Abstract
Objectives
To prospectively assess reproducibility, safety, and efficacy of microwave ablation (MWA) in the treatment of unresectable primary and secondary pulmonary tumors.
Methods
Patients with unresectable primary and metastatic lung tumors up to 4 cm were enrolled in a multicenter prospective clinical trial and underwent CT-guided MWA. Treatments were delivered using pre-defined MW power and duration settings, based on target tumor size and histology classifications. Patients were followed for up to 24 months. Treatment safety, efficacy, and reproducibility were assessed. Ablation volumes were measured at CT scan and compared with ablation volumes obtained on ex vivo bovine liver using equal treatment settings.
Results
From September 2015 to September 2017, 69 MWAs were performed in 54 patients, achieving technical success in all cases and treatment completion without deviations from the standardized protocol in 61 procedures (88.4%). Immediate post-MWA CT scans showed ablation dimensions smaller by about 25% than in the ex vivo model; however, a remarkable volumetric increase (40%) of the treated area was observed at 1 month post-ablation. No treatment-related deaths nor complications were recorded. Treatments of equal power and duration yielded fairly reproducible ablation dimensions at 48-h post-MWA scans. In comparison with the ex vivo liver model, in vivo ablation sizes were systematically smaller, by about 25%. Overall LPR was 24.7%, with an average TLP of 8.1 months. OS rates at 12 and 24 months were 98.0% and 71.3%, respectively.
Conclusions
Percutaneous CT-guided MWA is a reproducible, safe, and effective treatment for malignant lung tumors up to 4 cm in size.
Key Points
• Percutaneous MWA treatment of primary and secondary lung tumors is a repeatable, safe, and effective therapeutic option.
• It provides a fairly reproducible performance on both the long and short axis of the ablation zone.
• When using pre-defined treatment duration and power settings according to tumor histology and size, LPR does not increase with increasing tumor size (up to 4 cm) for both primary and metastatic tumors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Percutaneous thermal ablation is emerging as a safe and effective loco-regional alternative to surgery and stereotactic body radiation therapy (SBRT) for the treatment of either primary early-stage or metastatic pulmonary tumors [1, 2]. Alternating electric current circuits generating electromagnetic waves in the 400–500 kHz range (radiofrequency) have been mostly used for this purpose; they cause tissue heating through ionic agitation and Joule effect. Over the last decade, microwave ablation (MWA) has gained increasing popularity, partly supplanting RF ablation (RFA) for percutaneous thermal ablation of tumors in numerous anatomical districts [3,4,5,6,7]. MW heating is based on a dielectric mechanism, secondary to friction among molecular electric dipoles that are simultaneously rotating due to fast switching electromagnetic waves [3].
However, MWA is a relatively new technique compared with RFA, with a remarkably wider variability in device design and performance, more complex decision-making algorithms for treatment planning, and a less trivial correlation between the expected ablation size and any probe-related feature [4,5,6,7,8]. All these account for a lack of standardization in MWA treatment protocols, especially in extra-hepatic clinical applications such as the ablation of lung tumors. The coagulation charts provided by vendors are the most readily available guidelines to MWA planning, but they are based on ex vivo animal models (usually liver) that differ from the in vivo scenario. Very few published MWA clinical series contribute to build predictive models alternative to the vendors’ coagulation charts [9].
Our study (named “MALT,” i.e., Microwave Ablation of Lung Tumors) was designed to fill this gap of knowledge and to prospectively evaluate the performance repeatability of a commercially available 2450-MHz MWA system for the CT-guided percutaneous treatment of primary and metastatic pulmonary tumors up to 4 cm in diameter, using pre-defined treatment settings. We investigated the correlation between applied energy and ablation volume at post-procedural CT scan evaluations. Ablation volumes were also compared with those provided by the manufacturer’s ex vivo bovine liver coagulation chart in order to evaluate its capability in predicting clinical performance. Secondary endpoints were safety and clinical efficacy assessments, in terms of immediate and delayed complications and of overall (OS) and local progression-free survival (LPFS) rates.
Materials and methods
Study design
MALT study was designed as a prospective multicenter clinical trial enrolling consecutive patients with inoperable primary (“MALT1” study arm) or metastatic (“MALT2” study arm) lung cancers up to 4 cm in size. It was performed at three different centers, each with no less than 15 years of prior experience in percutaneous thermal ablation procedures on multiple target organs. The study was conducted in respect of the Declaration of Helsinki, according to the International Conference on Harmonization (ICH) Harmonized Tripartite Guideline for Good Clinical Practice. The Institutional Review Board of each participating center approved the study and all enrolled patients signed a specific written informed consent.
