Abstract
Objectives
To determine the diagnostic value of [68 Ga]Ga-DOTA-labeled-somatostatin analogue ([68 Ga]Ga-DOTA-SSA) PET/MRI for detecting liver metastasis in patients with neuroendocrine tumor (NET) and to compare it with [68 Ga]Ga-DOTA-SSA PET/CT.
Methods
A search of MEDLINE, EMBASE, and Cochrane was performed to identify original articles reporting the detection rate of [68 Ga]Ga-DOTA-SSA PET/MRI for liver metastasis in comparison with PET/CT. The pooled detection rates for liver metastasis on PET/MRI and PET/CT were calculated and compared using a restricted maximum likelihood estimation of random-effects model. The pooled added value of PET/MRI in comparison with PET/CT was calculated. Sensitivity analysis and subgroup analysis were performed to explore causes of study heterogeneity.
Results
In the six included studies (638 liver metastases), the pooled detection rates for liver metastasis on [68 Ga]Ga-DOTA-SSA PET/MRI and PET/CT were 93.5% (95% confidence interval [CI], 85.1–97.3%; I2 = 84.8%) and 76.8% (95% CI, 64.8–85.6%; I2 = 87.8%), respectively. PET/MRI had a significantly higher detection rate than PET/CT (p = 0.02), with 15.3% (95% CI, 8.0–27.4%) added value over PET/CT. After sensitivity analysis, the recalculated detection rates for liver metastasis were 94.8% (95% CI, 90.8–97.2%; I2 = 42.1%) for PET/MRI and 80.0% (95% CI, 65.3–89.5%; I2 = 90.0%) for PET/CT. The study location and the use of predefined imaging criteria for liver metastasis were associated with PET/MRI study heterogeneity.
Conclusion
[68 Ga]Ga-DOTA-SSA PET/MRI had good overall performance for detecting liver metastasis in patients with NET. Because of the small number of eligible studies, further studies are needed to validate the clinical usefulness of [68 Ga]Ga-DOTA-SSA PET/MRI.
Key Points
• [68 Ga]Ga-DOTA-SSA PET/MRI had a higher pooled detection rate for liver metastasis than PET/CT (93.5% vs. 76.8%).
• The added value of [68 Ga]Ga-DOTA-SSA PET/MRI for detecting liver metastasis in comparison with PET/CT was 15.3%.
•Study location and the predefined imaging criteria for liver metastasis were significant factors causing PET/MRI study heterogeneity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Positron emission tomography (PET) with the [68 Ga]Ga-labeled somatostatin receptor (SSTR) tracers, such as DOTA-Tyr(3)-octreotide (DOTA-TOC), DOTA-Tyr(3)-octreotate (DOTA-TATE), and DOTA-NaI(3)-octreotide (DOTA-NOC), is currently the preferred modality for functional imaging of neuroendocrine tumor (NET) [1]. Most NETs demonstrate increased SSTR expression in both primary and metastatic lesions. Through targeting of the SSTRs present on the cell surface of neuroendocrine cells, [68 Ga]Ga-DOTA-labeled-somatostatin analogue ([68 Ga]Ga-DOTA-SSA) PET provides high sensitivity and specificity for the diagnosis of well-differentiated NET, and leads to more accurate staging of NET and more appropriate treatment planning [2, 3]. Given these benefits of [68 Ga]Ga-DOTA-SSA PET, it is considered as a complementary imaging tool in patients with NET.
The most common organ for metastasis in patients with NET is the liver, with liver metastasis being observed in up to 85% of patients [4, 5]. Because liver metastasis is an important prognostic factor and is associated with markedly reduced survival in patients with NET [5, 6] (5-year overall survival rates are approximately 50% for patients with liver metastasis but 70–80% for those without liver metastasis), imaging workup for the correct diagnosis and localization of liver metastasis is essential. However, although [68 Ga]Ga-DOTA-SSA PET has good diagnostic performance for detecting primary and metastatic NET lesions, its ability to detect liver metastasis may be limited because of the moderately intense physiologic uptake in the liver resulting from nonspecific liver tissue handling of the [68 Ga]Ga-DOTA-SSA, as well as the SSTR uptake of NET tumor cells [3]. Therefore, there is a need to improve the performance of [68 Ga]Ga-DOTA-SSA PET for detecting liver metastasis in patients with NET.
