Abstract
Objectives
We aimed at investigating the origin of the correlations between tumor volume and 18F-FDG-PET texture indices in lung cancer.
Methods
Eighty-five consecutive patients with newly diagnosed non-small cell lung cancer (NSCLC) underwent a 18F-FDG-PET/CT scan before treatment. Seven phantom spheres uniformly filled with 18F-FDG, and covering a range of activities and volumes similar to that found in lung tumors, were also scanned. Established texture indices were computed for lung tumors and homogeneous spheres. The dependence between textural indices and volume in homogeneous spheres was modeled and then used to predict texture indices in lung tumors. Correlation analyses were carried out between predicted and texture features measured in lung tumors. Cox proportional hazards regression was used to investigate the associations between overall survival and volume-adjusted textural features.
Results
All textural features showed strong, non-linear correlations with volume, both in tumors and homogeneous spheres. Correlations between predicted versus measured texture features were very high for contrast (r2 = 0.91), dissimilarity (r2 = 0.90), ZP (r2 = 0.90), GLNN (r2 = 0.86), and homogeneity (r2 = 0.82); high for entropy (r2 = 0.50) and HILAE (r2 = 0.53); and low for energy (r2 = 0.30). Cox regressions showed that among volume-adjusted features, only HILAE was associated with overall survival (b = − 0.35, p = 0.008).
Conclusion
We have shown that texture indices previously found to be correlated with a number of clinically relevant outcomes might not provide independent information apart from that driven by their correlation with tumor volume, suggesting that these metrics might not be suitable as intratumor heterogeneity markers.
Key Points
• Associations between texture FDG-PET indices and overall survival have been widely reported in lung cancer, with tumor volume also being associated with overall survival, and therefore, it is still unclear whether the predictive power of textural indices is simply driven by this correlation.
• Our results demonstrated strong non-linear correlations between textural indices and volume, showing an analogous behavior for lung tumors from patients and homogeneous spheres inserted in phantoms.
• Our findings showed that texture FDG-PET indices might not provide independent information apart from that driven by their correlation with tumor volume.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Decades of research on tumor biology have revealed that tumors are heterogeneous entities at all scales (macroscopic, physiological, microscopic, genetic) [1, 2]. This tumor heterogeneity refers to the fact that different tumor cells can show distinct morphological and phenotypic profiles, including gene expression, metabolism, motility, proliferation, and metastatic potential [3,4,5]. Over the last few years, PET/CT has been proposed as a tool for noninvasive exploration of intratumor heterogeneity at the macroscopic scale [6,7,8], supposedly providing information about the biological features of tumors [9, 10]. Using a quantitative and automated approach termed as texture analysis, several prior studies have found significant correlations between tumor biology and heterogeneity measures derived from that texture analysis [11,12,13,14,15,16,17,18] in a number of different tumor types, such as breast [19, 20], pancreas [21], esophageal [22, 23], prostate [24], and especially numerous in lung cancer [25,26,27,28,29,30,31,32,33,34,35,36,37]. In this regard, several studies have reported that texture analysis can be correlated with clinically relevant outcomes such as overall survival [25, 27, 31, 34, 35], progression-free survival [27, 32, 37], and treatment response [26, 29, 36].
Although PET-based texture analysis seems to be an ideal tool for personalized medicine in oncology, numerous challenges need to be addressed before its reliable and interpretable use in the clinic [38]. There is still a need for a standardized quantification protocol to address all the widely reported issues related to the differences in the acquisition and reconstruction parameters, post-processing techniques, tumor segmentation methods or even the texture algorithm itself [38,39,40,41,42]. Nevertheless, the current major limitation of texture analysis is the lack of understanding of what PET texture indices actually represent in terms of the underlying real spatial distribution of radiotracer within the tumor. The complexity of the formulation of texture analysis makes it difficult to explain pervasive findings such as the correlations displayed between different texture indices [30, 31, 33,34,35, 43, 44], as well as the strong correlations between textural indices and tumor volume [45, 46]. Unraveling the biological substrate of these correlations is crucial to understand the complementary information provided by texture analysis, as well as to prove its clinical interpretation.
