Abstract
Objectives
Although ultrasound (US) is a standard modality for the assessment of cervical lymph node metastasis in patients with thyroid cancer, there is an increasing trend in the number of articles describing the use of contrast-enhanced computed tomography (CT). The purpose of this systematic review and meta-analysis was to evaluate the diagnostic performance of CT in the diagnosis of metastatic cervical lymph nodes and to identify the parameters responsible for heterogeneity in diagnostic performance.
Methods
Ovid-MEDLINE and EMBASE databases were searched up to May 22, 2018, for studies on the diagnostic performance of CT. The pooled sensitivity and specificity of all studies were calculated. In addition, subgroup analysis and meta-regression analysis were performed to evaluate factors responsible for heterogeneity.
Results
Seventeen (6378 patients, 11,590 lymph nodes) studies were included. The pooled sensitivity was 55% (95% CI, 47–63%), and the pooled specificity was 87% (95% CI, 90–95%). Higgins I2 statistic demonstrated substantial heterogeneity in the sensitivity (I2 = 96.3%) and specificity (I2 = 93.8%). In a per-neck level subgroup analysis, the Higgins I2 statistic demonstrated reduced heterogeneity in both sensitivity and specificity. In the meta-regression analysis, variation in the CT protocols, such as contrast amount, scan phase, and reconstruction slice thickness, was a statistically significant factor causing heterogeneity.
Conclusions
CT demonstrated acceptable diagnostic performance in the pre- and postoperative diagnosis of metastatic cervical lymph nodes in patients with thyroid cancer. Variation in the CT protocols was a main factor causing heterogeneity among the included studies.
Key Points
• The role of contrast-enhanced computed tomography (CT) needs to be reassessed.
• CT demonstrated acceptable diagnostic performance in the diagnosis of metastatic cervical lymph nodes in patients with thyroid cancer in the meta-analysis.
• Variation in the CT protocols was a main factor causing heterogeneity in the meta-regression analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Preoperatively, lymph node metastases in thyroid cancer are highly related with local recurrence and cancer-specific mortality [1, 2]. Otherwise, the rate of recurrence or persistent disease is reported to be up to 30% in post-thyroidectomy patients [3, 4], with the most common type being nodal metastasis [4, 5]. Therefore, detection of metastatic lymph nodes before and after thyroidectomy is important.
Ultrasound (US) is considered the modality of choice for the assessment of lymph node metastasis in thyroid cancer patients [6,7,8,9]. However, US is an operator-dependent and it may be difficult to evaluate retropharyngeal, retrosternal, and mediastinum [6, 10, 11]. In such cases, contrast-enhanced computed tomography (CT) has advantages over US [10, 12,13,14]. In addition, CT can deliver detailed anatomic information for surgeons, especially concerning nodal locations and relationships to anatomical landmarks [10]. There is an increasing trend in the number of articles describing the diagnostic performance of CT for cervical lymph node metastasis, especially against the background of “active surveillance” suggested by the new American Thyroid Association guidelines [6, 15, 16]. Several reports suggest that delaying radioactive iodine therapy because of the iodine content of CT contrast agent is not necessary, because the iodine clears up within 4–8 weeks, and the iodine content of the body is not an essential determinant for radioactive iodine therapy [17,18,19,20,21]. Therefore, recent guidelines suggest CT for the detection of metastatic lymph nodes in patients with thyroid cancer [9, 22]. Considering that, we consider that analysis of the diagnostic performance of thyroid CT is a timely and clinically important issue.
There is meta-analysis on the diagnostic performance of CT in the preoperative diagnosis of cervical lymph node metastasis [23]. However, this article includes only a small sample size, and it only evaluated preoperative diagnostic performance; it did not analyze the effects of different CT parameters and focused on a comparison of the diagnostic performances of US and CT.
Therefore, the purposes of the present study were to evaluate the diagnostic performance of CT in pre- and postoperative metastatic cervical lymph nodes in patients with thyroid cancer and to demonstrate the parameters influencing diagnostic performance.
Materials and methods
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [24]. Literature search and quality assessment are described in supplementary file.