To overcome this limitation, [68 Ga]Ga-DOTA-SSA PET combined with cross-sectional imaging such as CT or MRI can be considered; the PET provides molecular and functional information, and the cross-sectional imaging provides detailed anatomical information. Notably, of the available cross-sectional imaging modalities, many advances have recently been made in liver MRI, including the use of hepatobiliary contrast agents and diffusion-weighted imaging, and published studies have reported improved diagnostic performance of liver MRI for detecting liver metastasis [7, 8]. Recently, with the introduction of combined [68 Ga]Ga-DOTA-SSA PET and MRI, several studies have reported the performance of [68 Ga]Ga-DOTA-SSA PET/MRI for the detection of liver metastasis in patients with NET [9,10,11,12]. Although these studies generally agree on the advantage of [68 Ga]Ga-DOTA-SSA PET/MRI for the diagnosis of liver metastasis, the sample sizes of individual studies were relatively small for determining the added value of PET/MRI in comparison with PET/CT, and the reported added value of PET/MRI is quite variable, i.e., 6.3–17.6% [9, 12]).
Therefore, we performed a systematic review and meta-analysis with the aim of determining the diagnostic value of [68 Ga]Ga-DOTA-SSA PET/MRI for detecting liver metastasis in patients with NET, and to compare it with [68 Ga]Ga-DOTA-SSA PET/CT.
Methods
This systematic review and meta-analysis was performed in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13]. The following literature search, study selection, data extraction, and study quality assessment were independently conducted by two reviewers (S.H.C. and S.J.C.; both with ≥ 2 years of experience in performing systematic reviews and meta-analyses and ≥ 4 years of experience in liver MRI), and any disagreements were resolved in consensus.
Literature search strategy
A systematic search of MEDLINE, EMBASE, and Cochrane was performed to identify studies reporting the detection rate of [68 Ga]Ga-DOTA-SSA PET/MRI for liver metastasis of neuroendocrine tumor and comparing it with that of [68 Ga]Ga-DOTA-SSA PET/CT. The search terms included “DOTA-TOC”, “DOTA-TATE”, “DOTA-NOC”, “PET”, “MRI”, and “neuroendocrine tumor”, and a detailed list of the search terms is provided in Supplementary Table 1. Our search was limited to articles in English published between 1 January 2011 and 31 August 2021. The bibliographies of the identified articles were screened to search for other relevant articles.
Eligibility criteria
The inclusion criteria were as follows: (a) population: patients who had suspected liver metastasis from neuroendocrine tumor; (b) index test: [68 Ga]Ga-DOTA-SSA PET/MRI; (c) comparator: [68 Ga]Ga-DOTA-SSA PET/CT; (d) outcome: the detection rate of liver metastasis. Studies were excluded if they met any of the following criteria: (a) case reports, review articles, conference abstracts, editorials, letters, meta-analyses, and animal studies; (b) studies not relevant to the field of interest; (c) studies with overlapping patients and data.
Data extraction
The following data were recorded from the selected studies: (a) study characteristics: authors, published year, affiliation, country, study design; (b) subject characteristics: number of patients, age, and sex; (c) lesion characteristics: location of primary NET and number of liver metastases; (d) imaging techniques for [68 Ga]Ga-DOTA-SSA PET/MRI and PET/CT; type of DOTA-SSA, DOTA-SSA precursor dose, use of contrast agent, magnetic field strength, images used for [68 Ga]Ga-DOTA-SSA PET/MRI fusion or PET/CT, and fusion method; (e) image interpretation: number of readers, reader experience, characteristics, and use of predefined imaging criteria for liver metastasis; (f) reference standard: pathological or clinical diagnosis; (g) study outcome: detection rates of liver metastasis on [68 Ga]Ga-DOTA-SSA PET/MRI and PET/CT.
