Abstract
Objectives
We aimed to assess the ability of CT-determined resectability, as defined by a recent version of NCCN criteria, and associated CT findings to predict margin-negative (R0) resection in patients with PDAC after neoadjuvant FOLFIRINOX chemotherapy.
Methods
Sixty-four patients (36 men and 28 women; mean age, 58.8 years) with borderline resectable or unresectable PDAC who received neoadjuvant FOLFIRINOX were evaluated retrospectively. CT findings were independently assessed by two abdominal radiologists according to NCCN criteria (version 3. 2019). Tumor resectability was classified as resectable, borderline resectable, or unresectable, and change in resectability was classified as regression, stability, or progression. The associations of R0 resection rate with CT-determined resectability and change in resectability categories were evaluated, as were the sensitivity and specificity of NCCN criteria for R0 resection. Factors associated with R0 resection were identified by logistic regression analysis.
Results
R0 resection rate did not differ significantly among the resectable, borderline resectable, or unresectable PDAC (67–73%, p = 0.95) or among PDAC with regression, stability, or progression (56–77%, p = 0.39). The sensitivity and specificity for R0 resection were 67% and 37%, respectively, for resectability (resectable/borderline vs. unresectable) and 80% and 21%, respectively, for changes in resectability (regression/stable vs. progression). Low-contrast enhancement of soft tissue contacting artery (≤ 46.4 HU) was independently associated with R0 resection (p = 0.01).
Conclusion
CT-determined resectability after neoadjuvant FOLFIRINOX chemotherapy was relatively insensitive and non-specific for predicting R0 resection. Low-contrast enhancement of soft tissue contacting artery may increase the ability of CT to predict R0 resection.
Key Points
• Margin-negative resection rate of pancreatic cancer following FOLFIRINOX therapy did not differ among each resectability (67–73%, p = 0.95) based on NCCN criteria or changes in resectability categories (56–77%, p = 0.39).
• The sensitivity and specificity for margin-negative resection were 67% and 37% for resectability (resectable/borderline vs. unresectable) and 80% and 21% for changes in resectability (regression/stable vs. progression).
• Low-contrast enhancement of soft tissue contacting artery (≤ 46.4 HU) was independently associated with margin-negative resection (p = 0.01).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a lethal malignant neoplasm with a 5-year survival rate of 8% [1]. Although surgical resection remains the sole curative modality, only about 15% of patients present with resectable tumors, with the remaining 85% presenting with locally advanced or metastatic disease [2]. Patients with borderline resectable PDAC may be candidates for surgery, but resection is frequently margin-positive, resulting in a higher risk of tumor recurrence [3, 4]. Selected patients with borderline resectable or unresectable PDAC are therefore administered neoadjuvant chemotherapy to achieve margin-negative (R0) resection, with the FOLFIRINOX (5-fluorouracil/leucovorin/irinotecan/oxaliplatin) regimen exhibiting a higher R0 resection rate and longer overall survival than other neoadjuvant chemotherapy regimens [4,5,6].
Accurate determination of resectability is crucial for patient management. Imaging modalities, including computed tomography (CT) and magnetic resonance imaging (MRI), have been used to assess tumor resectability [7, 8]. The criteria formulated by the National Comprehensive Cancer Network (NCCN) are arguably the most prominent, with resectability classified as resectable, borderline resectable, or unresectable, based on vascular involvement. However, predicting resectability after neoadjuvant chemotherapy is challenging because of regional changes induced by chemotherapy [9,10,11], making predictions less accurate after than before chemotherapy [12].
Although several studies have evaluated the diagnostic accuracy of CT-determined resectability after neoadjuvant chemotherapy based on previous versions of the NCCN criteria [13,14,15,16,17], none to our knowledge has evaluated the diagnostic accuracy and clinical relevance of CT-determined resectability following neoadjuvant FOLFIRINOX therapy using the recently revised version (version 3. 2019). Therefore, the aim of this study was to evaluate the diagnostic performance of CT-determined resectability after neoadjuvant FOLFIRINOX chemotherapy in patients with borderline resectable and unresectable PDAC based on the recent version of the NCCN criteria. This study also evaluated CT features associated with R0 resection and assessed clinical relevance of CT-determined resectability in terms of oncological outcome.