Inclusion criteria
Studies that satisfied the following criteria were included: (1) involved patients with known thyroid cancer; (2) performed CT as the index test examination, regardless of the patients’ preoperative or postoperative state; (3) included reference standards based on histopathology tests; and (4) contained adequate information to reconstruct 2 × 2 tables to estimate the diagnostic performance of CT for the detection of metastatic cervical lymph nodes.
Exclusion criteria
The exclusion criteria were as follows: (1) case reports or case series including fewer than 10 patients; (2) letters, editorials, conference abstracts, and review articles; (3) articles focusing on a topic other than the diagnostic performance of CT for the detection of metastatic cervical lymph nodes in thyroid cancer; (4) articles with, or with suspicion of, overlapping populations: if articles had an overlapping population, the article with the largest population was included; (5) insufficient data for the reconstruction of 2 × 2 tables; and (6) articles without reference standards based on histopathology tests.
To assess the eligibility of the studies, the literature search and selection was independently performed by two radiologists (S.J.C. and C.H.S., both 1 year of experience in neuroradiology and thyroid radiology).
Data extraction
Data were extracted from the included studies using a standardized form, and included the following: (1) study characteristics: authors, year of publication, institution, country of origin, duration of patient recruitment, study design (prospective vs. retrospective, multicenter vs. single center, consecutive or not), time interval between index test (CT), and reference standard (the postoperative histological data in all 17 articles [10, 13, 14, 25,26,27,28,29,30,31,32,33,34,35,36,37,38] and 13 articles with additional data of fine needle aspiration regarded as standard references [10, 13, 14, 25, 26, 28,29,30,31,32,33,34,35,36]), blinding of radiologist to reference standard, characteristics of readers (number and experience), blinding of pathologist to image findings; (2) clinical and patient characteristics: number of patients, male to female ratio, mean patient age, number of lymph nodes, number of metastatic lymph nodes, the level of the metastatic lymph node (central (level VI) vs. lateral (levels II, III, IV, and V)), clinical setting (preoperative vs. postoperative state), final diagnosis (the type of thyroid cancer); (3) technical characteristics of CT and contrast enhancement: CT machine and vendor, detector number, kVp, mAs, reconstruction slice thickness, contrast model, quantity of injected contrast, scan delay time after contrast injection, flushing with saline or not, flushing saline flow rate; (4) the definition of CT image findings of a metastatic cervical lymph node; and (5) the diagnostic performance of CT.
Data synthesis and analyses
The primary outcome for the systematic review and meta-analysis was the diagnostic performance of pre- and postoperative CT for the detection of metastatic cervical lymph nodes in patients with thyroid cancer. A secondary aim was to identify parameters responsible for heterogeneity among the included studies by performing subgroup analyses according to specific clinical settings. Heterogeneity was determined using the Higgins I2 statistic [39]. The presence of a threshold effect caused by the heterogeneity was evaluated by visual assessment of coupled forest plots of sensitivity and specificity. In addition, the presence of a threshold effect (a positive correlation between sensitivity and false-positive rate) among the articles was evaluated, with a Spearman correlation coefficient of greater than 0.6 between the sensitivity and false-positive rates being considered to indicate a threshold effect [40].
The summary sensitivity and specificity, and their 95% CIs, were calculated using bivariate random-effects modeling [39, 41,42,43,44]. Additionally, the results are graphically presented by plotting hierarchic summary receiver operating characteristic (HSROC) curves with 95% confidence and prediction regions. Deeks’ funnel plot was used to assess publication bias, and Deeks’ asymmetry test was used to calculate the p value and determine statistical significance [45].
Subgroup analyses were performed to assess the diagnostic performance in specific clinical settings as follows: (1) preoperative CT analysis, (2) lateral neck lymph node analysis, and (3) central neck lymph node analysis. Meta-regression was performed using several covariates to identify the sources of heterogeneity among the studies as follows: (1) malignant lymph node rate (≥ median value vs. < median value), (2) study design (retrospective vs. prospective), (3) quantity of contrast injected (≥ 100 ml vs. < 100 ml), (4) scan delay time after contrast injection (arterial ≤ 45 s vs. venous > 60 s), (5) reconstruction slice thickness (≤ 3 mm vs. > 3 mm), and (6) blinding of the radiologist to the reference standard (yes or no).