Assessment of study quality
The methodologic quality of the studies was evaluated using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) criteria, including the risk of bias and the applicability of each study. The QUADAS-2 criteria consist of four domains: patient selection, index test, reference standard, and flow and timing.
Data synthesis
The detection rate for liver metastasis was defined as the number of liver metastases detected by the index test (i.e., [68 Ga]Ga-DOTA-SSA PET/MRI or PET/CT) divided by the total number of liver metastases. To determine the pooled detection rate for liver metastasis, the inverse variance method was used to calculate weights, and the percentages and their 95% confidence interval (CI) were obtained using a restricted maximum likelihood estimation of random-effects model. The pooled added value of [68 Ga]Ga-DOTA-SSA PET/MRI for detecting liver metastasis in comparison with PET/CT was also calculated, with the added value being defined as the difference in the detection rate between [68 Ga]Ga-DOTA-SSA PET/MRI and PET/CT. Study heterogeneity was assessed using Higgins’ I2 statistic, with a value > 50% being considered to indicate the presence of substantial heterogeneity. To evaluate the causes of study heterogeneity, sensitivity analysis was performed by recalculating the pooled detection rate of liver metastasis after excluding each individual study.
In addition, subgroup analysis was performed using meta-regression analysis including the following covariates: (a) study location (Europe vs. USA); (b) number of total liver metastases (> 100 vs. ≤ 100); (c) type of [68 Ga]Ga-DOTA-SSA (DOTA-TOC vs. DOTA-NOC); (d) CT scan used for fusion (dynamic image vs. single phase image); (e) MRI used for fusion (3.0 T vs. 1.5 T); (f) MRI contrast agent (hepatobiliary contrast vs. extracellular contrast); (g) [68 Ga]Ga-DOTA-SSA PET/MRI fusion method (simultaneous vs. retrospective); (h) reader characteristics (all radiologists vs. both radiologist and nuclear medicine physician); and (i) predefined imaging criteria for liver metastasis (used vs. not used).
Publication bias was evaluated using visual assessment of a funnel plot and Egger’s test (p < 0.05 indicating significant bias). Statistical analyses were conducted using the “meta” and “metafor” packages in R software (version 3.4.1; R Foundation for Statistical Computing).
Results
Literature search
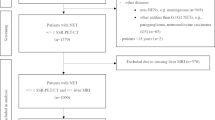
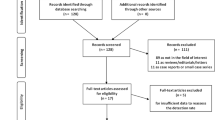
A total of 1226 articles were retrieved by the systematic search. Of these, 1203 articles were excluded after reviewing the titles and abstracts, including 246 review articles, 463 case reports, 11 scientific abstracts, 452 articles not in the field of interest, 16 meta-analyses, 8 articles concerning animals, 6 articles regarding pediatric patients, and 1 non-English article (Fig. 1). After full-text review, an additional 17 articles were excluded. Finally, 6 articles involving a total of 111 patients with 638 metastatic hepatic lesions were included in this study.
Study characteristics
The detailed characteristics of the six included studies are summarized in Table 1. All studies were performed prospectively. The number of patients ranged from 8 to 30, and the number of hepatic metastases ranged from 16 to 187. The liver metastases were from abdominal neuroendocrine tumors except for one adrenal, two parotid, two pulmonary NETs, and four of unknown origin [9, 11, 12, 14].