Materials and methods
The study protocol was approved by the institutional review board of our institution, which waived the requirement for informed patient consent because of the retrospective nature of this study.
Patients
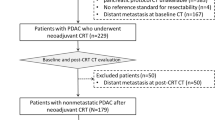
This study included 204 consecutive patients with a newly diagnosed PDAC who received neoadjuvant FOLFIRINOX chemotherapy from January 2013 to January 2017. Patients were considered eligible if they had undergone both pre- and post-chemotherapy multiphase CT with a pancreas protocol; had borderline resectable or unresectable PDAC, as determined by CT before chemotherapy according to the NCCN criteria; had available pathological results of resection margin status after surgery; and did not have distant metastases or any other suspicious lesions. Of the 204 patients, after excluding 128 patients who did not underwent surgery (chemotherapy as next treatment for 42, radiation treatment for 46, photodynamic therapy for 2, and no identified next treatment on medical record for 38) and 10 patients who underwent non-curative surgery, 64 patients finally met the inclusion criteria, including 36 men and 28 women (mean age ± standard deviation [SD], 58.84 ± 8.27 years) (Fig. 1).
CT technique
All included patients had undergone multiphase CT examinations with a pancreas protocol, consisting of unenhanced, arterial, and portal venous phases, before and after neoadjuvant chemotherapy using a 65-channel multi-detector row CT scanner (Discovery CT 750 HD; GE Healthcare, or Somatom Definition AS+ or Definition Edge; Siemens Healthineers). Axial unenhanced images of thickness 5.0 mm and axial and coronal, arterial and portal venous phase images of thickness 2.5–3.0 mm were reconstructed for image analysis. Detailed CT parameters are provided in the Supplementary Material.
Image analysis
All CT findings on both pre- and post-chemotherapy multiphase CT were independently evaluated by two board-certified abdominal radiologists (each with > 7-year experience in abdominal radiology), blinded to all information except that patients had undergone chemotherapy for PDAC. Findings evaluated were based on NCCN criteria (version 3. 2019; Supplementary Table 1) [18] for resectability and included (1) extent of soft tissue contacting arteries and veins (degree of vessel circumference, 0–360°); (2) depth of soft tissue invading arteries and veins, whether superficial (contact alone) or deep (focal luminal narrowing or contour irregularity of the vessel, partial thrombosis, or occlusion); (3) contrast enhancement (CE) of the tumor and of soft tissue surrounding arteries and veins, as determined by difference in Hounsfield units (HU) between portal venous phase images and unenhanced images; and (4) tumor size, as determined by its longest axis on axial or coronal images with the most conspicuous tumor. CE was evaluated by drawing regions of interest (ROI) on unenhanced and portal venous phase images, while avoiding areas of tumor necrosis. If multiple arteries or veins were involved, ROI was drawn at soft tissue with the largest extent contacting with artery or vein, respectively. Tumor size was measured in the same plane before and after FOLFIRINOX chemotherapy.
For CT-determined resectability according to the NCCN criteria [18], the extent of soft tissue contacting vessels was categorized as either abutment (≤ 180°) or encasement (> 180°). Any discrepancies regarding extent and depth of soft tissue contacting vessels between the two investigators were resolved on consensus after consultation with a third investigator (with 23-year experience in abdominal radiology). And then, CT-determined resectability was classified as resectable, borderline resectable, or unresectable on both pre- and post-chemotherapy CT [18]. After determining resectability, changes in CT-determined resectability from before to after chemotherapy were classified as regression, stable, or progression. Regression was defined as a change of an unresectable tumor to a borderline resectable or resectable tumor, or the change of a borderline resectable tumor to a resectable tumor. Progression was defined as the change of a borderline resectable tumor to an unresectable tumor. All other changes were regarded as stable. For degree of CE and tumor size, the mean values of measurements by the two investigators on both pre- and post-chemotherapy CT were used for analysis.
Clinical, surgical, and pathological analysis and follow-up
All treatment decisions were made by a multidisciplinary team, consisting of oncologists, surgeons, pathologists, and radiologists, based on the general condition and comorbidity of each patient and the results of imaging analysis. Patients were administered FOLFIRINOX every 2 weeks [19]. Multiphase CT was performed to evaluate tumor response, resectability, and presence of metastasis 2–4 weeks after completion of chemotherapy.