In this regard, the origin of the correlation of texture indices with tumor volume is particularly important in lung cancer. Associations between texture indices and overall survival have been consistently reported before by different studies [25, 27, 31, 34, 35]; however, tumor volume, which is also correlated with a more advanced disease stage, is strongly associated with survival [47,48,49], and therefore, it is unclear whether the predictive power of textural indices is simply driven by this correlation. In an attempt to account for this, some prior studies have statistically adjusted survival models for tumor volume [27, 46, 50]; however, correlations between texture indices and volume have been shown to be highly non-linear, leaving the efficacy of these approaches and, therefore, the real added value of texture indices, unclear.
In this study, we investigated the origin of the correlations between tumor volume and texture indices. For this, we employed a novel approach based on phantom data and studied the correlations between texture indices and volume in both real lung tumors and spheres with a homogeneous distribution of 18F-FDG.
Material and methods
Patient cohort
Our retrospective study included patients with newly diagnosed non-small lung cell carcinoma (NSCLC) referred to the Nuclear Medicine Department at Complexo Hospitalario Universitario de Santiago de Compostela (CHUS) for a pre-treatment 18FDG-PET/CT study from January to September 2018. Tumor stage was established according to the AJCC 7th Edition TNM classification [51].
Phantom data
NEMA image quality phantom [52] was used to mimic the shape of an upper human body including the commonly used hollow glass spheres with inner diameters of 3.7, 2.8, 1.7, 1.3, and 1 cm, and larger spheres specifically designed and manufactured for this work with inner diameters of 10 cm and 6 cm. In this way, our phantom study covered a range of sphere volumes from 0.29 to 294 cm3, which is similar to the range found in lung tumors [46].
Vereos PET/CT scanner
PET studies were carried out with digital Vereos PET/CT (Philips), a PET/CT scanner designed to improve small-lesion detection while reducing received dose and study time. The Digital Vereos PET/CT system is a digital photon counting PET scanner combined with a 128-channel CT system. The CT component, based on the Ingenuity CT, is a helical system with 40 mm axial coverage. The PET detector ring consists of 18 detector modules, each containing a 40 × 32 array of 4 × 4 × 19 mm3 LYSO crystals individually coupled to digital photon counters. The ring has a diameter of 764 mm and 164 mm axial length [53].
PET data acquisition
Patient preparation
Patients fasted for at least 6 h before the injection of 3.5 MBq/kg of 18F-FDG in order to ensure correct incorporation of the radiotracer, and blood glucose levels were checked and patients rested in a warm room 30 min before administration.
Phantom preparation
The phantom was prepared with two different sphere configurations: (a) spheres with 3.7, 2.8, 1.7, 1.3, and 1 cm diameter, and (b) spheres with 6 cm and 10 cm diameter. For each configuration, the phantom background was filled with a homogeneous 18F-FDG solution while spheres were filled with a higher 18F-FDG concentration to generate sphere-to-background ratios of approximately 4 and 6. Injected activity was selected so that the initial activity of the spheres was higher than that measured in the tumors with higher FDG uptake, thus covering, after decay for 8 h, the entire range of activities observed in our lung tumor sample (from 67 to 2.8 kBq/cm3).
Acquisition protocol
Patient and phantom data were acquired using exactly the same protocol for whole-body exams at our institution. Two minutes acquisition per bed were used, with an axial field of view of 16.4 cm for the first bed and 10 cm for subsequent ones, and the transaxial field of view was 576 mm. Phantom data was acquired using only one bed position centered on the phantom, with 2 min frames for 8 h. Those frames with activities below or above the observed activity range in tumors were discarded and not included in any of the analyses.
All images were reconstructed with the software provided by the manufacturer (Philips PET/Vereos version 2.0.2.26321) using an OSEM algorithm with 2 iterations and 10 subsets. Isotropic voxels of 2 mm size were used. Scatter, random, and attenuation corrections were turned on, but no PSF correction was performed.