Five studies used DOTA-TOC [9,10,11,12, 15] and one study used DOTA-NOC [14]. All six studies performed simultaneous PET/CT, whereas [68 Ga]Ga-DOTA-SSA PET/MRI was performed simultaneously in five studies [10,11,12, 14, 15], with retrospective fusion of PET/MRI performed in the remaining study [9]. In the study using retrospective fusion PET/MRI, the median interval between the PET/CT and PET/MRI was 0.6 days (range, 0–6 days) [9]. For the CT image fusion, five studies used dynamic images [9, 11, 12, 14, 15], whereas the other study used portal venous images only [10]. For the MRI image fusion, all six studies used dynamic images, and no study used arterial subtraction images. Five studies used 3.0-T MRI [10,11,12, 14, 15] and one study used 1.5-T MRI [9]. Regarding MRI contrast, four studies used a hepatobiliary contrast agent [9, 10, 14, 15] and two studies used an extracellular fluid contrast agent [11, 12]. Image interpretation was performed by radiologists only in two studies, who analyzed both CT/MRI and nuclear imaging [11, 15], and by both radiologists and nuclear medicine physicians in four studies [9, 10, 12, 14]. Five studies used predefined imaging criteria for liver metastasis such as 3- to 5-point scales for lesion characteristics/diagnostic confidence or specific imaging features for liver metastasis on PET/CT or PET/MRI [9, 11, 12, 14, 15], whereas the other study was unclear about how liver metastasis was defined on CT/MRI (Supplementary Table 2) [10]. Regarding the reference standard for NET liver metastasis, a composite standard of reference for NET liver metastasis was used in four studies [9, 11, 12, 14], imaging follow-up was used in one study [15], and the remaining study was unclear about how liver metastasis was determined [10].
Study quality according to QUADAS-2
Of the four domains assessed, a high risk of bias was most frequently noted in the flow and timing domain, because patients did not receive the same reference standard in three studies [9, 11, 12] and information about the reference standard was unavailable in one study [10] (Supplementary Fig. 1). In the reference standard domain, all six studies were unclear as to whether the reference standard results were interpreted without knowledge of the results of the index test [9,10,11,12, 14, 15], and the reference standard used to correctly classify the target condition was unclear in three studies [10, 14, 15]. In the index test domain, three studies were unclear about whether the index test results were interpreted without knowledge of the results of the reference standard [9, 10, 12]. In the patient selection domain, two studies had an unclear risk of bias because they were unclear as to whether patients were consecutively enrolled or not [10, 12].
Detection rates for liver metastasis on [ 68 Ga]Ga-DOTA-SSA PET/MRI and PET/CT
The detection rates for liver metastasis of the individual studies ranged from 76.2% to 100% on [68 Ga]Ga-DOTA-SSA PET/MRI, and 56.6% to 93.7% on PET/CT (Table 2). The pooled detection rate for liver metastasis on PET/MRI was 93.5% (95% CI, 85.1–97.3%; Fig. 2), which was higher than that of 76.8% (95% CI, 64.8–85.6%; Fig. 2) on PET/CT. Compared with PET/CT, PET/MRI had 15.3% (95% CI, 8.0–27.4%) added value for detecting liver metastasis, with a significant difference between PET/MRI and PET/CT (p = 0.02). Substantial study heterogeneity was noted for both PET/MRI (I2 = 84.8%) and PET/CT (I2 = 87.8%).
The results of the sensitivity analysis are summarized in Supplementary Table 3. When the study of Hope et al. was excluded, no substantial study heterogeneity was noted for PET/MRI (I2 < 50%), whereas substantial study heterogeneity remained for PET/CT (I2 = 90.0%). The recalculated detection rates for liver metastasis were 94.8% (95% CI, 90.8–97.2%) for PET/MRI and 80.0% (95% CI, 65.3–89.5%) for PET/CT.
False-positive results on [ 68 Ga]Ga-DOTA-SSA PET/MRI and PET/CT
Of the six included studies, three studies reported the false-positive results on PET/MRI and PET/CT. No false-positive result was noted on PET/MRI in two studies [11, 14], whereas three false-positive results were noted in one study [9]. No false-positive result was noted on PET/CT in one study [14], whereas one and eight were noted in the other two studies [9, 11]. Therefore, the specificity ranged from 95.6% to 100% on PET/MRI and 88.2% to 100% on PET/CT.