Surgical resection was performed 1–37 days (median, 3 days) after post-chemotherapy CT by one of four surgeons in our institution, each of whom had ≥ 8 years of experience in pancreatic surgery and performed approximately 50–150 operations for pancreatic cancer annually. Margin status was evaluated on serial sections of each surgical specimen. Negative resection margin was defined as the absence of tumor cells at the resection margin [20].
Patients were followed-up every 3–6 months after surgery by blood tests including serum carbohydrate antigen (CA) 19-9 concentrations, and imaging modalities, such as CT and/or MRI. Whenever possible, recurrent and metastatic tumors were confirmed by biopsy; if pathological results were not available, these lesions were confirmed by sequential enlargement of a lesion on imaging modalities and hypermetabolism on PET/CT scans.
Statistical analysis
Clinical and pathological characteristics of patients and tumors before chemotherapy between groups of patients with borderline resectable and unresectable PDAC and CT findings before and after chemotherapy were compared using t test or Wilcoxon signed rank test for continuous variables and Fisher’s exact test or McNemar test for categorical variables. The correlations of R0 resection rate with CT resectability category and change in CT resectability category were determined using linear-by-linear association. The sensitivity and specificity of CT-determined resectability and of its change after chemotherapy for predicting R0 resection were evaluated. Inter-observer agreement for CT findings was evaluated using intraclass correlation coefficient (ICC, two-way random effects model; absolute agreement) [21].
CT and laboratory findings associated with R0 resection, in all patients and in patients with borderline resectable and unresectable PDAC after chemotherapy, were evaluated by univariate logistic regression analysis followed by multiple logistic regression analysis using backward elimination. Continuous variables were categorized each into two groups, with degree of CE of soft tissue surrounding arteries and veins categorized as low or high according to the cutoff value determined by Youden’s index [22]; tumor size classified as ≤ 2 cm or > 2 cm based on TNM stage [23]; and CA 19-9 concentration classified as ≤ 200 U/mL or > 200 U/mL [24].
Recurrence-free survival (RFS) and cancer-specific survival (CSS) were defined as the time from the date of surgery to the date of disease recurrence or death from any cause and to the date of disease progression or primary cancer–related death, respectively. According to the CT-determined resectability category and change in CT-determined resectability category, RFS and CSS curves in groups of patients were assessed using the Kaplan-Meier method and compared using the log-rank test. All statistical analyses were performed using a statistical software package (MedCalc version 16.8, MedCalc Software), with a 2-sided p value < 0.05 considered statistically significant.
Results
Patients and tumor characteristics
Table 1 shows the baseline characteristics of patients and tumors before chemotherapy. A higher percentage of patients with borderline resectable PDAC (68.9%, 31/45) than with unresectable PDAC (42.1%, 8/19) underwent extended surgery (p = 0.04). Rate of R0 resection in patients with borderline resectable PDAC was higher than that of unresectable PDAC (77.8% vs. 52.6%, p = 0.04).
Changes in CT findings and CT-determined resectability after chemotherapy
Following chemotherapy, the extent of soft tissue contacting arteries and veins decreased significantly (p < 0.01 each; Table 2). The percentage of tumors showing deep venous invasion by soft tissue was significantly lower after (41.7%) than before (68.1%) chemotherapy (p < 0.01). The magnitude of CE of soft tissue surrounding arteries (mean change, − 6.4 ± 14.3 HU) and veins (mean change, − 9.5 ± 13.6 HU) was also significantly lower after than before chemotherapy (p < 0.01). Median tumor diameter and level of CA 19-9 also decreased significantly (p < 0.01).
After chemotherapy, nine, 33, and 22 patients had resectable, borderline resectable, and unresectable PDAC, respectively. Sixteen patients were classified as experiencing tumor regression, including seven who changed from unresectable tumors to borderline resectable tumors, three who changed from unresectable to resectable tumors, and six who changed from borderline resectable to resectable tumors. In addition, 35 patients had stable tumors, including 26 with borderline resectable and nine with unresectable tumors, and 13 experienced tumor progression (Fig. 2).
