions of gastrointestinal carcinoma. We found that [68Ga]FAPI-04 was superior to [18F]FDG in the detection of primary tumour, postoperative overall recurrence and metastases, and peritoneal carcinomatosis. The higher TBR and TLR of [68Ga]FAPI-04 enhanced the contrast of lesions against background tissue, making them easier to see and reducing the rate of missed diagnoses.
FAPI, as a new imaging agent, presents a promising alternative for gastrointestinal carcinoma. Pang et al reported detection sensitivities of 100% for [68Ga]FAPI-04 and 53% for [18F]FDG PET/CT in the diagnosis of primary tumour in patients with gastrointestinal cancer, with [68Ga]FAPI-04 showing higher TBR than [18F]FDG (7.6 vs. 2.2) [11]. Jiang et al also found sensitivities of 100% for [68Ga]FAPI-04 and 82% for [18F]FDG PET in the detection of primary gastric cancer, but found no significant difference between the SUVmax of the two tracers. However, the TBR of [68Ga]FAPI-04 was obviously higher than that of [18F]FDG [12]. In our study, [68Ga]FAPI-04 resulted in detection of more primary tumours than did [18F]FDG (94.44% vs. 61.11%), with higher TBR and TLR. In patients with SRCC and MAC, [68Ga]FAPI-04 detected more primary tumours than did [18F]FDG, although there was no significant difference because of the small number of cases. Compared with CT and/or MRI, [68Ga]FAPI-04 had obvious advantages in finding small primary lesions of gastric and appendiceal cancer; moreover, because [68Ga]FAPI-04 detected new and/or more lesions or a decreasing disease extent, the treatment was changed in 13 (27.7%) patients, similar to a recent study (22.9%) [20]. A high TBR is one of the most important requirements for clear display of lesions, which is crucial for accurate staging and determination of treatment strategies.
Accurate lymph node staging is crucial for treatment and prognosis in patients with gastrointestinal cancer. Kuten et al found that the detection rate of regional lymph node involvement in gastric cancer was comparable between [18F]FDG and [68Ga]FAPI-04, with a similar SUVmax (4.3 vs. 7.9) [14]. Pang et al found that [68Ga]FAPI-04 showed more lymph node metastases than did [18F]FDG PET/CT (79% vs. 54% for lesion-based sensitivity) in patients with gastrointestinal cancers, with a much higher median SUVmax (6.7 vs. 2.4) [11]. Our results are partially consistent with previous studies; in patient-based analysis, the sensitivity of [68Ga]FAPI-04 (81.82%) was similar to that of [18F]FDG (54.55%) for the detection of lymph node involvement. However, the detection rate of [68Ga]FAPI-04 (88.89%) was significantly higher than that of [18F]FDG (55.56%) in lesion-based analysis, with a similar median SUVmax and SUVmean but higher TBR and TLR. In general, [68Ga]FAPI-04 PET/CT could detect more lymph node metastases than [18F]FDG.
The peritoneum frequently shows metastatic involvement in gastrointestinal carcinoma. The peritoneal cancer index score is an important evaluation indicator of tumour resectability and curability, and it is strongly associated with patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy [21]. Both CT and [18F]FDG PET have limited diagnostic reliability for peritoneal carcinomatosis, leading to gross underestimations in the extent of peritoneal carcinomatosis, especially in small miliary lesions (< 0.5 cm) on peritoneal surfaces and in mucinous tumours [22,23,24,25]. Zhao et al reported that the diagnostic sensitivity for peritoneal carcinomatosis from various tumour types was significantly higher on [68Ga]FAPI-04 than on [18F]FDG (97.67% vs. 72.09%) and found significantly higher uptake of [68Ga]FAPI-04 than [18F]FDG in gastric cancer (8.05 vs. 3.44) and colorectal cancer (10.14 vs. 3.86) [19]. Our results also demonstrated that [68Ga]FAPI-04 was superior to [18F]FDG in the detection of peritoneal metastasis. Moreover, the uptake value of [68Ga]FAPI-04 was significantly higher than that of [18F]FDG, which is similar to the findings of previous studies [11, 19]. Additionally, because there is no [68Ga]FAPI-04 uptake in the normal gastrointestinal tract, peritoneal lesions were shown more clearly. In our study, we also found false-positive [68Ga]FAPI-04 accumulation in peritoneal lesions in patients with postoperative gastrointestinal carcinoma. In addition to overexpression in CAFs, FAP is also selectively expressed in stromal cells and mesenchymal stem cells during wound healing, fibrotic reactions, and inflammatory conditions [26]. Accordingly, we should pay more attention to reviewing [68Ga]FAPI-04 PET/CT images in routine clinical practice, especially in postoperative patients. Overall, compared with [18F]FDG, [68Ga]FAPI-04 shows much higher diagnostic sensitivity and accuracy for detection of peritoneal involvement in patients with gastrointestinal cancer, which is more conductive to patient stratification management and could guide surgeons in the resection of peritoneal lesions.