PET data analysis
To investigate which part of the heterogeneity measured comes from biological reality of the tumor and which from other factors, we calculated textural features from PET images of NSCLC patients and then compared them with such textural features from PET images of homogeneous spheres. The same procedure was performed for patient and phantom data, following the general scheme illustrated in Fig. 1. Each tumor/sphere was manually enclosed in a cropping box by an experienced nuclear physician and automatic segmentation was performed inside the box applying a threshold of 0.45 × SUVmax [54]. The textural features were obtained using in-house implemented algorithms in MATLAB (The MathWorks, Inc.). To facilitate comparison with previously published literature, we computed exactly the same indices as in [46], and further computed 4 extra textural features that have been previously found to be associated with clinically relevant outcomes among lung cancer patients [33]. This set of indices includes first-order statistics features and textural features, derived from co-occurrence matrix (CM) [12], and size zone matrix (SZM) [55]. We adopted the same definition for the CM as in [46], in which we used a single matrix taking into account all 13 directions simultaneously to compute the elements P(I,J) of the CM, using a neighbor distance of n = 1 pixel. The selected features calculated from the CM [12] were entropy, dissimilarity, energy, contrast, and homogeneity. The selected features calculated from SZM [55] were high intensity large area emphasis (HILAE), Gray Level Non-Uniformity Normalized (GLNN), and zone percentage (ZP). Features were calculated after quantization with 64 gray levels as previously described [45, 46, 56,57,58,59]. A more detailed description of the textural indices is given in the Supplementary Material. Entropy, dissimilarity, HILAE, and ZP have shown robustness with respect to partial volume effects and segmentation [56], and reconstruction settings [42], as well as high reproducibility [57].
Statistical analysis
For descriptive statistics, mean ± standard deviation was used. We used a smoothing spline to model the dependence between texture indices and volume in homogeneous spheres. The smoothing parameter was set using cross-validation. Resulting spline models were then used to predict texture indices in lung tumors, using tumor volume as the only predictor. We additionally reported root mean-squared errors (RMSE) to provide a measure of fit quality. Finally, Pearson correlation analyses were performed between predicted and measured texture features to estimate the fraction of variance (r2) that is explained by the phantom-based model. To facilitate spline regression fitting, HILAE was log-transformed due to its wide range of values (~ 700 to 22000) and to reduce skewness of the residuals. Cox proportional hazards regression was used to investigate the associations between textural features and overall survival. These analyses were performed with both raw textural features and volume-adjusted textural features by subtracting volume dependence using the phantom-based spline model. All the analyses were performed using MATLAB (The MathWorks, Inc.).
Results
Patients’ characteristics
Table 1 shows the tumor characteristics of the patients included in this study. Eighty-five patients met clinical and imaging criteria for inclusion. The median age was 68 ± 10 years, being 19 females (12/19 smokers) and 62 males (62/62 smokers). Tumor stage was determined in 75/85 patients, with 11 patients in stage I, 2 patients in stage II, 28 patients in stage III, and 34 patients in stage IV. All tumors were correctly segmented, and tumor volumes ranged from 0.35 to 399.7 cm3. As an example, Fig. 2 shows PET scans from three representative patients.
Textural features and volume
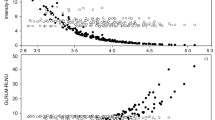
Figure 3 shows the dependence of textural features measured in tumors and phantom spheres with volume. All textural features showed strong, non-linear correlations with volume, both in tumors and phantom spheres. Moreover, the measured texture indices in tumors and phantom spheres displayed a significant overlap, particularly for dissimilarity (Fig. 3b), ZP (Fig. 3d), contrast (Fig. 3f), GLNN (Fig. 3g), and homogeneity (Fig. 3h). Entropy (Fig. 3a), HILAE (Fig. 3c), and energy (Fig. 3e) showed slight differences between spheres and tumors for larger volumes, but still the overlap was significant. For large volumes, entropy and energy measured in tumors was higher than entropy and energy measured in spheres, while HILAE measured in tumors was lower than HILAE in spheres.