The MIDAS and METANDI modules in STATA 15.0 (StataCorp) and the Mada package in R version 3.2.3 (The R Foundation for Statistical Computing) were used for the statistical analyses, which were performed by one of the authors (C.H.S., with 5 years of experience performing systematic reviews and meta-analyses).
Results
Literature search
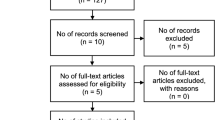
The detailed article selection process is described in Fig. 1. An initial systematic search identified 757 articles. After removing 215 duplicates, screening of the remaining 542 titles and abstracts resulted in the exclusion of a further 506 articles. No additional articles were identified in the searches of the bibliographies of the relevant articles. Full-text reviews of 36 provisionally eligible articles were performed, and 19 articles were excluded because of the following reasons: 15 articles were not in the field of interest [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60], three articles included patient cohorts partially overlapping with other studies [61,62,63], and one article contained 10 or fewer enrolled patients [64]. Finally, 17 studies were included in this meta-analysis [10, 13, 14, 25,26,27,28,29,30,31,32,33,34,35,36,37,38].
Characteristics of the included studies
The detailed patient characteristics are listed in Table 1. The total study population was 6378, with individual studies ranging from 20 to 3668 patients. The studies had mean patient ages ranging from 34.1 to 65.6 years. The number of lymph nodes ranged from 85 to 6557, with a total sum of 11,590. In 15 articles, the population consisted of patients with proven papillary thyroid carcinoma (PTC) [10, 14, 25,26,27,28,29,30,31,32,33,34,35], while in the remaining two articles, the study population consisted of patients with both PTC and medullary thyroid carcinoma [37, 38]. Four of the included studies were prospective in design [10, 33,34,35], while the other 13 studies were retrospective [13, 14, 25,26,27,28,29,30,31,32, 36,37,38]. Fourteen of the included articles evaluated the preoperative diagnostic performance of CT [10, 14, 25,26,27,28, 30,31,32,33,34,35,36, 38], two articles evaluated it postoperatively [29, 37], and one article evaluated it both pre- and postoperatively [13].
The detailed CT characteristics are summarized in Table 2. The CT vendors and scanners were heterogeneous, with four studies having used only Siemens [10, 13, 25, 28], three studies only GE [34, 35, 38], two studies only Philips [26, 37], one study both GE and Siemens [14], one study both Siemens and Philips [27], and one study GE, Siemens, and Philips [30]. The contrast material used for CT was iopromide in five studies [13, 14, 25, 28, 30], iohexol in four studies [26, 35,36,37], iopamidol in one study [34], and a non-specified nonionic contrast agent in two studies [10, 38]. The injection rates for the contrast agent were 3 ml/s in seven studies [14, 25, 26, 28, 30, 36, 38], 2 ml/s in two studies [35, 37], 1.2 ml/s in one study [10], 3–3.5 ml/s in one study [33], 3.5 ml/s in one study [13], and 4 ml/s in one study [34]. The quantities of contrast agent injected were 90 ml in six studies [14, 25, 28, 30, 33, 38], 120 ml in two studies [26, 36], 100 ml in two studies [13, 37], 65 ml in one study [10], and 60 ml in one study [35]. Of the five studies reporting the use of a saline flush after contrast injection, three used a saline flush rate of 3 ml/s [25, 30, 33], one reported using 50 ml of saline [13], and one did not give information on the quantity or rate of saline injected [28]. Image slices were reconstructed at a thickness of 3 mm in five studies [10, 25, 28, 36,37,38], 5 mm in three studies [34, 35, 38], 2.5–3 mm in three studies [14], and 0.5 mm in one study [26]. The CT criteria for cervical metastatic lymph nodes were mostly similar across the studies that were almost morphologic criteria including size, central necrosis or cystic change, dense or heterogeneous enhancement by measurement of CT attenuation, and calcification described in Table 2.