Subgroup analysis
The results of subgroup analyses are summarized in Table 3. Study location and predefined imaging criteria for liver metastasis were significantly associated with the study heterogeneity affecting PET/MRI (p < 0.01). Studies from Europe and studies that used predefined imaging criteria for liver metastasis had a significantly higher pooled detection rate (94.8% vs. 76.2%) for liver metastasis than the study from America and the study with unclear imaging criteria. Regarding PET/CT, the total number of liver metastases and the type of [68 Ga]Ga-DOTA-SSA were significantly associated with study heterogeneity (p ≤ 0.02). Studies that included more than 100 liver metastases showed a lower detection rate than those that included less than 100 liver metastases (65.1% vs. 88.8%). PET/CT with DOTA-TOC had a lower detection rate than that with DOTA-NOC (71.1% vs. 92.8%).
Publication bias
There was no significant publication bias associated with either [68 Ga]Ga-DOTA-SSA PET/MRI (p = 0.23; Supplementary Fig. 2A) or PET/CT (p = 0.07; Supplementary Fig. 2B).
Discussion
Our meta-analysis found that [68 Ga]Ga-DOTA-SSA PET/MRI had a high overall detection rate for liver metastasis in patients with NET (93.5%, [95% CI, 85.1–97.3%]). Compared with PET/CT, the detection rate for liver metastasis in PET/MRI was significantly higher (93.5% vs. 76.8%), indicating that PET/MRI has 15.3% added value (p = 0.02). Because our meta-analysis included a relatively large number of liver metastases, the meta-analytic pooled estimation would be more powerful and relevant.
Previous literature generally agrees that [68 Ga]Ga-DOTA-SSA PET/MRI has better diagnostic performance for detecting liver metastasis than PET/CT [9,10,11,12], but the reported added values of PET/MRI are quite variable. Our meta-analysis found 15.3% added value for [68 Ga]Ga-DOTA-SSA PET/MRI in comparison with PET/CT. The higher detection rate for liver metastasis on [68 Ga]Ga-DOTA-SSA PET/MRI than on PET/CT found in our meta-analysis is in line with the results of previous studies, which reported that contrast-enhanced MRI had higher sensitivity for detecting liver metastasis than contrast-enhanced CT (95.2% vs. 78.5%) [16]. Generally, dynamic CT or MRI sequences are valuable for detecting liver metastasis from NET, because most liver metastases in patients with NET show hypervascularity on arterial phase imaging [17] 18. However, recent technical advances, including diffusion-weighted imaging (DWI) and hepatobiliary phase (HBP) imaging using a hepatobiliary contrast agent, have led to improved diagnostic performance of MRI in the detection of liver metastasis [7, 19]. Notably, because DWI may visualize small sub-centimeter liver lesions below the resolution limit of PET/CT, and those lesions that lack sufficient SSTR expression [20], combined DWI and HBP can lead to the best performance for detecting liver metastasis in patients with NET (86% sensitivity and 94% specificity by Hayoz et al.) [21]. Therefore, the higher detection rate for liver metastasis on [68 Ga]Ga-DOTA-SSA PET/MRI in comparison with PET/CT can be explained by the high lesion-to-liver conspicuity of HBP and the detection of additional small lesions on DWI. Generally, as increasing the sensitivity of a diagnostic test comes at the expense of the specificity [22], the high detection rate of PET/MRI may be due to false-positive results. Although the specificities of PET/MRI in three available studies were high overall at 95.6–100%, our results might have limitations for determining the performance of PET/MRI because of incomplete pathological reference standards, i.e., four studies used imaging follow-up as well as pathology as a reference standard for liver metastasis.
Sensitivity analysis indicated that the study of Hope et al. was the cause of PET/MRI study heterogeneity. This study had a relatively high proportion of lesions smaller than 1 cm, i.e., 62.4% in Hope et al. vs. 37.0% in Schreiter et al. Given that small liver metastases show low radiotracer activity on PET and low lesion conspicuity on MRI [9, 10], the lower detection rate for liver metastasis in this study is understandable. In addition, the detection rates for liver metastasis on [68 Ga]Ga-DOTA-SSA PET/MRI differed significantly according to study location (Europe vs. North America) and the use of predefined imaging criteria for liver metastasis. Because all five studies from Europe used predefined imaging criteria for liver metastasis (whereas the one study from North America did not use them) and there was no overall difference in demographic characteristics (i.e., patient number or age) between the two study locations (Europe vs. North America), the use of predefined imaging criteria for liver metastasis, which can affect the diagnostic accuracy of the index test [23], would appear be a reasonable explanation for the different detection rates of [68 Ga]Ga-DOTA-SSA PET/MRI between the two study locations. Considering our results from both sensitivity analysis and subgroup analysis, the 94.8% detection rate can be regarded as a general summary estimate of PET/MRI.