Correlations between tumor and sphere texture features
Figure 4 displays textural feature values predicted using tumor volume with the phantom-based spline model versus the measured textural features in tumors. Strong and significant correlations were found in all the studied features (r > 0.7, p < 0.0001). Correlations between predicted versus tumor texture indices were very high for dissimilarity (r = 0.95, r2 = 0.90, RMSE = 0.993), contrast (r = 0.95, r2 = 0.91, RMSE = 28.83), ZP (r = 0.94, r2 = 0.90, RMSE = 0.055), GLNN (r = 0.93, r2 = 0.86, RMSE = 0.0393), and homogeneity (r = 0.90, r2 = 0.82, RMSE = 0.0197), and high for entropy (r = 0.70, r2 = 0.50, RMSE = 0.261) and the log (HILAE) (r = 0.73, r2 = 0.53, RMSE = 0.192). On the other hand, we find a low correlation only in the case of energy (r = 0.55, r2 = 0.30, RMSE = 0.00046). r2 values indicate that 90% of the variance of dissimilarity, contrast, and ZP was driven by non-heterogeneity information, and 80% for GLNN and homogeneity, while this fraction of variance was reduced to more than 50% for entropy and HILAE and close to 30% for energy.
Predicted versus measured textural features in lung tumors. Predicted textural features were computed using a spline model describing the dependence of textural features on lesion volume in homogeneous spheres. Thus, the model is entirely built on phantom data and only uses tumor volume for the estimation of the textural features. The analyzed textural features were (a) entropy, (b) dissimilarity, (c) HILAE, (d) ZP, (e) energy, (f) contrast, (g) GLNN, and (h) homogeneity. Squared Pearson correlation coefficients are reported as r2 and interpreted as the fraction of variance described by the phantom-based model
Survival analysis
We now tested whether the observed correlations with volume could drive or obscure associations with overall survival. Table 2 summarizes the results of univariable Cox regressions for each textural feature, both with and without subtraction of the volume effect estimated with our phantom-based model. We found that, among volume-unadjusted features, only entropy and energy were associated with survival (b = − 0.90, p = 0.01; b = 510, p = 0.01, respectively). These associations disappeared when adjusting for the effect of volume as estimated by the phantom-based model (b = − 0.43, p = 0.20; b = 3.6, p = 0.98); however, HILAE emerged as associated with survival (b = − 0.35, p = 0.008).
Discussion
Although texture analysis in lung cancer has shown promising performance for some clinically relevant tasks [25,26,27,28,29,30,31,32,33,34,35], its widespread use is still limited due to the lack of understanding of what texture features actually measure [40,41,42]. Aimed at shedding light on the biological correlates of texture analysis, we studied the correlations displayed between texture indices and lesion volumes [45, 46], both in lung tumor and phantom spheres, to estimate the physiological and non-physiological contributions to the observed correlations. Our strategy based on multiple patient-to-phantom comparisons was able to identify a strong non-physiological contribution to the correlation between volume and eight robust and well-established textural features (entropy, dissimilarity, HILAE, ZP, energy, contrast, GLNN, and homogeneity). Strikingly, this non-biologically driven correlation derived from phantom data was able to explain between 30 and 90% of the observed correlation between tumor volume and texture indices in real tumors.
Our findings demonstrated strong non-linear correlations between textural features and volume, showing an analogous behavior for spheres and tumors. This fact was clearly evident for dissimilarity, ZP, contrast, GLNN, and homogeneity, which showed a virtually complete overlapping between texture indices in tumors and texture indices in phantom’s spheres, suggesting that these metrics might not be useful for heterogeneity assessment. This indicates that prior findings linking these texture indices with survival or biological features of lung tumors are likely driven by this spurious correlation [26, 50, 60]. Moreover, given the strong non-linear dependencies with volume, it is far from clear that conventional linear techniques for confounder adjustment have effectively removed this spurious effect. Less severe overlapping was found in the trends of spheres and tumors for entropy, energy, and HILAE; being entropy and energy from tumors higher than that from homogeneous spheres; and HILAE from tumors lower than that from homogeneous spheres. This indicates that entropy, energy, and HILAE might provide biologically driven information beyond the non-biological information driven by volume. Nevertheless, our correlation analyses demonstrated that, still, the spurious correlation with volume explains more than 30% of the observed correlation. Moreover, entropy displayed a clear non-monotonic relationship with volume, which further complicates potential interpretability of this measure as a marker of intratumor heterogeneity. Future studies are warranted to further understand the technical factors that lead to these complex associations. Taken together, our findings suggest that (1) the ability to measure real intratumor heterogeneity of the studied indices is severely confounded by tumor volume and (2) the correlation between tumor volume and texture indices is mostly non-biologically driven.