A multidisciplinary tumor board deemed patients unresectable and candidates to percutaneous thermal ablation treatment. Inclusion and exclusion criteria for MALT study are listed in Table 1. Pre-treatment work-up included routine physical examination, laboratory tests, and re-evaluation by contrast-enhanced chest computed tomography (CT) when needed. All demographic and clinical data were collected in a study-specific case report form.
In each study arm (MALT1 and MALT2), lesions were classified according to size (group 1: less than 2 cm; group 2: between 2 and 3 cm; group 3: between 3 and 4 cm).
MWA treatment
All thermal ablation treatments were performed via percutaneous approach, under CT guidance, using the HS AMICA™ 2.45-GHz microwave ablation system HS Hospital Service SpA), composed of a solid-state microwave generator with output of up to 140 W (AMICA-GEN, either model AGN-H-1.0 or AGN-3.0), and 14 G internally cooled interstitial applicators (AMICA-PROBE). A single antenna was used in all MWA treatments (Figs.1 and 2).
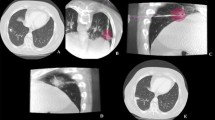
A 74-year-old man with a 2.8-cm primary tumor in the right upper lobe treated with MWA (60W × 10 min) (a, d: pre-procedural CT-images) with a large ablation volume obtained at 1-month follow-up (b, e: 1 month CT images). Primary tumor disappeared at 6 months follow-up CT images (c, f), without procedural nor delayed complications
A 70-year-old female with two 3-cm metastases in the middle (a), and right lower lobe (b), treated with MW ablations (70 W × 10 min), in supine (c) and right lateral-prone (d) decubitus, respectively. Apparent complete ablation of both tumors obtained at 1-month post-treatment CT scan, with sufficient safety margin without procedural complications (e, f). At 9-month CT follow-up, complete ablation was maintained in the peripheral metastasis (g), while a recurrence occurred on the hilar side of the central lesion, possibly due to heat sink effect of relatively large adjacent vessels (h)
Each lesion was treated inserting the antenna along the major lesion axis, placing the tip 1 to 5 mm beyond the tumor distal edge in order to obtain a sufficient (5–10 mm) safety margin (Fig. 2). Treatment protocol was standardized to continuous MW energy delivery at a pre-defined power rate (P) for a pre-defined time (T = 10 min), without neither probe re-positioning nor power variations during the treatment. P was pre-determined based on the lesion histology (considering that primary lung tumors need a wider safety margin than metastases [10,11,12,13]) and size, namely P = 40 W-60 W-80 W for treating tumors belonging to groups 1-2-3 of the MALT1 arm and P = 30 W-50 W-70 W for treating tumors belonging to groups 1-2-3 of the MALT2 arm. Treatment protocols were selected to warrant full coverage of the nodules (including the required safety margin) and equal energy delivery (Ed = P*T in seconds) in all lesions belonging to the same study group (Ed = 24 kJ-36 kJ-48 kJ for groups 1-2-3 of MALT1 and Ed = 18 kJ-30 kJ-42 kJ for groups 1-2-3 of MALT2). In the case of patients with multiple lesions, these were treated in the same session if located in the same lobe or lung (Fig. 2), or in separate sessions otherwise. All procedural data tracked by the generator during MWA procedures were recorded and attached to the correspondent case report form.
Treatments not complying with all listed procedural rules were excluded from the study for the evaluation of treatment repeatability and for comparison with the ex vivo animal model, while being still considered for secondary purposes (complications and clinical success rates).
Ex vivo (ablation chart)/in vivo comparison
Ex vivo coagulation charts are often used as a reference for MWA treatment planning in clinical practice. As for the MWA system used in the MALT study, the coagulation chart provided by the manufacturer is the results of laboratory experiments conducted on ex vivo bovine liver at room temperature using various settings (P = 20, 40, 60, 80, and 100 W; T = 3, 5, 10, and 15 min). The chart reports the long-axis (Lev, i.e., along the MWA probe trajectory) and short-axis (Dev, i.e., perpendicular to the MWA probe) measures of the coagulation zone produced in liver tissue. Data at 30 W, 50 W, and 70 W were obtained by linear interpolation.