In our subgroup analysis, there was no significantly different detection rate for liver metastasis between [68 Ga]Ga-DOTA-SSA PET/MRI with hepatobiliary contrast and that with extracellular contrast (92.3% vs. 95.9%, p = 0.51). After the study of Hope et al. was excluded in the sensitivity analysis, the recalculated detection rate was similar (94.8% vs. 95.9%, p = 0.68). However, our results differ from those of a previous study that reported that PET/MRI with a hepatobiliary contrast agent increased the detection of liver metastasis on HBP images [24]. Because of the small number of eligible studies, the results of our study are limited for determining whether the diagnostic performance of PET/MRI can be improved by the use of hepatobiliary contrast agents, and further study is needed to validate this.
Five studies obtained PET/MRI using simultaneous acquisitions, whereas the remaining study obtained PET/MRI by a retrospective fusion of PET images with MRI. Compared with simultaneous acquisition, retrospective fusion has the advantages of reducing the cost for new technology or having no need for prepared imaging protocols [25]. However, in spite of no significantly different detection rates for liver metastasis between the two fusion methods in our meta-analysis, a retrospective fusion may be particularly challenging in the case of different patient positions, various scanners, or anatomic complexity. These limitations can be mitigated by simultaneous acquisition because it is free from the problems of misalignment or local misregistration [26].
Our study has several limitations. First, although we robustly investigated the eligible studies through a systematic review (approximately 1200 articles), the comparison between [68 Ga]Ga-DOTA-SSA PET/MRI and PET/CT may be statistically underpowered because of the small number of included studies. Furthermore, no significantly different detection rate was found between [68 Ga]Ga-DOTA-TOC and [68 Ga]Ga-DOTA-NOC PET/MRIs, whereas [68 Ga]Ga-DOTA-NOC PET/CT had a significantly higher detection rate than [68 Ga]Ga-DOTA-TOC PET/CT. Considering that the affinity for SSTR subtypes differs according to the type of tracer, i.e., [68 Ga]Ga-DOTA-TATE has high affinity for SSTR subtype 2, [68 Ga]Ga-DOTA-TOC for SSTR subtypes 2 and 5, and [68 Ga]Ga-DOTA-NOC for SSTR subtypes 2, 3, and 5 [3], the broad SSTR binding profile of [68 Ga]Ga-DOTA-NOC might lead to the better performance in the detection of liver metastasis [27]. However, only one [68 Ga]Ga-DOTA-NOC PET/CT study was available in our meta-analysis, which was not sufficient to compare performance between the two tracers. Further studies with a large sample size are needed to compare the diagnostic performance of PET/MRI with that of PET/CT. Second, substantial study heterogeneity was noted in both PET/MRI and PET/CT. To minimize the effect of study heterogeneity, we robustly performed sensitivity and subgroup analyses and identified factors associated with study heterogeneity. However, as our meta-analysis could not analyze the effect of factors on study heterogeneity at the patient level, the result of sensitivity and subgroup analyses may have a limitation, and future individual patient data meta-analysis is needed to analyze all possible interactions. Third, because we focused on the diagnostic value of [68 Ga]Ga-DOTA-SSA PET/MRI for detecting liver metastasis in patients with NET, we could not determine the impact of [68 Ga]Ga-DOTA-SSA PET/MRI on patient management, i.e., changes to their treatment strategy. As [68 Ga]Ga-DOTA-SSA PET/CT altered management in 19–71% of patients with NET by detecting more lesions and more involved organs [3, 28], the additional detection rate of [68 Ga]Ga-DOTA-SSA PET/MRI for liver metastasis may have an impact on treatment plans. However, because of a lack of evidence for the impact of [68 Ga]Ga-DOTA-SSA PET/MRI on patient management, future study is needed.