In order to explore the implications of our findings, we conducted a survival analysis to investigate whether the observed correlations with the volume could drive or obscure associations with overall survival. For this, we studied the association between texture indices and overall survival, considering both volume-adjusted and volume-unadjusted texture indices as estimated from our phantom model. Our results showed that only entropy was associated with survival in the volume-unadjusted model [46]. Remarkably, this association disappeared when removing volume dependence through our phantom model and, in contrast, a HILAE effect emerged [60]. This finding suggests the potential of spurious volume correlations for obscuring biologically driven associations in texture analysis, highlighting the need for novel approaches that effectively remove artificial volume dependencies in textural features.
From an interpretability point of view, our findings challenge the established (but weakly supported) notion that the correlation between tumor volume and texture indices arises as a reflection of higher intratumor heterogeneity due to tumor growth [46]. The fact that we were able to describe more than 75% of the variance for some textural features in tumors with a model entirely based on phantom data excludes the possibility of a biological origin, while casts doubt on the suitability of these indices for assessing intratumor heterogeneity. These findings are consistent with those from a prior report in which the limited ability of texture indices for the assessment of intratumor heterogeneity was suggested using phantom and simulation data [61]. Here, we crucially add to these results by performing direct comparisons between phantom lesions and tumors, observing a strong overlap between the texture indices and confirming these previous findings. Overall, our findings suggest that a significant number of texture indices previously found to be correlated with clinically relevant outcomes might not provide any useful information apart from that driven by its correlation with tumor volume.
Our study has some strengths. We performed the first systematic comparison between texture indices measured in real lung tumors and in activity-matched homogeneous phantoms, allowing us to determine texture indices’ values in the absence of heterogeneity. Moreover, these analyses were carried out in a single PET/CT scan under highly controlled and routinely used settings. Compared to previous studies that also analyzed textural feature dependence on volume in a purely correlational way [46], we were able to isolate non-biological contributions to textural features in lung tumors, demonstrating the large impact on texture indices of these non-heterogeneity-driven correlations, and allowed a better understanding of what textural features actually reflect. This study also has some limitations. First, we only analyzed one specific type of tumor in a group of patients that came from the same hospital. Second, we only included a relatively small number of heterogeneity quantification metrics. However, these eight textural features included here have been widely studied previously [33,34,35, 46] and also been shown to have a prognostic value in different cancer types [46, 56, 57, 61]. Third, we employed a widely used approach for tumor segmentation based on intensity thresholding. Other approaches might lead to different results; however, we kept the same approach for both tumors and phantoms, and therefore, any potential bias should cancel out in direct comparisons between tumor and phantom data.
Conclusion
We have shown that textural features previously found to be correlated with a number of clinically relevant outcomes present strong, non-biologically driven correlations with tumor volume. Textural features measured in homogeneous phantom spheres were highly predictive of textural features measured in tumors of similar volume. Our findings suggest that these spurious correlations might hamper reliable measurement of intratumor heterogeneity with texture analysis.