A 61-year-old man with pancreatic head cancer who underwent R0 resection. (a, b) Axial and coronal pre-chemotherapy CT images show a hypoattenuating mass in the pancreatic head (arrowheads) and soft tissue (arrows) contacting the superior mesenteric artery (*) as much as 270°, which was classified as unresectable cancer according to NCCN criteria. (c, d) Axial and coronal post-chemotherapy CT images show the soft tissue (arrows) contacting the superior mesenteric artery (*) more than 180° despite decreased volume of the soft tissue. It was classified as unresectable and stable cancer. However, the degree of contrast enhancement of the soft tissue contacting the superior mesenteric artery was low (33.4 HU)
ICCs for each CT finding before (0.75–0.96) and after (0.64–0.95) chemotherapy showed that inter-observer agreement ranged from moderate to excellent (Supplementary Table 2).
Accuracy of CT-determined resectability after chemotherapy
R0 resection after chemotherapy was achieved in 67% (6/9), 73% (24/33), and 68% (15/22) of patients with resectable, borderline resectable, and unresectable PDAC, respectively (p = 0.95). CT-determined resectability according to NCCN criteria (resectable and borderline resectable vs. unresectable) had a sensitivity of 66.7% (95% confidence interval [CI], 51.1–80.0%), a specificity of 36.8% (95% CI, 16.3–61.6%), and a positive predictive value of 71.4% (95% CI, 62.6–78.9%), for predicting R0 resection.
Of the patients in the regression, stable, and progression groups, 56% (9/16), 77% (27/35), and 69% (9/13), respectively, underwent R0 resection (p = 0.39). Change in CT-determined resectability according to NCCN criteria (regression and stable vs. progression) had a sensitivity of 80.0% (95% CI, 65.4–90.4%), a specificity of 21.1% (6.1–45.6%), and a positive predictive value of 70.6% (95% CI, 64.6–76.0%) for predicting R0 resection (Supplementary Table 3).
Factors associated with R0 resection after chemotherapy
Univariate analysis showed that, for all patients with borderline resectable and unresectable PDAC before chemotherapy, CE of soft tissue contacting arteries and veins was significantly associated with R0 resection after neoadjuvant FOLFIRINOX chemotherapy (Table 3). By contrast, CT-determined resectability and change in CT-determined resectability were not significantly associated with R0 resection. The optimal cutoff values based on Youden’s index for CE of soft tissue contacting arteries and veins after chemotherapy were 46.4 HU (sensitivity, 70%; specificity, 65%) and 42.5 HU (sensitivity, 61%; specificity, 86%), respectively. Multivariate analysis showed low CE of soft tissue contacting arteries (≤ 46.4 HU) was independently associated with R0 resection (adjusted odds ratio = 7.4; p = 0.01) (Fig. 2).
Subgroup analysis of patients with borderline resectable and unresectable PDAC after chemotherapy showed that low CE of soft tissue contacting arteries was the only factor significantly associated with R0 resection (adjusted odds ratio = 9.1; p = 0.01). Of patients with borderline resectable PDAC after chemotherapy, 84% (16/19) with low CE and 40% (4/10) with high CE of soft tissue contacting arteries underwent R0 resection (p = 0.03). Of patients with unresectable PDAC after chemotherapy, 73% (8/11) with low CE and 60% (6/10) with high CE of soft tissue contacting arteries underwent R0 resection (p = 0.66).
Recurrence-free and cancer-specific survival
RFS and CSS were assessed postoperatively for 2–56 months until disease recurrence, death, or last evaluation (median, 9 months). Of the 64 patients, 44 (68.8%) experienced tumor recurrences and eight (12.5%) died. RFS was significantly longer in patients with resectable than with unresectable PDAC after chemotherapy (p = 0.01) (Fig. 3). RFS was also significantly longer in patients who did not than in those who did experience tumor progression after neoadjuvant chemotherapy (p = 0.01). CSS was also significantly higher in patients without progression than in those with progression (p < 0.01) (Supplementary Fig. 1).