The liver is a common metastatic location for gastrointestinal carcinoma, and accurate identification of the degree of liver metastasis from gastrointestinal cancer is critical for subsequent treatment management and the patient’s prognosis [27, 28]. [68Ga]FAPI-04 shows advantages over [18F]FDG in the detection of liver metastasis from gastrointestinal cancer, with reported patient-based sensitivities of 96.6% and 70.8% and lesion-based sensitivities of 96.8% and 80.2% [29]. Although there was no significant difference in the SUVmax of [68Ga]FAPI-04 versus [18F]FDG in liver metastases, the TLR was significantly higher on [68Ga]FAPI-04 than on [18F]FDG [29]. In line with previous studies [13, 29], our results showed that the TLR of the SUVmax was higher on [68Ga]FAPI-04 than on [18F]FDG. Our patient- and lesion-based analyses revealed that [68Ga]FAPI-04 allowed detection of more liver metastases, although the difference was not statistically significant, which may be because of the small number of liver metastases. Mao et al reported that for colorectal cancer liver metastases (less than 10 mm), both early and delayed [18F]FDG PET/CT imaging had significantly lower sensitivity (26.42% vs. 47.17%) [30]. Four metastatic liver lesions were missed on [18F]FDG, which may be because of high [18F]FDG uptake in liver blood pool and partial volume effect. Overall, our results suggest that [68Ga]FAPI-04 is superior to [18F]FDG in diagnosing liver metastasis.
This study had several limitations. First, we included patients with a wide range of cancers. Patient selection bias may have affected this study. Second, this was a retrospective single-centre study, and some metastatic lesions were not confirmed by histopathological findings. Third, the number of patients with distant metastases (ovary, lung, and bone) was small, and the diagnostic efficiency of the two imaging modalities may not be representative. A future prospective study with a larger number of participants would provide a more comprehensive overview of the usefulness of [68Ga]FAPI-04 PET/CT in gastrointestinal carcinoma.
Conclusions
Our study demonstrated that [68Ga]FAPI-04 outperformed [18F]FDG PET/CT in detecting primary tumour, postoperative recurrence and metastases, and peritoneal carcinomatosis in patients with gastrointestinal carcinoma. Moreover, [68Ga]FAPI-04 PET/CT displayed lesions more clearly because of its higher TBR.
Abbreviations
- CAF:
-
Cancer-associated fibroblast
- CT:
-
Computed tomography
- FAP:
-
Fibroblast activation protein
- FAPI:
-
Fibroblast activation protein inhibitor
- FDG:
-
Fluorodeoxyglucose
- IQR:
-
Interquartile range
- MAC:
-
Mucinous adenocarcinoma
- MRI:
-
Magnetic resonance imaging
- PET/CT:
-
Positron emission tomography/computed tomography
- SRCC:
-
Signet ring cell carcinoma
- SUV:
-
Standardised uptake value
- TBR:
-
Tumour-to-mediastinal blood pool ratio
- TLR:
-
Tumour-to-normal liver parenchyma ratio
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Kitajima K, Nakajo M, Kaida H et al (2017) Present and future roles of FDG-PET/CT imaging in the management of gastrointestinal cancer: an update. Nagoya J Med Sci 79:527–543
Seko-Nitta A, Nagatani Y, Murakami Y et al (2021) (18)F-fluorodeoxyglucose uptake in advanced gastric cancer correlates with histopathological subtypes and volume of tumor stroma. Eur J Radiol 145:110048
Zi F, He J, He D, Li Y, Yang L, Cai Z (2015) Fibroblast activation protein alpha in tumor microenvironment: recent progression and implications (review). Mol Med Rep 11:3203–3211
Lindner T, Loktev A, Giesel F, Kratochwil C, Altmann A, Haberkorn U (2019) Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm Chem 4:16
Peltier A, Seban RD, Buvat I, Bidard FC, Mechta-Grigoriou F (2022) Fibroblast heterogeneity in solid tumor: from single cell analysis to whole-body imaging. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2022.04.008
Kratochwil C, Flechsig P, Lindner T et al (2019) (68)Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med 60:801–805
Loktev A, Lindner T, Burger EM et al (2019) Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J Nucl Med 60:1421–1429
Koerber SA, Staudinger F, Kratochwil C et al (2020) The role of (68)Ga-FAPI PET/CT for patients with malignancies of the lower gastrointestinal tract: first clinical experience. J Nucl Med 61:1331–1336
Qin C, Shao F, Gai Y et al (2022) (68)Ga-DOTA-FAPI-04 PET/MR in the evaluation of gastric carcinomas: comparison with (18)F-FDG PET/CT. J Nucl Med 63:81–88