Quality assessment
The overall quality of the included studies according to the QUADAS-2 criteria was moderate, with the exception of three articles that satisfied fewer than four of the seven items (Fig. 2) [31, 32, 35]. In the patient selection domain, 11 studies showed an unclear risk of bias due to unclear information about consecutive patient enrollment [10, 13, 25, 27, 28, 31, 32, 35,36,37,38]. In the index test domain, there was an unclear risk of bias in seven studies where the index test was interpreted without the operators being blinded to knowledge of the results [26, 27, 29, 31, 32]. In terms of the reference standard, all included studies were considered to have an unclear risk of bias because of unclear blindness to knowledge of the index test during interpretation of the reference standard test [10, 13, 14, 25,26,27,28,29,30,31,32,33,34,35,36,37,38]. In the flow and timing domain, one study was considered to have a low risk of bias [27], while the others considered unclear or high risk. Ten studies were considered to have an unclear risk of bias because the intervals between the index test and reference standard were not specified [13, 25, 26, 28, 31,32,33,34,35, 38]. However, all studies were considered to have low applicability in the patient selection, index test, and reference standard domains [10, 13, 14, 25,26,27,28,29,30,31,32,33,34,35,36,37,38].
Diagnostic performance of CT
The sensitivity and specificity of the individual included studies were variable, ranging from 23 to 83% and 64 to 94%, respectively. Heterogeneity was present (p < 0.01) in the Q test, and the Higgins I2 statistic demonstrated substantial heterogeneity in sensitivity (I2 = 96.3%) and specificity (I2 = 93.8%); however, there was no threshold effect (Spearman correlation coefficient of < 0.6). For all the included 17 studies, the pooled sensitivity was 55% (95% CI, 47–63%) and the pooled specificity was 87% (95% CI, 90–95%) (Fig. 3). In the HSROC curve, there was a relatively large difference between the 95% confidence and prediction region, indicating heterogeneity between the studies (Fig. 4), while the area under the HSROC curve was 0.82 (95% CI, 0.79–0.85). According to the Deeks’ funnel plot, the likelihood of publication bias was low, with a p value of 0.41 for the slope coefficient (Fig. 5).
Subgroup analysis
We performed several subgroup analyses to evaluate various clinical scenarios. For preoperative CT analysis (n = 14), the pooled sensitivity was 54% (95% CI, 45–64%) and the pooled specificity was 87% (95% CI, 83–90%). In the per-neck level analysis, the summary sensitivity for lateral neck lymph nodes was 69% (95% CI, 59–77%) and the specificity was 91% (95% CI, 87–94%), while the summary sensitivity for central neck lymph nodes was 43% (95% CI, 32–55%) and the specificity was 91% (95% CI, 83–94%).
In the per-neck level subgroup analysis, the heterogeneity in both sensitivity and specificity, as demonstrated by the Higgins I2 statistic, was reduced, with the I2 values being 86.2% for sensitivity and 84.3% for specificity for lateral neck lymph nodes, and 90.4% for sensitivity and 90% for specificity for central neck lymph nodes.
Meta-regression
We performed meta-regression analysis to determine the causes of heterogeneity (Table 3). Among the potential covariates, three were shown to be significantly associated with study heterogeneity in the joint model, with these being contrast amount, scan phase, and reconstruction slice thickness. Sensitivity was higher in the arterial scan phase than in the venous scan phase, and was also higher with thin slices (≤ 3 mm) than with thick slices (> 3 mm). Apart from these three covariates, no other factor was demonstrated to significantly affect heterogeneity.
Discussion
This systematic review and meta-analysis showed that CT for pre- and postoperative diagnosis of metastatic cervical lymph nodes in patients with thyroid cancer had a pooled sensitivity of 55% (95% CI, 47–63%), a pooled specificity of 87% (95% CI, 90–95%), and an area under the HSROC curve of 0.82 (95% CI, 0.79–0.85). The Higgins I2 statistic demonstrated substantial heterogeneity in the sensitivity (I2 = 96.3%) and specificity (I2 = 93.8%); however, in the per-neck level subgroup analysis, this heterogeneity was reduced. Therefore, our meta-analysis demonstrated that CT showed acceptable diagnostic performance. In the meta-regression analysis, variations in the CT protocols such as contrast amount, scan phase, and reconstruction slice thickness were significant factors influencing the heterogeneity in diagnostic performance. Therefore, optimization and standardization of CT protocols are required in the future.