Ex vivo ablation measures (Lev, Dev, and the ablation sphericity index Sev = Dev/Lev) were compared with their counterparts in the MALT study (Liv, Div, and Siv = Div/Liv, respectively, i.e., the radiological measurements of the ablation zones achieved clinically using the same MWA parameters) to assess the predictive accuracy of the manufacturer’s chart.
Deviations of the in vivo clinical results from ex vivo predictions were calculated as follows: ΔL = (Liv − Lev)/Lev and ΔD = (Div − Dev)/Dev for the ablation long and short axis, respectively.
Post-treatment and follow-up studies
All patients underwent an unenhanced chest CT scan immediately after the removal of the antenna and a baseline and contrast-enhanced chest CT scan within 48 h post-MWA, to assess volume of ablation (V0) and exclude complications. Post-treatment chest CT scans and clinical examinations were scheduled at 1, 3, 6, 12, 18, and 24 months post-MWA, according to our standard of care, for the evaluation of the local efficacy and possible delayed complications (Figs. 1 and 2).
All examinations were carried out using 64-channel multidetector-row CT systems (Lightspeed VCT XT, GE; Brilliance 64, Philips) and 120 kV and 100 mA tube voltage and current, respectively. All studies were acquired from the pulmonary apices to the diaphragm, with 1.25-mm-slice thickness reconstruction. A total of 0.4 gI/kg of iodinate low-osmolar non-ionic contrast medium (iopromide, 370 mgI/mL, Ultravist) was injected with a flow rate of 3 mL/s, followed by 60 mL of saline. Arterial phase was acquired at 35 s whereas delayed phase at 60 s [10].
At each post-MWA CT scan, the following were evaluated: size of the ablation zone along three planes (D1, D2, and D3, see below), including the post-ablation peripheral zone of ground-glass opacity (GGO) when present, and occurrence of local or distal tumor progression (LP and DP, respectively).
Measurements were performed by direct visual inspection of the post-ablation CT scans, using the ordinary tools provided by the CT scan viewer software suite. As ablated areas are well approximated by ellipsoids with rotational symmetry around the microwave probe axis, ablation volumes were obtained from linear measurements by applying the ellipsoid formula (π/6*D1*D2*D3, where D1, D2, and D3 are the longest ablation diameters in the xy, yz, and xz planes of an xyz orthogonal Cartesian coordinate system).
Normalized ablation volumes (Vn = Vt/V0, where Vt is the measured volume at time t post-MWA) were calculated at each follow-up control, to identify the time constant τ characteristic of tissue repair mechanisms, i.e., the mean time to complete “re-absorption” of the thermal lesion.
Treatment-related morbidity and mortality rates were based on the incidence of complications during the first 7 days post-treatment. Complications were assigned a severity grade using the SIR latest classification [14]: 0 = no complications; 1 = mild adverse event (requiring no therapy or non-substantial therapy); 2 = moderate adverse events (with moderate escalation of care, requiring substantial treatment); 3 = severe adverse events (with marked escalation of care); 4 = life-threatening or disabling event; 5 = patient death. The same severity scale was also used for delayed complications (later than 7 days post-MWA). Complication score, defined as mean severity grade, was calculated for all treatments in MALT1/MALT2 groups.
Based on the follow-up CT scans, treatment efficacy was evaluated as complete ablation (CA) (Figs. 1 and 2) or partial ablation (PA), according to CT analysis of lesion size and absence or persistent enhancement. Persistence or appearance (after CA) of enhancing areas was classified as LP (Fig. 2). Local and distant disease progression rates (LPR and DPR, respectively) and time to local and distant progression (TLP and TDP) were recorded. Finally, OS and LPFS curves were estimated.
Statistical analysis
A specific statistical analysis plan, to obtain a sample sizing, was not pre-designed due to lack of preliminary information from pilot studies.
Quantitative variables were reported with average (AVG) value, standard deviation (SD), minimum (MIN) value, maximum (MAX) value, and coefficient of variation (CV = SD/AVG).
The p value threshold for statistical significance was set to p = 0.05, based on a two-tailed, unpaired, heteroskedastic Student’s t test. The analyses of OS and LPFS were conducted using the Kaplan-Meier method. All calculations were performed using Excel worksheets and validated with software SPSS v16.0 (IBM).