Recurrence-free survival (RFS) curves of all study patients according to (a) CT-determined resectability category after chemotherapy and (b) change in CT-determined resectability category from before to after chemotherapy. RFS was compared between each pair of two CT-determined resectability categories (i.e., resectable vs. borderline resectable, borderline resectable vs. unresectable, and resectable vs. unresectable) and between non-progression group (regression and stable) and progression group using log-rank tests
Discussion
This study of patients with PDAC who received FOLFIRINOX neoadjuvant chemotherapy found that R0 resection rates did not differ significantly in groups of patients with resectable, borderline resectable, and unresectable tumors, as defined by NCCN criteria (version 3. 2019). R0 resection rates also did not differ in patients who experienced tumor regression, stability, and progression following FOLFIRINOX treatment. Although patients with resectable and borderline resectable PDAC were considered optimal candidates for R0 resection, the NCCN criteria were relatively insensitive and non-specific in predicting R0 resection in these patients. Among various CT findings, low CE of soft tissue contacting arteries was independently associated with R0 resection. RFS was significantly longer in patients who did not experience tumor progression than in those who did.
The extent of soft tissue in contact with arteries and veins was significantly reduced after neoadjuvant chemotherapy, resulting in CT-determined tumor regression in 16 (25%) patients and resectable PDAC in nine (14%). Compared with the previously reported 46% rate of R0 resection following first-line surgery in patients with borderline resectable and unresectable PDAC [25], R0 resection was achieved by 45 (70%) patients in our study after chemotherapy. These quantitative and qualitative changes in CT parameters and improvements in R0 resection rate indicate that neoadjuvant FOLFIRINOX therapy is effective in patients with borderline resectable and unresectable PDAC. These results are consistent with those of recent meta-analyses of patients with borderline resectable and unresectable PDACs, which showed R0 resection rates of all resected PDACs after neoadjuvant FOLFIRINOX ranging from 78 to 93% [5, 6].
R0 resection rates after neoadjuvant chemotherapy did not differ significantly among groups of patients with the three resectability categories (resectable, borderline resectable, and unresectable), with rates ranging from 67 to 73%. If borderline resectable patients are considered resectable, then CT-determined resectability after chemotherapy was less sensitive (67%) and non-specific (37%) in predicting R0 resection. These results are in accordance with those of previous studies using older versions of the NCCN criteria [13,14,15], which reported that R0 resection rates did not differ significantly among resectability categories and a sensitivity of 80–90% and a specificity of 13–46% after chemotherapy. The limited accuracy of CT-determined resectability after chemotherapy may be mainly due to difficulties differentiating between benign treatment-related changes, such as pancreatitis and fibrosis, and residual tumor infiltration [11, 26]. This may lead to overestimates of vascular involvement of tumors when assessing resectability after chemotherapy. Our finding that sensitivity was lower in the present study than in previous reports may be due to several factors. First, we used a different version of the NCCN criteria. The recent version used in this study also classify tumors contacting the first jejunal branch of the superior mesenteric vein as unresectable [7], whereas the older versions used in previous studies only classified tumors contacting the superior mesenteric vein as unresectable PDACs [27]. Differences in the accuracy of CT-determined resectability may also have been due to differences in time intervals between post-chemotherapy CT and completion of neoadjuvant therapy or surgery and to differences in chemotherapy regimens or total numbers of chemotherapy cycles.
We also found that R0 resection rates were similar (56–77%) in groups of patients with tumor regression, stability, and progression following neoadjuvant chemotherapy. Change in CT-determined resectability (i.e., regression and stable vs. progression) was relatively sensitive (80%) but not specific (21%) for diagnosing R0 resection. Similarly, identical R0 resection rates were observed in groups of patients with tumor regression (85.7%) and stability (85.2%) following chemotherapy, with no patient experiencing tumor progression [14]. Another study of resectability (non-progression vs. progression), as assessed by change in tumor-vascular circumferential contact showed a similar sensitivity (78%), but a higher specificity (67%) [26]. That study, however, used its own modified NCCN criteria and included only patients with initially borderline resectable PDAC.