Pang Y, Zhao L, Luo Z et al (2021) Comparison of (68)Ga-FAPI and (18)F-FDG uptake in gastric, duodenal, and colorectal cancers. Radiology 298:393–402
Jiang D, Chen X, You Z et al (2022) Comparison of [(68) Ga]Ga-FAPI-04 and [(18)F]-FDG for the detection of primary and metastatic lesions in patients with gastric cancer: a bicentric retrospective study. Eur J Nucl Med Mol Imaging 49:732–742
Gundogan C, Komek H, Can C et al (2022) Comparison of 18F-FDG PET/CT and 68Ga-FAPI-04 PET/CT in the staging and restaging of gastric adenocarcinoma. Nucl Med Commun 43:64–72
Kuten J, Levine C, Shamni O et al (2022) Head-to-head comparison of [(68)Ga]Ga-FAPI-04 and [(18)F]-FDG PET/CT in evaluating the extent of disease in gastric adenocarcinoma. Eur J Nucl Med Mol Imaging 49:743–750
Wang FH, Zhang XT, Li YF et al (2021) The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond) 41:747–795
Wang X, Liang P, Lv P, Li R, Hou P, Gao J (2022) Clinical characteristics and CT features of hepatic epithelioid haemangioendothelioma and comparison with those of liver metastases. Insights Imaging 13:9
Low RN (2007) Abdominal MRI advances in the detection of liver tumours and characterisation. Lancet Oncol 8:525–535
Sivesgaard K, Larsen LP, Sorensen M et al (2018) Diagnostic accuracy of CE-CT, MRI and FDG PET/CT for detecting colorectal cancer liver metastases in patients considered eligible for hepatic resection and/or local ablation. Eur Radiol 28:4735–4747
Zhao L, Pang Y, Luo Z et al (2021) Role of [(68)Ga]Ga-DOTA-FAPI-04 PET/CT in the evaluation of peritoneal carcinomatosis and comparison with [(18)F]-FDG PET/CT. Eur J Nucl Med Mol Imaging 48:1944–1955
Qin C, Song Y, Gai Y et al (2022) Gallium-68-labeled fibroblast activation protein inhibitor PET in gastrointestinal cancer: insights into diagnosis and management. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-022-05847-0
Sugarbaker PH, Jelinek JS (2021) The radiologist’s role in the multidisciplinary team for patients with colon cancer peritoneal metastases. Surg Oncol 40:101690
Koh JL, Yan TD, Glenn D, Morris DL (2009) Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol 16:327–333
Pasqual EM, Bertozzi S, Bacchetti S et al (2014) Preoperative assessment of peritoneal carcinomatosis in patients undergoing hyperthermic intraperitoneal chemotherapy following cytoreductive surgery. Anticancer Res 34:2363–2368
Low RN, Barone RM (2018) Imaging for peritoneal metastases. Surg Oncol Clin N Am 27:425–442
Gertsen EC, Brenkman HJF, van Hillegersberg R et al (2021) 18F-fludeoxyglucose-positron emission tomography/computed tomography and laparoscopy for staging of locally advanced gastric cancer: a multicenter prospective dutch cohort study (PLASTIC). JAMA Surg 156:e215340
Altmann A, Haberkorn U, Siveke J (2021) The latest developments in imaging of fibroblast activation protein. J Nucl Med 62:160–167
Hori S, Honda M, Kobayashi H et al (2021) A grading system for predicting the prognosis of gastric cancer with liver metastasis. Jpn J Clin Oncol 51:1601–1607
Engstrand J, Nilsson H, Stromberg C, Jonas E, Freedman J (2018) Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer 18:78
Sahin E, Elboga U, Celen YZ, Sever ON, Cayirli YB, Cimen U (2021) Comparison of (68)Ga-DOTA-FAPI and (18)FDG PET/CT imaging modalities in the detection of liver metastases in patients with gastrointestinal system cancer. Eur J Radiol 142:109867
Mao W, Zhou J, Qiu L, Yin H, Tan H, Shi H (2020) The added value of dual-time-point (18)F-FDG PET/CT imaging in the diagnosis of colorectal cancer liver metastases. Abdom Radiol (NY) 45:1075–1081
Funding
This study has received funding by the Improvement Project for Theranostic Ability on Difficulty Miscellaneous Disease (tumour) (No. ZLYNXM202007), the National Natural Science Foundation of China (No. 82171986), the Medical Sci-Tech Innovation Platform of Zhongnan Hospital of Wuhan University (PTXM2022013), and Science, Technology and Innovation Seed Fund of Zhongnan Hospital of Wuhan University (CXPY2020045).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is prof. Yong He, MD, PhD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 586 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Tian, Y., Chen, J. et al. Usefulness of [68Ga]FAPI-04 and [18F]FDG PET/CT for the detection of primary tumour and metastatic lesions in gastrointestinal carcinoma: a comparative study. Eur Radiol 33, 2779–2791 (2023). https://doi.org/10.1007/s00330-022-09251-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09251-y