Although ultrasound and ultrasound-guided FNA are first-line diagnosis procedures for the detection of pathological nodes in the neck, the number of articles reporting the performance of CT for the diagnosis of cervical lymph node metastasis in thyroid cancer patients in routine clinical practice is increasing. This is because of the following reasons. First, the new American Thyroid Association guidelines suggest “active surveillance” for some recurrent patients, according to the risk stratification system [6, 15, 16]. Therefore, detection of preoperative lymph node metastasis is important before active surveillance. Second, there was concern over the use of iodinated contrast agents for thyroid CT for detecting metastatic lymph nodes in pre-op evaluation. However, recent studies reported that contrast-enhanced CT does not decrease the effect of subsequent radioactive iodine therapy [17,18,19,20,21]. Third, CT is an objective technique that is a less operator-dependent modality than US. Moreover, CT provides more anatomical information and improved detection of deeply located lesions, such as those in the retropharyngeal, retrosternal, and mediastinal areas [6, 10, 11]. Fourth, CT provides added value to US [23]. Fifth, in a postoperative setting, US diagnosis has many challenging clinical aspects, including anatomical distortion, postoperative fibrosis, and patients’ resistance for re-operation [65]. Finally, minimally invasive treatments such as radiofrequency ablation (RFA) and laser ablation are increasingly being used to treat recurrent thyroid cancers [66, 67], and current RFA guidelines suggest both CT and US detect lymph nodes before RFA, and to then evaluate the effect of RFA. On the basis of this background, this analysis of the diagnostic performance of CT is timely and clinically important.
Although one meta-analysis on the performance of CT has been published [23], several original articles on the diagnostic performance of CT have been newly published since 2017 [13, 27,28,29, 31, 33, 38]. Moreover, the previously published meta-analysis contained relatively small sample size, and it evaluated only preoperative CT performance (no postoperative CT examinations). Therefore, we considered there to be a need to update the literature and add the newly available information to a systematic review and meta-analysis, including a larger sample size (926 to 6378 patients), the addition of postoperative CT, and the analysis of various CT parameters.
The result of per-neck level subgroup analysis indirectly suggests that the level of the lymph node could be a cause of heterogeneity. In the meta-regression analysis, the thyroid CT protocol was revealed to be the main factor causing heterogeneity. Sensitivity was higher in the arterial scan phase than in the venous scan phase, which is in agreement with a study that demonstrated that arterial phase CT may be helpful for improving the detection of lymph node metastasis [13]. In addition, sensitivity was also higher with thin slices (≤ 3 mm) than with thick slices (> 3 mm). These additional results indicating that variation in the CT parameters causes heterogeneity suggest that optimization and unification of CT protocols could be crucial for improving the performance of CT.
This study has several limitations. First, not all subtypes of thyroid cancer were enrolled in this study. The number of medullary thyroid cancer patients was small, and patients with follicular thyroid cancer and anaplastic thyroid cancer were not enrolled. The criteria to define the metastatic lymph node were not exactly same, even though that were mostly similar across the studies. In addition, most studies (14 of 17) were retrospective, resulting in a risk of bias in patient selection, with the possibility of increased diagnostic sensitivity [68].
In conclusion, CT demonstrated acceptable diagnostic performance in the pre- and postoperative diagnosis of metastatic cervical lymph nodes in patients with thyroid cancer. Variation in the CT protocols is a main factor behind the heterogeneity across the included studies. Therefore, this study could be considered to indicate the need for optimization and unification of CT protocols in patients with thyroid cancer.