Results
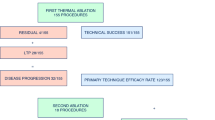
From September 2015 to September 2017, 54 patients with a total of 69 nodules were enrolled in our study; more than one tumor was ablated in 11 treatment sessions, with a maximum number of 3 tumors per session.
The main features of patients and tumors are reported in Table 2. Technical success (i.e., complete tumor ablation, with margin) was achieved in all cases (69/69). However, the planned treatment protocol, based on the use of a single antenna without probe re-positioning nor power variations, was performed in 61 out of the overall 69 treatments (88.4%). In the other 8 treatments (11.6%), two consecutive overlapping ablations were performed for ensuring complete tumor coverage with safety margins, due to the impossibility of precisely accessing the target tumor along its longest axis. These 8 treatments were excluded from the evaluation of MWA repeatability and comparability with the ex vivo bovine liver model and retained only for safety and efficacy assessments.
The onset of GGO at the ablation site, partially or completely encompassing the tumor, was reported in the majority of cases (61.5% of overall treated lesions), with a statistically significant difference between the MALT1 arm (50.0%) and the MALT2 arm (69.2%) (p = 0.0034).
In terms of MWA repeatability assessment (Table 3), the 48-h CT study showed a wider variability in the long axis (CV = 25.3 ± 5.5%) than in the short axis (CV = 17.5 ± 7.3%) of the ablated zone. Table 3 also shows how ablation sizes measured in the clinical series were systematically smaller than those in the ex vivo model for all investigated settings, nearly to the same extent in the long and short axes (ΔL = − 24.0 ± 21.8% vs ΔD = − 27.0 ± 15.6%, p = 0.27).
Figure 3 shows the dependence of the ablation measures (L and D) on deposited energy Ed, both in the ex vivo model and in the MALT series. While Lev and Liv grow almost equally fast with increasing Ed, in terms of the short ablation axis the performance gap between ex vivo and in vivo appears to reduce as Ed grows.
At 1-month post-treatment CT scan, volume of ablation was on average 40% larger than at immediate post-MWA CT.
Figure 4 depicts how the ablated tissue shrinks over time, as monitored through the follow-up CT scans: normalized ablation volumes appear to undergo an exponential decrease (i.e., Vn = V0 * e−t/τ), with a time constant τ of approximately 9.2 months.
Volume of the treated tissue area (Vn, normalized with respect to the ablation volume measured immediately after treatment) as a function of time (T, expressed in months post-MWA). Black circles: average of Vn values measured from follow-up CT scans at 1, 3, 6, 12, and 18 months post-MWA; error bars: standard deviation of measurements at each time point; dashed curve: exponential data fit
Safety and clinical efficacy
No treatment-related deaths were recorded. Two (2.9%, on a per treatment basis) grade-2 peri-procedural complications occurred (pleural effusions requiring drainage, both in the MALT2 arm). A pneumothorax was reported in 29/69 procedures (42.0%): 17/28 in MALT1 (60.7%) and 12/41 in MALT2 (29.3%); chest tube placing or prolonged hospital stay was never needed, but post-procedural pneumothorax drainage was performed in 4 cases, all belonging to the MALT 2 arm. An overall treatment-related complication score of 0.49 ± 0.55 was reported (MALT1: 0.64 ± 0.48; MALT2: 0.39 ± 0.58; p = 0.056). Only 1 (1.4%) grade-2 delayed complication occurred (a bronchial fistula, in the MALT1 arm). The overall delayed complication score was 0.25 ± 0.47. A statistically significant difference (p = 0.023) was observed between MALT1 and MALT2 in terms of delayed complication scores (0.42 vs 0.12, respectively, p = 0.033).
All patients were hospitalized overnight and discharged within 2 days post-MWA, except for the two subjects with grade-2 complications (2 and 3 days additional hospital stay, respectively).
LPR and DPR of 24.7% and 33.3% were reported (TLP: 8.1 ± 6.0 months; TDP: 9.0 ± 7.0 months); a remarkably higher DPR was registered in MALT2 (54.3% vs 4%). Subgroups analysis for LPR is shown in Table 4.