In conclusion, [68 Ga]Ga-DOTA-SSA PET/MRI had overall good performance for detecting liver metastasis in patients with NET, and had 15.3% added value in comparison with PET/CT. Therefore, [68 Ga]Ga-DOTA-SSA PET/MRI may be clinically useful for detecting liver metastasis in patients with NET. Although it can be considered as a diagnostic tool for liver metastasis, further studies are needed to validate its clinical usefulness because of the small number of eligible studies and a lack of evidence for the impact of PET/MRI on patient management.
Abbreviations
- CI:
-
Confidence interval
- HBP:
-
Hepatobiliary phase
- NET:
-
Neuroendocrine tumor
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QUADAS:
-
Quality Assessment of Diagnostic Accuracy Studies
- SSA:
-
Somatostatin analogue
References
Miederer M, Seidl S, Buck A et al (2009) Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging 36:48–52
Ruf J, Heuck F, Schiefer J et al (2010) Impact of multiphase 68Ga-DOTATOC-PET/CT on therapy management in patients with neuroendocrine tumors. Neuroendocrinology 91:101–109
Hofman MS, Lau WF, Hicks RJ (2015) Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics 35:500–516
Norton JA (2005) Endocrine tumours of the gastrointestinal tract. Surgical treatment of neuroendocrine metastases. Best Pract Res Clin Gastroenterol 19:577–583
Tomassetti P, Campana D, Piscitelli L et al (2005) Endocrine pancreatic tumors: factors correlated with survival. Ann Oncol 16:1806–1810
Madeira I, Terris B, Voss M et al (1998) Prognostic factors in patients with endocrine tumours of the duodenopancreatic area. Gut 43:422–427
Giesel FL, Kratochwil C, Mehndiratta A et al (2012) Comparison of neuroendocrine tumor detection and characterization using DOTATOC-PET in correlation with contrast enhanced CT and delayed contrast enhanced MRI. Eur J Radiol 81:2820–2825
Morse B, Jeong D, Thomas K, Diallo D, Strosberg JR (2017) Magnetic resonance imaging of neuroendocrine tumor hepatic metastases: does hepatobiliary phase imaging improve lesion conspicuity and interobserver agreement of lesion measurements? Pancreas 46:1219–1224
Schreiter NF, Nogami M, Steffen I et al (2012) Evaluation of the potential of PET-MRI fusion for detection of liver metastases in patients with neuroendocrine tumours. Eur Radiol 22:458–467
Hope TA, Pampaloni MH, Nakakura E et al (2015) Simultaneous (68)Ga-DOTA-TOC PET/MRI with gadoxetate disodium in patients with neuroendocrine tumor. Abdom Imaging 40:1432–1440
Sawicki LM, Deuschl C, Beiderwellen K et al (2017) Evaluation of (68)Ga-DOTATOC PET/MRI for whole-body staging of neuroendocrine tumours in comparison with (68)Ga-DOTATOC PET/CT. Eur Radiol 27:4091–4099
Beiderwellen KJ, Poeppel TD, Hartung-Knemeyer V et al (2013) Simultaneous 68Ga-DOTATOC PET/MRI in patients with gastroenteropancreatic neuroendocrine tumors: initial results. Invest Radiol 48:273–279
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Berzaczy D, Giraudo C, Haug AR et al (2017) Whole-body 68Ga-DOTANOC PET/MRI versus 68Ga-DOTANOC PET/CT in patients with neuroendocrine tumors: a prospective study in 28 patients. Clin Nucl Med 42:669–674
Jawlakh H, Velikyan I, Welin S, Sundin A (2021) (68) Ga-DOTATOC-PET/MRI and (11) C-5-HTP-PET/MRI are superior to (68) Ga-DOTATOC-PET/CT for neuroendocrine tumour imaging. J Neuroendocrinol 33:e12981
Dromain C, de Baere T, Lumbroso J et al (2005) Detection of liver metastases from endocrine tumors: a prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J Clin Oncol 23:70–78