Change history
23 December 2020
A Correction to this paper has been published: https://doi.org/10.1007/s00330-020-07619-6
Abbreviations
- CM:
-
Co-occurrence matrix
- GLNN:
-
Gray Level Non-Uniformity Normalized
- HILAE:
-
High intensity large area emphasis
- NSCLC:
-
Non-small lung cell carcinoma
- SZM:
-
Size zone matrix
- ZP:
-
Zone percentage
References
Gerlinger M, Rowan AJ, Horswell S et al (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366:883–892
McGranahan N, Swanton C (2015) Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell 27:15–26
Tellez-Gabriel M, Ory B, Lamoureux F, Heymann M-F, Heymann D (2016) Tumour heterogeneity: the key advantages of single-cell analysis. Int J Mol Sci 20;17(12):2142
Michor F, Polyak K (2010) The origins and implications of intratumor heterogeneity. Cancer Prev Res (Phila) 3:1361–1364
Visvader JE (2011) Cells of origin in cancer. Nature. 469:314–322
Krause BJ, Schwarzenböck S, Souvatzoglou M (2013) FDG PET and PET/CT. Recent Results Cancer 187:351–369
Davnall F, Yip CSP, Ljungqvist G, Selmi M, Ng F (2012) Sanghera B, et al Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3:573–589
Chicklore S, Goh V, Siddique M, Roy A, Marsden PK, Cook GJR (2013) Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging 40:133–140
Fonti R, Conson M, Del Vecchio S (2019) PET/CT in radiation oncology. Semin Oncol 46:202–209
Miller TR, Pinkus E, Dehdashti F, Grigsby PW (2003) Improved prognostic value of 18F-FDG PET using a simple visual analysis of tumor characteristics in patients with cervical cancer. J Nucl Med 44:192–197
Bailly C, Bodet-Milin C, Bourgeois M et al (2019) Exploring tumor heterogeneity using PET imaging: the big picture. Cancers (Basel) 11(9):1282
Hatt M, Tixier F, Visvikis D, Cheze Le Rest C (2017) Radiomics in PET/CT: more than meets the eye? J Nucl Med 58:365–366
Tixier F, Hatt M, Valla C, et al (2014) Visual versus quantitative assessment of intratumor 18F-FDG PET uptake heterogeneity: prognostic value in non-small cell lung cancer. J Nucl Med 55(8):1235–1241
Haralick RM, Shanmugam K, Dinstein I (1973) Textural features for image classification. IEEE Trans Syst Man Cybern SMC-3:610–621
Amadasun M, King R (1989) Textural features corresponding to textural properties. IEEE Trans Syst Man Cybern 19:1264–1274
Alic L, Niessen WJ, Veenland JF (2014) Quantification of heterogeneity as a biomarker in tumor imaging: a systematic review. PLoS One 9:e110300
Materka A, Strzelecki M (1998). Texture analysis methods – a review. Technical University of Lodz, Institute of Electronics, COST B11 report, Brussels
Castellano G, Bonilha L, Li LM, Cendes F (2004) Texture analysis of medical images. Clin Radiol 59:1061–1069
Moscoso A, Ruibal Á, Domínguez-Prado I et al (2018) Texture analysis of high-resolution dedicated breast 18 F-FDG PET images correlates with immunohistochemical factors and subtype of breast cancer. Eur J Nucl Med Mol Imaging 45:196–206
Groheux D, Martineau A, Teixeira L et al (2017) 18FDG-PET/CT for predicting the outcome in ER+/HER2- breast cancer patients: comparison of clinicopathological parameters and PET image-derived indices including tumor texture analysis. Breast Cancer Res 19:3
Kamitaka Y, Miwa K, Nishii R et al (2019) Textural analysis of 18F-FDG PET/CT to predict tumor response of carbon-ion radiotherapy in patients with locally advanced pancreas cancer. J Nucl Med 60:3005–3005
Tixier F, Le Rest CC, Hatt M et al (2011) Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med 52:369–378
Wang S, Wang S, Liu E-T (2019) Application value of 18F-FDG PET/CT and texture analysis in evaluating the M staging of esophageal cancer. J Nucl Med 60:219–219
Lue K-H, Chen Y-H, Lin H-H, Liu S-H, Kao C-H, Chuang K-S (2019) Relationship between prostate-specific antigen kinetics and radiomic features of F-18 fluorocholine PET in patients with prostate cancer. J Nucl Med 60:3004–3004
Song J, Dong D, Huang Y, Liu Z, Tian J (2016) Association between tumor heterogeneity and overall survival in patients with non-small cell lung cancer. 2016 IEEE 13th Int Symp Biomed Imaging ISBI