Abbreviations
- CT:
-
Contrast-enhanced computed tomography
- HSROC:
-
Hierarchic summary receiver operating characteristic
- PTC:
-
Papillary thyroid carcinoma
- RFA:
-
Radiofrequency ablation
- US:
-
Ultrasound
References
Sivanandan R, Soo KC (2001) Pattern of cervical lymph node metastases from papillary carcinoma of the thyroid. Br J Surg 88:1241–1244
Randolph GW, Duh QY, Heller KS et al (2012) The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 22:1144–1152
Johnson NA, Tublin ME (2008) Postoperative surveillance of differentiated thyroid carcinoma: rationale, techniques, and controversies. Radiology 249:429–444
Robenshtok E, Fish S, Bach A, Dominguez JM, Shaha A, Tuttle RM (2012) Suspicious cervical lymph nodes detected after thyroidectomy for papillary thyroid cancer usually remain stable over years in properly selected patients. J Clin Endocrinol Metab 97:2706–2713
Kim H, Kim TH, Choe JH et al (2017) Patterns of initial recurrence in completely resected papillary thyroid carcinoma. Thyroid 27:908–914
Haugen BR, Alexander EK, Bible KC et al (2016) 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133
Yeh MW, Bauer AJ, Bernet VA et al (2015) American Thyroid Association statement on preoperative imaging for thyroid cancer surgery. Thyroid 25:3–14
Gharib H, Papini E, Paschke R et al (2010) American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. Endocr Pract 16:468–475
Shin JH, Baek JH, Chung J et al (2016) Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol 17:370–395
Lesnik D, Cunnane ME, Zurakowski D et al (2014) Papillary thyroid carcinoma nodal surgery directed by a preoperative radiographic map utilizing CT scan and ultrasound in all primary and reoperative patients. Head Neck 36:191–202
Moon HJ, Kim EK, Yoon JH, Kwak JY (2012) Differences in the diagnostic performances of staging US for thyroid malignancy according to experience. Ultrasound Med Biol 38:568–573
King AD (2008) Imaging for staging and management of thyroid cancer. Cancer Imaging 8:57–69
Park JE, Lee JH, Ryu KH et al (2017) Improved diagnostic accuracy using arterial phase CT for lateral cervical lymph node metastasis from papillary thyroid cancer. AJNR Am J Neuroradiol 38:782–788
Ahn JE, Lee JH, Yi JS et al (2008) Diagnostic accuracy of CT and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J Surg 32:1552–1558
Kim TY, Shong YK (2017) Active surveillance of papillary thyroid microcarcinoma: a mini-review from Korea. Endocrinol Metab (Seoul) 32:399–406
Tufano RP, Clayman G, Heller KS et al (2015) Management of recurrent/persistent nodal disease in patients with differentiated thyroid cancer: a critical review of the risks and benefits of surgical intervention versus active surveillance. Thyroid 25:15–27
Padovani RP, Kasamatsu TS, Nakabashi CC et al (2012) One month is sufficient for urinary iodine to return to its baseline value after the use of water-soluble iodinated contrast agents in post-thyroidectomy patients requiring radioiodine therapy. Thyroid 22:926–930
Sohn SY, Choi JH, Kim NK et al (2014) The impact of iodinated contrast agent administered during preoperative computed tomography scan on body iodine pool in patients with differentiated thyroid cancer preparing for radioactive iodine treatment. Thyroid 24:872–877
Ho JD, Tsang JF, Scoggan KA, Leslie WD (2014) Urinary iodine clearance following iodinated contrast administration: a comparison of euthyroid and postthyroidectomy subjects. J Thyroid Res 2014:580569
Mishra A, Pradhan PK, Gambhir S, Sabaretnam M, Gupta A, Babu S (2015) Preoperative contrast-enhanced computerized tomography should not delay radioiodine ablation in differentiated thyroid carcinoma patients. J Surg Res 193:731–737
Tala Jury HP, Castagna MG, Fioravanti C, Cipri C, Brianzoni E, Pacini F (2010) Lack of association between urinary iodine excretion and successful thyroid ablation in thyroid cancer patients. J Clin Endocrinol Metab 95:230–237
Leenhardt L, Erdogan MF, Hegedus L et al (2013) 2013 European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J 2:147–159
Suh CH, Baek JH, Choi YJ, Lee JH (2017) Performance of CT in the preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid cancer: a systematic review and meta-analysis. AJNR Am J Neuroradiol 38:154–161
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W65–W94