Over the entire MALT cohort, estimated OS and LPFS rates at 12 and 24 months were 98.0% and 71.3%, and 80.2% and 64.7%, respectively (Fig. 5). MALT1 resulted in OS = 95.7% and LPFS = 71.4% at 12 months, and OS = 78.1% and LPFS = 62.5% at 24 months; MALT2 yielded OS = 100.0% and LPFS = 68.6% at 12 months, and OS = 81.6% and LPFS = 64.6% at 24 months. No statistically significant difference was demonstrated between the two arms (p = 0.456 for OS and 0.461 for LPFS).
However, differences in oncological management between primary and secondary tumors were remarkable, considering the higher number of patients subject to systemic therapy in the MALT2 arm.
Discussion
MALT trial confirmed the clinical viability of percutaneous MWA in the treatment of lung tumors up to 4 cm in size, with performance variability comparable to that reported in previous series, reporting the results of treatment of hepatic metastases and hepatocellular carcinomas with the same MWA system [15]. The cause of residual variability, despite the strict control of MW energy dosing provided by the MALT protocol, is probably intrinsic to the relatively coarse classification criteria used for this study, in which target tumors were grouped only according on histology (primary or metastatic) and size. Additional and more refined stratification criteria—e.g., anatomic factors, characteristics of surrounding parenchyma, and prior systemic or regional treatments—are probably needed. Moreover, the different responses to MWA between primary and metastatic lesions in terms of immediate appearance of GGO pattern at the treatment site may be due to the differences of the respective tissue backgrounds.
CT scans performed within 48 h after MWA completion clearly showed an ablation size inferior by about 25% in each linear dimension compared with what was predicted by the ex vivo coagulation chart. This may be due to the tissue shrinkage phenomenon induced by thermal treatments: the MWA contraction ratio (i.e., pre-MWA to post-MWA ratio of a tissue segment length comprised in the treated volume) reported in the literature for lung parenchyma (~ 50%) is appreciably higher than its counterpart for liver parenchyma (~ 30%) [16,17,18,19,20,21].
Standard deviations of ΔL (21.8%) and ΔD (15.6%) are comparable with the intrinsic MWA performance variability observed in our study. Therefore, we have to conclude that, when cutting down all table entries by 25%, the standard ex vivo coagulation chart may still be used as a valid reference when planning pulmonary MWA treatments.
It is worthwhile to note that, at 1-month post-treatment CT scan, the ablation volume was approximately 40% larger in volume than that at previous post-MWA CT scan: delayed necrotizing mechanisms, secondary to thermal treatment, may cause further enlargement of the radiologically detectable ablation zone in the short term. These findings should be taken into account in the clinical practice for the timely and correct assessment of MWA of lung tumors.
Both rate and type of complications of percutaneous MWA reported in MALT study are very similar to those of previously published studies [21, 22]. Globally, MALT trial confirmed safety and tolerability of percutaneous lung MWA. A trend towards increased likelihood and/or severity of complications when treating primary compared with metastatic tumors has emerged, probably due to the increased respiratory co-morbidities in patients with primary tumors [23,24,25,26,27].
The LPRs observed in the MALT study are slightly lower than those reported in the literature for RFA series, particularly with regard to the treatment of pulmonary metastases [28, 29]. Increasing tumor size up to 4 cm did not negatively affect LPR: this may be interpreted as an indirect proof of the capability of MWA, compared with RFA, of achieving good locale disease control also when dealing with large tumors, possibly raising the bar of ablatable lesion size from 3 cm at least up to 4 cm [30,31,32]. The increased heat sinking rejection, the higher intra-tumoral temperatures, and the local tissue inhomogeneity ensured by MWA provide a robust physical explanation to our findings [33, 34].
Nevertheless, several partial ablations occurred, possibly also due to some procedural rules imposed by MALT protocol, such as the mandatory use of a single probe in a single position, at a pre-set power, for a pre-set time. In fact, the use of one specific MWA technology and strict procedural protocols were key for the assessment of performance repeatability. On the other hand, these features circumscribe the scope of the study to the explored MWA system and settings only. Ad hoc procedural adjustments in routine clinical practice may indeed help to further improve outcomes in terms of local disease control. The other main limitation of the MALT study is the relatively low number of enrolled patients and treated tumors, especially in view of the further sub-grouping according to tumor histology and dimensional class.
Conclusions
The prospective multicenter MALT trial confirmed that percutaneous MWA treatment of primary and metastatic lung tumors using a 2450-MHz ablation system is a repeatable, reproducible, safe, and effective therapeutic option. Local progression of treated tumors up to 4 cm did not correlate to their initial size.