The degree of CE of soft tissue contacting vessels after neoadjuvant chemotherapy may help in determining resectability on CT. The odds ratio of R0 resection was significantly higher (7.4–9.1) in patients with low (≤ 46.4 HU) than high (> 46.4 HU) CE of soft tissue contacting arteries after chemotherapy. The R0 resection rate in patients with borderline resectable PDAC after chemotherapy was also significantly higher in those with low than high CE of periarterial soft tissue (84% vs. 40%, p = 0.03). In addition to classifying patients by CT-determined resectability, this finding may enable a further stratification of patients who would be candidates for R0 resection after neoadjuvant chemotherapy. Because CE tends to be low in soft tissue lesions with high proportions of post-treatment change, such as fibrosis or edema, to residual tumor [28], CE of soft tissue contacting arteries may be helpful in differentiating treatment-related changes from residual tumor infiltration, especially for patients with borderline resectable PDAC after chemotherapy. However, considering wide standard deviation of change in CE degree (− 6.4 ± 14.3 for artery and − 9.5 ± 13.6 for vein), wide range of CI of hazard ratio for CE (1.5–37.0 for all patients), and moderate inter-observer agreement for CE degree for veins (0.75 and 0.64) in our study, additional studies in larger populations are needed to verify the clinical value of CE degree and to determine optimal CE cutoff of soft tissue contacting vessels predictive of R0 resection.
CT-determined resectability using the NCCN criteria may also be relevant to oncological outcomes after neoadjuvant chemotherapy, despite similar R0 resection rates. Reduced vascular involvement by tumor and a resectable PDAC on post-chemotherapy CT have been associated with favorable oncological outcomes following surgery [14, 29, 30]. In the present study, RFS and CSS were significantly better in patients who did not show progression after chemotherapy than in those who did (p ≤ 0.01). Larger studies are needed to assess whether CT-determined resectability category and change in this category are independently prognostic in patients with PDAC.
This study had several limitations. First, the number of included patients was relatively small because this study only included patients treated with neoadjuvant FOLFIRINOX chemotherapy. This avoided the possible confounding effects of other neoadjuvant chemotherapy regimens or radiation. Second, this study only included patients who underwent radical surgery, which may have resulted in an overestimate of R0 resection rate. Considering a subset of 138 patients did not undergo radical surgery and were not analyzed in this study, our results might have limited value in clinical practice. However, this was required to determine resection margin status. Third, this study was retrospective in design, which may have resulted in selection biases with respect to clinical and radiological findings. In cases of margin-positive resection, pathological correlations with significant radiological findings could not be performed due to insufficient detail data on pathological margin. In addition, there might be limitation in direct head-to-head comparison of HU before and after chemotherapy because CT parameters were not standardized. Fourth, new NCCN guideline (version 1. 2020) and R0 definition with wider margin (> 1 mm of negative tumor margin) have been recently introduced. Future study to investigate diagnostic accuracy of CT-determined resectability with these new version and reference standard is required.
In conclusion, CT-determined resectability according to the recent version of the NCCN criteria was relatively insensitive and non-specific for predicting R0 resection in patients with PDAC after FOLFIRINOX therapy. The addition of low CE of soft tissue contacting arteries to CT-determined resectability may be helpful in predicting R0 resection. Regression and stability in CT-determined resectability after chemotherapy may be associated with improved RFS after surgery.
Abbreviations
- CA:
-
Carbohydrate antigen
- FOLFIRINOX:
-
Fluorouracil, leucovorin, irinotecan, and oxaliplatin
- HU:
-
Hounsfield unit
- ICC:
-
Intraclass correlation coefficient
- NCCN:
-
The National Comprehensive Cancer Network
- PDAC:
-
Pancreatic ductal adenocarcinoma
- R0 resection:
-
Margin-negative resection
- RFS:
-
Recurrence-free survival
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30
Hidalgo M (2010) Pancreatic cancer. N Engl J Med 362:1605–1617
Ryan DP, Hong TS, Bardeesy N (2014) Pancreatic adenocarcinoma. N Engl J Med 371:1039–1049
Versteijne E, Vogel JA, Besselink MG et al (2018) Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg 105:946–958