Lovinfosse P, Janvary ZL, Coucke P et al (2016) FDG PET/CT texture analysis for predicting the outcome of lung cancer treated by stereotactic body radiation therapy. Eur J Nucl Med Mol Imaging 43:1453–1460
Cook GJR, Yip C, Siddique M et al (2013) Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med 54:19–26
Giesel FL, Schneider F, Kratochwil C et al (2017) Correlation between SUVmax and CT radiomic analysis using lymph node density in PET/CT-based lymph node staging. J Nucl Med 58:282–287
Bashir U, Siddique MM, Mclean E, Goh V, Cook GJ (2016) Imaging heterogeneity in lung cancer: techniques, applications, and challenges. AJR Am J Roentgenol 207:534–543
Moon SH, Kim J, Joung J-G et al (2019) Correlations between metabolic texture features, genetic heterogeneity, and mutation burden in patients with lung cancer. Eur J Nucl Med Mol Imaging 46:446–454
Scrivener M, de Jong EEC, van Timmeren JE, Pieters T, Ghaye B, Geets X (2016) Radiomics applied to lung cancer: a review. Transl Cancer Res 5:398–409
Cherezov D, Goldgof D, Hall L et al (2019) Revealing tumor habitats from texture heterogeneity analysis for classification of lung cancer malignancy and aggressiveness. Sci Rep 9:4500
Han S, Woo S, Suh CH, Kim YJ, Oh JS, Lee JJ (2018) A systematic review of the prognostic value of texture analysis in 18F-FDG PET in lung cancer. Ann Nucl Med 32:602–610
Jensen GL, Yost CM, Mackin DS et al (2018) Prognostic value of combining a quantitative image feature from positron emission tomography with clinical factors in oligometastatic non-small cell lung cancer. Radiother Oncol 126:362–367
Kirienko M, Cozzi L, Antunovic L et al (2018) Prediction of disease-free survival by the PET/CT radiomic signature in non-small cell lung cancer patients undergoing surgery. Eur J Nucl Med Mol Imaging 45:207–217
Dong X, Sun X, Sun L et al (2016) Early change in metabolic tumor heterogeneity during chemoradiotherapy and its prognostic value for patients with locally advanced non-small cell lung cancer. PLoS One 11:e0157836
Park S, Ha S, Lee S-H et al (2018) Intratumoral heterogeneity characterized by pretreatment PET in non-small cell lung cancer patients predicts progression-free survival on EGFR tyrosine kinase inhibitor. PLoS One 13:e0189766
Hatt M, Tixier F, Pierce L, Kinahan PE, Le Rest CC, Visvikis D (2017) Characterization of PET/CT images using texture analysis: the past, the present… any future? Eur J Nucl Med Mol Imaging 44:151–165
Depeursinge A, Foncubierta-Rodriguez A, Van De Ville D, Müller H (2014) Three-dimensional solid texture analysis in biomedical imaging: review and opportunities. Med Image Anal 18:176–196
Altman DG, Lausen B, Sauerbrei W, Schumacher M (1994) Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 86:829–835
Chalkidou A, O’Doherty MJ, Marsden PK (2015) False discovery rates in PET and CT studies with texture features: a systematic review. PLoS One 10:e0124165
Yan J, Chu-Shern JL, Loi HY et al (2015) Impact of image reconstruction settings on texture features in 18F-FDG PET. J Nucl Med 56:1667–1673
Lemarignier C, Martineau A, Teixeira L et al (2017) Correlation between tumour characteristics, SUV measurements, metabolic tumour volume, TLG and textural features assessed with 18F-FDG PET in a large cohort of oestrogen receptor-positive breast cancer patients. Eur J Nucl Med Mol Imaging 44:1145–1154
Orlhac F, Soussan M, Maisonobe J-A, Garcia CA, Vanderlinden B, Buvat I (2014) Tumor texture analysis in 18F-FDG PET: relationships between texture parameters, histogram indices, standardized uptake values, metabolic volumes, and total lesion glycolysis. J Nucl Med 55:414–422
Brooks FJ, Grigsby PW (2014) The effect of small tumor volumes on studies of intratumoral heterogeneity of tracer uptake. J Nucl Med 55:37–42
Hatt M, Majdoub M, Vallières M et al (2015) 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J Nucl Med 56:38–44