Abbreviations
- AVG:
-
Average
- CA:
-
Complete ablation
- COPD:
-
Chronic obstructive pulmonary disease
- CT:
-
Computed tomography
- CV:
-
Coefficient of variation
- D :
-
Ablation short axis (i.e., diameter perpendicular to the microwave probe)
- DP:
-
Distant progression
- DPR:
-
Distant progression rate
- E d :
-
Microwave energy delivered to the patient
- EV:
-
Ex vivo data series (i.e., ablations on ex vivo bovine liver)
- GGO:
-
Ground-glass opacity
- IV:
-
In vivo data series (i.e., ablations on MALT patients)
- L :
-
Ablation long axis (i.e., longest diameter, along the microwave probe track)
- LP:
-
Local progression
- LPFS:
-
Local progression-free survival
- LPR:
-
Local tumor progression rate
- MALT:
-
Microwave Ablation of Lung Tumors (study acronym)
- MALT1:
-
MALT study arm related to primary lung tumors
- MALT2:
-
MALT study arm related to metastatic lung tumors
- MAX:
-
Maximum value
- MIN:
-
Minimum value
- MW:
-
Microwave radiation (2450 MHz)
- MWA:
-
Microwave ablation
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- P :
-
Power of ablation treatment
- PA:
-
Partial ablation
- RFA:
-
Radiofrequency ablation
- S :
-
Ablation sphericity index
- SBRT:
-
Stereotactic body radiation therapy
- SD:
-
Standard deviation
- T :
-
Duration of ablation treatment
- TDP:
-
Time to distant progression
- TLP:
-
Time to local progression
- V 0 :
-
Ablation volume measured pre-MWA
- V n :
-
Normalized ablation volume
- V t :
-
Ablation volume measured at time t post-MWA
References
Prud’homme C, Deschamps F, Moulin B et al (2019) Image-guided lung metastasis ablation: a literature review. Int J Hyperthermia 36:37–45
Jiang B, Mcclure MA, Chen T, Chen S (2018) Efficacy and safety of thermal ablation of lung malignancies: a network meta-analysis. Ann Thorac Med 13:243–250
Skinner MG, Iizuka MN, Kolios MC, Sherar MD (1998) A theoretical comparison of energy sources—microwave, ultrasound and laser—for interstitial thermal therapy. Phys Med Biol 43:3535–3547
Brace CL, Hinshaw JL, Laeseke PF, Sampson LA, Lee FT (2009) Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology 251:705–711
Aufranc V, Farouil G, Abdel-Rehim M et al (2019) Percutaneous thermal ablation of primary and secondary lung tumors: Comparison between microwave and radiofrequency ablation. Diagn Interv Imaging 100:781–791
Izzo F, Granata V, Grassi R et al (2019) Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist. 24(10):e990–e1005. https://doi.org/10.1634/theoncologist.2018-0337
Shakeri S, Raman SS (2019) Trends in percutaneous thermal ablation therapies in the treatment of T1a renal cell carcinomas rather than partial nephrectomy/radical nephrectomy. Semin Intervent Radiol 36:183–193
Xiong L, Dupuy DE (2016) Lung ablation: what’s new? J Thorac Imaging 31:228–237
Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT Jr, Brace CL (2014) Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why? Radiographics 34:1344–1362
Iezzi R, Larici AR, Franchi P et al (2017) Tailoring protocols for chest CT applications: when and how? Diagn Interv Radiol 23:420–427
Anderson EM, Lees WR, Gillams AR (2009) Early indicators of treatment success after percutaneous radiofrequency of pulmonary tumors. Cardiovasc Intervent Radiol 32:478–483
de Baère T (2011) Lung tumor radiofrequency ablation: where do we stand? Cardiovasc Intervent Radiol 34:241–251
Vogl TJ, Worst TS, Naguib NN, Ackermann H, Gruber-Rouh T, Nour-Eldin NE (2013) Factors influencing local tumor control in patients with neoplastic pulmonary nodules treated with microwave ablation: a risk-factor analysis. AJR Am J Roentgenol 200(3):665–667
Khalilzadeh O, Baerlocher MO, Shyn PB et al (2017) Proposal of a new adverse event classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol 28:1432–1437
Amabile C, Ahmed M, Solbiati L et al (2017) Microwave ablation of primary and secondary liver tumours: ex vivo, in vivo, and clinical characterisation. Int J Hyperthermia 33:34–42