Ronot M, Cuccioli F, Dioguardi Burgio M et al (2017) Neuroendocrine liver metastases: vascular patterns on triple-phase MDCT are indicative of primary tumour location. Eur J Radiol 89:156–162
Dromain C, de Baere T, Baudin E et al (2003) MR imaging of hepatic metastases caused by neuroendocrine tumors: comparing four techniques. AJR Am J Roentgenol 180(1):121–128
Sankowski AJ, Ćwikla JB, Nowicki ML et al (2012) The clinical value of MRI using single-shot echoplanar DWI to identify liver involvement in patients with advanced gastroenteropancreatic-neuroendocrine tumors (GEP-NETs), compared to FSE T2 and FFE T1 weighted image after i.v. Gd-EOB-DTPA contrast enhancement. Med Sci Monit 18:Mt33–Mt40
Mayerhoefer ME, Ba-Ssalamah A, Weber M et al (2013) Gadoxetate-enhanced versus diffusion-weighted MRI for fused Ga-68-DOTANOC PET/MRI in patients with neuroendocrine tumours of the upper abdomen. Eur Radiol 23:1978–1985
Hayoz R, Vietti-Violi N, Duran R, Knebel JF, Ledoux JB, Dromain C (2020) The combination of hepatobiliary phase with Gd-EOB-DTPA and DWI is highly accurate for the detection and characterization of liver metastases from neuroendocrine tumor. Eur Radiol 30:6593–6602
Bossuyt PM, Irwig L, Craig J, Glasziou P (2006) Comparative accuracy: assessing new tests against existing diagnostic pathways. BMJ 332:1089–1092
Whiting PF, Rutjes AWS, Westwood ME, Mallett S (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536
Kirchner J, Sawicki LM, Deuschl C et al (2017) 18 F-FDG PET/MR imaging in patients with suspected liver lesions: value of liver-specific contrast agent gadobenate dimeglumine. PLoS One 12:e0180349
Donati OF, Hany TF, Reiner CS et al (2010) Value of retrospective fusion of PET and MR images in detection of hepatic metastases: comparison with 18F-FDG PET/CT and Gd-EOB-DTPA-enhanced MRI. J Nucl Med 51:692–699
Monti S, Cavaliere C, Covello M, Nicolai E, Salvatore M, Aiello M (2017) An evaluation of the benefits of simultaneous acquisition on PET/MR coregistration in head/neck imaging. J Healthc Eng 2017:2634389
Wild D, Bomanji JB, Benkert P et al (2013) Comparison of 68Ga-DOTANOC and 68Ga-DOTATATE PET/CT within patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med 54:364–372
Deppen SA, Liu E, Blume JD et al (2016) Safety and efficacy of 68Ga-DOTATATE PET/CT for diagnosis, staging, and treatment management of neuroendocrine tumors. J Nucl Med 57:708–714
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (grant number: NRF-2019R1G1A1099743).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Sang Hyun Choi.
Conflict of interest
Sang Hyun Choi receives research funding from Bayer Healthcare outside the submitted work. The other authors have no conflicts of interest to declare.
Statistics and biometry
Ji Sung Lee has significant statistical expertise.
Informed consent
Written informed consent was not required for this study because of the study nature of meta-analysis.
Ethical approval
Institutional review board approval was not required because of the study nature of meta-analysis.
Methodology
• Systematic review
• Meta-analysis
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Choi, S.J., Choi, S.H., Lee, D.Y. et al. Diagnostic value of [68 Ga]Ga-DOTA-labeled-somatostatin analogue PET/MRI for detecting liver metastasis in patients with neuroendocrine tumors: a systematic review and meta-analysis. Eur Radiol 32, 4628–4637 (2022). https://doi.org/10.1007/s00330-021-08527-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08527-z