Abstract
Objectives
To investigate the prognostic role of early post-infarction cardiac magnetic resonance (CMR) on long-term risk stratification of ST segment elevation myocardial infarction (STEMI) patients with preserved left ventricular ejection fraction (LVEF).
Methods
Seventy-seven STEMI patients treated by primary percutaneous coronary intervention (PCI) and LVEF > 50% at CMR were included. The median time between STEMI and CMR was 5 days (IQR 2–8). LV volumes and function, area at risk (on T2 weighted images), infarcted myocardium (on late enhanced images), intramyocardial hemorrhage, and early and late microvascular obstruction (MVO) were detected and measured. CMR tissue determinants were correlated with the incidence of major adverse cardiovascular events (MACEs) over a 5-year follow-up.
Results
During median follow-up of 4 years (range 3 to 5 years), eight (10%) patients experienced MACE, yielding an annualized event rate of 2.1%. All CMR tissue markers were not significantly different between MACE and no-MACE patients, except for the presence of late MVO (50% vs. 16%, respectively; p = 0.044) and its extent (2.30 ± 1.64 g vs. 0.18 ± 0.12 g, respectively; p = 0.000). From receiver-operating characteristic (ROC) curve (area under the curve 0.89; 95% confidence interval, 0.75–1.0; p = 0.000), late MVO extent > 0.385 g was a strong independent predictor of MACE at long-term follow-up (sensitivity = 87%, specificity = 90%; hazard ratio = 2.24; 95% confidence interval, 1.51–3.33; p = 0.000).
Conclusions
Late MVO extent after primary PCI on CMR seems to be a strong predictor of MACE at 5-year follow-up in patients with LVEF > 50%. Noticeably, late MVO extent > 0.385 g provided relevant prognostic insights leading to improved long-term risk stratification.
Key Points
• Tissue markers provided by cardiac magnetic resonance aid in prognostic stratification after myocardial infarction
• The occurrence of late microvascular obstruction after acute myocardial infarction increases risk of major adverse events at 5-year follow-up.
• The greater microvascular obstruction extent on late gadolinium enhanced images is related to an increased risk of adverse events in patients with myocardial infarction and preserved left ventricular function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Left ventricular ejection fraction (LVEF) is a well-documented strong predictor of major adverse cardiovascular events (MACE) and mortality in patients with previous acute myocardial infarction (AMI) [1,2,3]. According to the current cardiac chamber quantifications by echocardiography guidelines, LVEF values of < 50% are suggestive of abnormal systolic left ventricular (LV) function [4]. Data from epidemiological studies and registries show that left ventricular systolic dysfunction (LVSD) is present in 46–52% of AMI patients within 5 days of infarct, with a significantly higher incidence of post-infarction LVSD in patients with ST segment elevation myocardial infarction (STEMI) versus patients with non ST segment elevation myocardial infarction (NSTEMI, 48% vs. 36.5%, respectively; p = 0.001) [5, 6]. Compared to AMI patients with normal LV function, those with severely impaired LVEF have higher 1-year rates of net adverse clinical events, MACE, cardiac death, and all-cause mortality (hazard ratio = 4.49) [1, 3]. Therefore, the prognosis of patients with LVEF < 50% has been deeply investigated, and the clinical and therapeutic management is well defined. However, no studies have specifically investigated the prediction of post-infarction MACE in patients with preserved LVEF after AMI. A better understanding of factors involved in the potential development of MACE in those patients would help to identify high-risk individuals that could benefit from more aggressive treatment.
Cardiac magnetic resonance (CMR) represents a well-established and reproducible diagnostic tool, which provides a combined assessment of the global and regional LV function and infarction-related myocardial tissue changes, and represents the non-invasive gold standard for in vivo visualization and characterization of myocardial damage [7]. Many CMR parameters have been shown to improve the overall risk stratification of AMI patients with LVSD, including infarct size [7], microvascular obstruction (MVO) [8], myocardial salvage [9], post-reperfusion myocardial hemorrhage [10], and right ventricular (RV) involvement [11].
The present study was designed to investigate the prognostic value of these CMR tissue determinants measured early after AMI in a cohort of STEMI patients with preserved LVEF during long-term follow-up.