Farina L, Nissenbaum Y, Cavagnaro M, Goldberg SN (2018) Tissue shrinkage in microwave thermal ablation: comparison of three commercial devices. Int J Hyperthermia 34:382–391
Amabile C, Farina L, Lopresto V et al (2017) Tissue shrinkage in microwave ablation of liver: an ex vivo predictive model. Int J Hyperthermia 33:101–109
Farina L, Weiss N, Nissenbaum Y et al (2014) Characterisation of tissue shrinkage during microwave thermal ablation. Int J Hyperthermia 30:419–428
Sommer CM, Sommer SA, Mokry T et al (2013) Quantification of tissue shrinkage and dehydration caused by microwave ablation: experimental study in kidneys for the estimation of effective coagulation volume. J Vasc Interv Radiol 24:1241–1248
Liu D, Brace CL (2019) Evaluation of tissue deformation during radiofrequency and microwave ablation procedures: influence of output energy delivery. Med Phys 46:4127–4134
Brace CL, Diaz TA, Hinshaw JL, Lee FT Jr (2010) Tissue contraction caused by radiofrequency and microwave ablation: a laboratory study in liver and lung. J Vasc Interv Radiol 21:1280–1286
Belfiore G, Ronza F, Belfiore MP et al (2013) Patients’ survival in lung malignancies treated by microwave ablation: our experience on 56 patients. Eur J Radiol 82:177–181
Ferguson CD, Luis CR, Steinke K (2017) Safety and efficacy of microwave ablation for medically inoperable colorectal pulmonary metastases: single-centre experience. J Med Imaging Radiat Oncol 61:243–249
Zheng A, Wang X, Yang X et al (2014) Major complications after lung microwave ablation: a single-center experience on 204 sessions. Ann Thorac Surg 98:243–248
Ierardi AM, Coppola A, Lucchina N, Carrafiello G (2017) Treatment of lung tumours with high-energy microwave ablation: a single-centre experience. Med Oncol 34:5
Wolf FJ, Grand DJ, Machan JT, DiPetrillo TA, Mayo-Smith WW, Dupuy DE (2008) Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 247:871–879
Healey TT, March BT, Baird G, Dupuy DE (2017) Microwave ablation for lung neoplasms: a retrospective analysis of long-term results. J Vasc Interv Radiol 28:206–211
Hiraki T, Gobara H, Iishi T et al (2007) Percutaneous radiofrequency ablation for pulmonary metastases from colorectal cancer: midterm results in 27 patients. J Vasc Interv Radiol 18:1264–1269
Yan TD, King J, Sjarif A et al (2007) Treatment failure after percutaneous radiofrequency ablation for nonsurgical candidates with pulmonary metastases from colorectal carcinoma. Ann Surg Oncol 14:1718–1726
Gillams AR, Lees WR (2008) Radiofrequency ablation of lung metastases: factors influencing success. Eur Radiol 18:672–677
Hiraki T, Sakurai J, Tsuda T et al (2006) Risk factors for local progression after percutaneous radiofrequency ablation of lung tumors: evaluation based on a preliminary review of 342 tumors. Cancer 107:2873–2880
Lu Q, Cao W, Huang L et al (2012) CT-guided percutaneous microwave ablation of pulmonary malignancies: results in 69 cases. World J Surg Oncol 10:80
Ierardi AM, Floridi C, Fontana F et al (2013) Microwave ablation of liver metastases to overcome the limitations of radiofrequency ablation. Radiol Med 118:949–961
Baisi A, De Simone M, Raveglia F, Cioffi U (2013) Thermal ablation in the treatment of lung cancer: present and future. Eur J Cardiothorac Surg 43:683–686
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Roberto Iezzi.
Conflict of interest
One author of this manuscript (NT) declares relationships with the following companies: Nevio Tosoratti is an employee of H.S. Hospital Service SpA, the company manufacturing the MWA apparatus used in the study.
The other authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• case-control study/experimental study
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iezzi, R., Cioni, R., Basile, D. et al. Standardizing percutaneous Microwave Ablation in the treatment of Lung Tumors: a prospective multicenter trial (MALT study). Eur Radiol 31, 2173–2182 (2021). https://doi.org/10.1007/s00330-020-07299-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07299-2