Choi JS, Kim J, Kwak JY, Kim MJ, Chang HS, Kim EK (2009) Preoperative staging of papillary thyroid carcinoma: comparison of ultrasound imaging and CT. AJR Am J Roentgenol 193:871–878
Choi YJ, Yun JS, Kook SH, Jung EC, Park YL (2010) Clinical and imaging assessment of cervical lymph node metastasis in papillary thyroid carcinomas. World J Surg 34:1494–1499
Chong A, Ha JM, Han YH et al (2017) Preoperative lymph node staging by FDG PET/CT with contrast enhancement for thyroid cancer: a multicenter study and comparison with neck CT. Clin Exp Otorhinolaryngol 10:121–128
Eun NL, Son EJ, Kim JA, Gweon HM, Kang JH, Youk JH (2018) Comparison of the diagnostic performances of ultrasonography, CT and fine needle aspiration cytology for the prediction of lymph node metastasis in patients with lymph node dissection of papillary thyroid carcinoma: a retrospective cohort study. Int J Surg 51:145–150
Kang JH, Jung DW, Pak KJ et al (2018) Prognostic implication of fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography in patients with recurrent papillary thyroid cancer. Head Neck 40:94–102
Kim E, Park JS, Son KR, Kim JH, Jeon SJ, Na DG (2008) Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid 18:411–418
Kim SK, Woo JW, Park I et al (2017) Computed tomography-detected central lymph node metastasis in ultrasonography node-negative papillary thyroid carcinoma: is it really significant? Ann Surg Oncol 24:442–449
Lee DW, Ji YB, Sung ES et al (2013) Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol 39:191–196
Lee Y, Kim JH, Baek JH et al (2018) Value of CT added to ultrasonography for the diagnosis of lymph node metastasis in patients with thyroid cancer. Head Neck. https://doi.org/10.1002/hed.25202
Liu X, Ouyang D, Li H et al (2015) Papillary thyroid cancer: dual-energy spectral CT quantitative parameters for preoperative diagnosis of metastasis to the cervical lymph nodes. Radiology 275:167–176
Morita S, Mizoguchi K, Suzuki M, Iizuka K (2010) The accuracy of (18)[F]-fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography, ultrasonography, and enhanced computed tomography alone in the preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid carcinoma. World J Surg 34:2564–2569
Na DK, Choi YJ, Choi SH, Kook SH, Park HJ (2015) Evaluation of cervical lymph node metastasis in thyroid cancer patients using real-time CT-navigated ultrasonography: preliminary study. Ultrasonography 34:39–44
Seo YL, Yoon DY, Baek S et al (2012) Detection of neck recurrence in patients with differentiated thyroid cancer: comparison of ultrasound, contrast-enhanced CT and (18)F-FDG PET/CT using surgical pathology as a reference standard: (ultrasound vs. CT vs. (18)F-FDG PET/CT in recurrent thyroid cancer). Eur Radiol 22:2246–2254
Zhao Y, Li X, Li L et al (2017) Preliminary study on the diagnostic value of single-source dual energy CT in diagnosing cervical lymph node metastasis of thyroid carcinoma. J Thorac Dis 9:4758–4766
Kim KW, Lee J, Choi SH, Huh J, Park SH (2015) Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part I. General guidance and tips. Korean J Radiol 16:1175–1187
Deville WL, Buntinx F, Bouter LM et al (2002) Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2:9
Suh CH, Park SH (2016) Successful publication of systematic review and meta-analysis of studies evaluating diagnostic test accuracy. Korean J Radiol 17:5–6
Lee J, Kim KW, Choi SH, Huh J, Park SH (2015) Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part II. Statistical methods of meta-analysis. Korean J Radiol 16:1188–1196
Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH (2005) Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 58:982–990
Rutter CM, Gatsonis CA (2001) A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med 20:2865–2884
Deeks JJ, Macaskill P, Irwig L (2005) The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 58:882–893
Ahmed S, Ghazarian MP, Cabanillas ME et al (2017) Imaging of anaplastic thyroid carcinoma. AJNR Am J Neuroradiol. https://doi.org/10.3174/ajnr.A5487
AlNoury MK, Almuhayawi SM, Alghamdi KB, Al-Noury KI (2015) Preoperative imaging modalities to predict the risk of regional nodal recurrence in well-differentiated thyroid cancers. Int Arch Otorhinolaryngol 19:116–120
Byun BH, Jeong UG, Hong SP et al (2012) Prediction of central lymph node metastasis from papillary thyroid microcarcinoma by 18F-fluorodeoxyglucose PET/CT and ultrasonography. Ann Nucl Med 26:471–477