Materials and methods
Study population
One hundred seventy-three consecutive STEMI patients, treated by primary percutaneous coronary intervention (PCI) within 12 h after symptoms onset, who undergone CMR in the early post-infarction phase (within 8 days from symptoms onset) between January 2006 and April 2016, were retrospectively evaluated for study inclusion (Fig. 1).
Study flow chart. One hundred seventy-three consecutive STEMI patients treated by primary PCI were evaluated for study inclusion. We excluded patients with LVEF < 50% and CMR contraindications (claustrophobia). Shown are the numbers of patients who were considered eligible, followed up during the study period, and enrolled in the final cohort. CMR, cardiac magnetic resonance; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; STEMI, ST segment elevation myocardial infarction
Exclusion criteria were LVEF < 50% assessed by CMR, Killip class > III at admission, history of previous heart failure (HF) or coronary revascularization, in-hospital HF, prior AMI, and CMR contraindications (claustrophobia, hemodynamic instability, and impaired renal function). Clinical variables including creatine kinase and troponin I measurements, cardiovascular risk factors, and pharmacological treatment were collected before the CMR scan. Coronary intervention and periprocedural treatment have been accomplished according to the current European Society of Cardiology (ESC) guidelines [12].
CMR acquisition protocol
CMR studies were conducted by using a 1.5-T scanner (Magnetom Avanto, Siemens Healthcare) equipped with a multichannel phase-array cardiac coil. A standardized CMR protocol, including the ECG-gated cine steady-state free precession (cine-MR), T2-weighted short tau inversion recovery (T2w-STIR), early and late gadolinium enhanced (EGE and LGE, respectively) sequences, was performed in all patients.
Cine-MR sequence details were TE 1.21 ms, TR 51.3 ms, flip angle 45°, slice thickness 8 mm, matrix 256 × 256, field of view (FoV) ranging from 340 to 400 mm, and in-plane resolution ranging from 1.3 mm × 1.3 mm to 1.6 mm × 1.6 mm. For T2w-STIR imaging, breath-hold black-blood segmented TSE technique was adopted by the use of triple inversion recovery sequences: TE 75 ms, TR 2 R-to-R intervals, TI 140 ms to null fat signal, flip angle 150°, slice thickness 8 mm, matrix of 256 × 256, FoV 340–400 mm, and spatial resolution ranging from 1.3 mm × 1.3 mm to 1.6 mm × 1.6 mm [13]. EGE and LGE images were obtained by acquiring segmented T1-weighted inversion recovery (IR) sequences 1 to 4 and 10 to 15 min after intravenous injection of contrast agent (gadolinium-BOPTA, Multihance, Bracco Diagnostics Inc.; 0.1 mmol/kg body weight at 2 ml/s), respectively; sequence parameters were TE 2.3 ms, TR 5 ms, optimized TI 250/300 ms to null normal myocardium, flip angle 15°, slice thickness 8 mm, matrix 256 × 256, FoV 340–400 mm, and spatial resolution ranging from 1.3 mm × 1.3 mm to 1.6 mm × 1.6 mm. All sequences were acquired during short breath holds, in the same vertical and horizontal long axis, and on short-axis views completely encompassing the LV.
Image analysis
All images were analyzed by consensus of two CMR-experienced operators (M.F. and I.C. with 16 and 17 years of experience, respectively) blinded to clinical, laboratory, and angiographic findings. Left and right ventricular end-systolic volume (ESV), end-diastolic volume (EDV), myocardial mass, and ejection fraction (EF) were derived from the short-axis cine-MR images by manually tracing the endocardial and epicardial borders using a dedicated software (Cvi42, Circle Cardiovascular Imaging Inc.). A LVEF cut-off value of 50% was used to distinguish patients with preserved LVEF (> 50%) from those with LVSD (< 50%) [4]. The left ventricle was divided into 17 segments, based on the standardized AHA model, to assess wall motion patterns [14]. Functional impairment was expressed qualitatively, assigning to each segment a motion score from 1 to 4 (1 = normal, 2 = hypokinesia, 3 = akinesia, and 4 = dyskinesia); the wall motion score index (WMSI) was then obtained by dividing the sum of all segment scores for the number of segments.