Werner-Wasik M, Swann RS, Bradley J et al (2008) Increasing tumor volume is predictive of poor overall and progression-free survival: secondary analysis of the Radiation Therapy Oncology Group 93-11 phase I-II radiation dose-escalation study in patients with inoperable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 70:385–390
Huang W, Fan M, Liu B et al (2014) Value of metabolic tumor volume on repeated 18F-FDG PET/CT for early prediction of survival in locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy. J Nucl Med 55:1584–1590
Kurtipek E, Çayci M, Düzgün N et al (2015) (18)F-FDG PET/CT mean SUV and metabolic tumor volume for mean survival time in non-small cell lung cancer. Clin Nucl Med 40:459–463
Chaddad A, Desrosiers C, Toews M, Abdulkarim B (2017) Predicting survival time of lung cancer patients using radiomic analysis. Oncotarget. 8:104393–104407
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474
Performance measurements of positron emission tomographs (PET) [Internet]. [cited 2020 May 27]. Available from: https://www.nema.org
Vereos P. Advanced molecular imaging. 12. https://www.usa.philips.com/healthcare/solutions/advanced-molecular-imaging
Bashir U, Azad G, Siddique MM et al (2017) The effects of segmentation algorithms on the measurement of 18F-FDG PET texture parameters in non-small cell lung cancer. EJNMMI Res 7:60
Texture indexes and gray level size zone matrix application to cell nuclei classification. Pattern Recogn Inf Process. 2009. Available from: https://www.scienceopen.com/document?vid=2c91747d-b5c9-4a39-8751-9e17e9776f22
Hatt M, Tixier F, Cheze Le Rest C, Pradier O, Visvikis D (2013) Robustness of intratumour 18F-FDG PET uptake heterogeneity quantification for therapy response prediction in oesophageal carcinoma. Eur J Nucl Med Mol Imaging 40:1662–1671
Tixier F, Hatt M, Le Rest CC, Le Pogam A, Corcos L, Visvikis D (2012) Reproducibility of tumor uptake heterogeneity characterization through textural feature analysis in 18F-FDG PET. J Nucl Med 53:693–700
van Velden FHP, Cheebsumon P, Yaqub M et al (2011) Evaluation of a cumulative SUV-volume histogram method for parameterizing heterogeneous intratumoural FDG uptake in non-small cell lung cancer PET studies. Eur J Nucl Med Mol Imaging 38:1636–1647
Groheux D, Majdoub M, Tixier F et al (2015) Do clinical, histological or immunohistochemical primary tumour characteristics translate into different 18F-FDG PET/CT volumetric and heterogeneity features in stage II/III breast cancer? Eur J Nucl Med Mol Imaging 42:1682–1691
Takeda K, Takanami K, Shirata Y et al (2017) Clinical utility of texture analysis of 18F-FDG PET/CT in patients with stage I lung cancer treated with stereotactic body radiotherapy. J Radiat Res 58:862–869
Yang F, Young LA, Johnson PB (2018) Quantitative radiomics: validating image textural features for oncological PET in lung cancer. Radiother Oncol 129:209–217
Acknowledgments
Special thanks to the technical staff of the Nuclear Medicine Department, who provided all the required information on the acquisition and reconstruction protocol and management of patients.
Funding
This study has received funding by the public project DTS17/00138 co-funded by FEDER and Beca SEMNIM 2020 (con el patrocinio de Advanced Accelerator Applications).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Pablo Aguiar.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
AM-R has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• prognostic study/observational/experimental
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The information that Manuel Piñeiro-Fiel and Alexis Moscoso have contributed equally to this work was missing.
Manuel Piñeiro-Fiel and Alexis Moscoso contributed equally to this work.
Supplementary Information
ESM 1
(DOCX 433 kb)
Rights and permissions
About this article
Cite this article
Piñeiro-Fiel, M., Moscoso, A., Lado-Cacheiro, L. et al. Is FDG-PET texture analysis related to intratumor biological heterogeneity in lung cancer?. Eur Radiol 31, 4156–4165 (2021). https://doi.org/10.1007/s00330-020-07507-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07507-z