Petrelli F, Coinu A, Borgonovo K et al (2015) FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas 44:515–521
Suker M, Beumer BR, Sadot E et al (2016) FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 17:801–810
Tempero MA, Malafa MP, Al-Hawary M et al (2017) Pancreatic Adenocarcinoma, Version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 15:1028–1061
Papavasiliou P, Chun YS, Hoffman JP (2013) How to define and manage borderline resectable pancreatic cancer. Surg Clin North Am 93:663–674
Morgan DE, Waggoner CN, Canon CL et al (2010) Resectability of pancreatic adenocarcinoma in patients with locally advanced disease downstaged by preoperative therapy: a challenge for MDCT. AJR Am J Roentgenol 194:615–622
Kim YE, Park MS, Hong HS et al (2009) Effects of neoadjuvant combined chemotherapy and radiation therapy on the CT evaluation of resectability and staging in patients with pancreatic head cancer. Radiology 250:758–765
Cassinotto C, Mouries A, Lafourcade JP et al (2014) Locally advanced pancreatic adenocarcinoma: reassessment of response with CT after neoadjuvant chemotherapy and radiation therapy. Radiology 273:108–116
Cassinotto C, Cortade J, Belleannee G et al (2013) An evaluation of the accuracy of CT when determining resectability of pancreatic head adenocarcinoma after neoadjuvant treatment. Eur J Radiol 82:589–593
Marchegiani G, Todaro V, Boninsegna E et al (2018) Surgery after FOLFIRINOX treatment for locally advanced and borderline resectable pancreatic cancer: increase in tumour attenuation on CT correlates with R0 resection. Eur Radiol 28:4265–4273
Wagner M, Antunes C, Pietrasz D et al (2017) CT evaluation after neoadjuvant FOLFIRINOX chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur Radiol 27:3104–3116
Kim BR, Kim JH, Ahn SJ et al (2019) CT prediction of resectability and prognosis in patients with pancreatic ductal adenocarcinoma after neoadjuvant treatment using image findings and texture analysis. Eur Radiol 29:362–372
Somers I, Bipat S (2017) Contrast-enhanced CT in determining resectability in patients with pancreatic carcinoma: a meta-analysis of the positive predictive values of CT. Eur Radiol 27:3408–3435
Garces-Descovich A, Beker K, Jaramillo-Cardoso A, James Moser A et al (2018) Applicability of current NCCN guidelines for pancreatic adenocarcinoma resectability: analysis and pitfalls. Abdom Radiol (NY) 43:314–322
National Comprehensive Cancer Network Pancreatic Adenocarcinoma (Version 3. 2019). Available via https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed 2 July 2019
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Habibzadeh F, Habibzadeh P, Yadollahie M (2016) On determining the most appropriate test cut-off value: the case of tests with continuous results. Biochem Med (Zagreb) 26:297–307
Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK (2017) AJCC Cancer Staging Manual, 8th edn. Springer, New York
Ferrone CR, Finkelstein DM, Thayer SP et al (2006) Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol 24:2897–2902
Hong SB, Lee SS, Kim JH et al (2018) Pancreatic Cancer CT: prediction of resectability according to NCCN criteria. Radiology 289:710–718
Joo I, Lee JM, Lee ES et al (2018) Preoperative MDCT assessment of resectability in borderline resectable pancreatic cancer: effect of neoadjuvant chemoradiation therapy. AJR Am J Roentgenol 210:1059–1065
Tempero MA, Arnoletti JP, Behrman SW et al (2012) Pancreatic adenocarcinoma, version 2.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 10:703–713
Hattori Y, Gabata T, Matsui O et al (2009) Enhancement patterns of pancreatic adenocarcinoma on conventional dynamic multi-detector row CT: correlation with angiogenesis and fibrosis. World J Gastroenterol 15:3114–3121
Tran Cao HS, Balachandran A, Wang H et al (2014) Radiographic tumor-vein interface as a predictor of intraoperative, pathologic, and oncologic outcomes in resectable and borderline resectable pancreatic cancer. J Gastrointest Surg 18:269–278 discussion 278
Hayasaki A, Isaji S, Kishiwada M et al (2018) Survival analysis in patients with pancreatic ductal adenocarcinoma undergoing chemoradiotherapy followed by surgery according to the international consensus on the 2017 definition of borderline resectable cancer. Cancers (Basel) 10:65
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jae Ho Byun.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Jong Keon Jang) has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 331 kb)
Rights and permissions
About this article
Cite this article
Jang, J.K., Byun, J.H., Kang, J.H. et al. CT-determined resectability of borderline resectable and unresectable pancreatic adenocarcinoma following FOLFIRINOX therapy. Eur Radiol 31, 813–823 (2021). https://doi.org/10.1007/s00330-020-07188-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07188-8