Çaǧlayan S, Urhan M, Sildiroǧlu O, Kurt Y, Önde ME, Narin Y (2009) Management of thyroid cancer associated with elevated serum thyroglobulin and negative radioiodine scanning. Turkish J Med Sci 39:693–699
Eisenkraft BL, Som PM (1999) The spectrum of benign and malignant etiologies of cervical node calcification. AJR Am J Roentgenol 172:1433–1437
Giraudet AL, Vanel D, Leboulleux S et al (2007) Imaging medullary thyroid carcinoma with persistent elevated calcitonin levels. J Clin Endocrinol Metab 92:4185–4190
Goyal N, Pakdaman M, Kamani D, Caragacianu D, Goldenberg D, Randolph GW (2017) Mapping the distribution of nodal metastases in papillary thyroid carcinoma: where exactly are the nodes? Laryngoscope 127:1959–1964
Kim H, Na KJ, Choi JH, Ahn BC, Ahn D, Sohn JH (2016) Feasibility of FDG-PET/CT for the initial diagnosis of papillary thyroid cancer. Eur Arch Otorhinolaryngol 273:1569–1576
Miki H, Oshimo K, Inoue H et al (1993) Diagnosis and surgical treatment of small papillary carcinomas of the thyroid gland. J Surg Oncol 54:78–80 discussion 80-71
Rubello D, Mazzarotto R, Casara D (2000) The role of technetium-99m methoxyisobutylisonitrile scintigraphy in the planning of therapy and follow-up of patients with differentiated thyroid carcinoma after surgery. Eur J Nucl Med 27:431–440
Rubello D, Rampin L, Nanni C et al (2008) The role of 18F-FDG PET/CT in detecting metastatic deposits of recurrent medullary thyroid carcinoma: a prospective study. Eur J Surg Oncol 34:581–586
Szakall S Jr, Esik O, Bajzik G et al (2002) 18F-FDG PET detection of lymph node metastases in medullary thyroid carcinoma. J Nucl Med 43:66–71
Mulla M, Schulte KM (2012) Terminology inaccuracies in the interpretation of imaging results in detection of cervical lymph node metastases in papillary thyroid cancer. Endocr Connect 1:78–86
Soler ZM, Hamilton BE, Schuff KG, Samuels MH, Cohen JI (2008) Utility of computed tomography in the detection of subclinical nodal disease in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg 134:973–978
Wu CW, Dionigi G, Lee KW et al (2012) Calcifications in thyroid nodules identified on preoperative computed tomography: patterns and clinical significance. Surgery 151:464–470
Jeong HS, Baek CH, Son YI et al (2006) Integrated 18F-FDG PET/CT for the initial evaluation of cervical node level of patients with papillary thyroid carcinoma: comparison with ultrasound and contrast-enhanced CT. Clin Endocrinol (Oxf) 65:402–407
Lee DH, Kang WJ, Seo HS et al (2009) Detection of metastatic cervical lymph nodes in recurrent papillary thyroid carcinoma: computed tomography versus positron emission tomography-computed tomography. J Comput Assist Tomogr 33:805–810
Yoon JH, Kim JY, Moon HJ et al (2011) Contribution of computed tomography to ultrasound in predicting lateral lymph node metastasis in patients with papillary thyroid carcinoma. Ann Surg Oncol 18:1734–1741
Jadvar H, McDougall IR, Segall GM (1998) Evaluation of suspected recurrent papillary thyroid carcinoma with [18F]fluorodeoxyglucose positron emission tomography. Nucl Med Commun 19:547–554
Sippel RS, Chen H (2009) Controversies in the surgical management of newly diagnosed and recurrent/residual thyroid cancer. Thyroid 19:1373–1380
Baek JH, Kim YS, Sung JY, Choi H, Lee JH (2011) Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol 197:W331–W336
Mauri G, Cova L, Ierace T et al (2016) Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol 39:1023–1030
Deeks JJ (2001) Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ 323:157–162
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jung Hwan Baek.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Chong Hyun Suh) has significant statistical expertise (5 years of experience in a systematic review and meta-analysis).
Informed consent
Written informed consent was not required for this study because of the nature of our study, which was a systemic review and meta-analysis.
Ethical approval
Institutional Review Board approval was not required because of the nature of our study, which was a systemic review and meta-analysis.
Methodology
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Cho, S.J., Suh, C.H., Baek, J.H. et al. Diagnostic performance of CT in detection of metastatic cervical lymph nodes in patients with thyroid cancer: a systematic review and meta-analysis. Eur Radiol 29, 4635–4647 (2019). https://doi.org/10.1007/s00330-019-06036-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06036-8