Area at risk (AAR) extent was quantified on short-axis T2w-STIR images as myocardium with a signal intensity > 2SD above the mean signal of healthy, remote myocardium, as previously described [15]. Similarly, the infarcted myocardial mass was measured from the short-axis LGE images with a threshold-based method (signal intensity > 5SD of unenhanced, remote myocardium) [16].
MVO is defined as a subendocardially located dark area within the hyperenhanced myocardium [17]; it was assessed on EGE and LGE images (early and late MVO, respectively) and quantified by manual contouring the hypointense zone within the enhanced areas [18]. Finally, on T2w-STIR images, hemorrhagic infarcts were identified as a hypointense core surrounded by a peripheral hyperintense rim [19].
Follow-up assessment
Follow-up was started at the time of CMR and collected by periodic phone interviews and review of outpatient clinics or hospitalization records. MACEs were defined as reinfarction, cardiac death, coronary revascularization, re-hospitalization for angina pectoris, congestive heart failure, or arrhythmias.
The primary endpoint was to assess the prognostic value of CMR tissue features in predicting MACE in STEMI patients with preserved LVEF at 5-year follow-up.
Statistical analysis
Continuous variables are reported as mean ± standard deviation or median with the corresponding interquartile range (25th to 75th percentile) as appropriate; categorical data are expressed as the number and percentage of patients. Annualized event rates are expressed as the number of patients experiencing MACE divided by the number of patient-years follow-up. Comparison of CMR parameters between patients with MACE or no-MACE was performed with the Student’s independent samples t test for normally distributed continuous variables and with the Fischer’s exact test for categorical variables, when an expected cell count was less than five. Receiver-operating characteristic (ROC) curve analysis was performed to determine an area under the curve (AUC) for continuous variables with significant difference between MACE and no-MACE patients, whereas the optimal cut-off for these parameters was identified with the Youden’s index, maximizing sensitivity and specificity. Survival curves were obtained by Kaplan-Meier analysis with Mantel-Cox log-rank comparison to illustrate the time-dependent occurrence of the primary endpoint (MACE) in relation to the late MVO extent cut-off value. Univariate Cox regression analysis was used to estimate the hazard ratios (HR) for the primary endpoint. Risk factors for the occurrence of the primary endpoint were identified by performing a multivariate analysis.
Statistical tests were performed with SPSS software, version 21.0. All tests were two-tailed, with p < 0.05 considered as statistically significant; and all confidence intervals (CI) were calculated to the 95th percentile.
Results
Among 79 eligible patients, two (2.5%) were lost during follow-up, yielding a final cohort of 77 patients (mean age 60 ± 2 years; 63 males, 82%). Baseline clinical and CMR characteristics are summarized in Table 1. More than half of patients had hypertension (n = 43, 56%) and smoking habits (n = 50, 65%), with a family history of CAD (n = 44, 57%). The overall median door-to-balloon time was 110 min (IQR 60–300), and the majority of patients had TIMI flow grades 0 or 1 before PCI (n = 73, 95%). Cardiovascular medications at hospital discharge were ACE inhibitors/ARB in 83% (n = 64), β-blockers in 74% (n = 57), statins in 88% (n = 68), aspirin in 58% (n = 45), and clopidogrel in 40% (n = 31) of patients.
Median duration of follow-up was 48 months (range 3 to 5 years). The primary endpoint occurred in eight patients (10.38%), yielding an annualized event rate of 2.16%. Three patients (3.89%) were hospitalized due to decompensated congestive heart failure, and five patients (6.49%) underwent coronary revascularization (4 PCI and 1 CABG).
The median time between STEMI and CMR was 5 days (IQR 2–8). Mean LVEF was 56.8 ± 1.0%, and the infarct size determined by the extent of LGE was 11.4 ± 2.4 g; early and late MVOs, defined as CMR no-reflow phenomenon, were detected in 32 (42%) and 15 (19%) patients, respectively.
No statistically significant differences regarding extent of AAR, infarct size, LVWMSI and the presence of hemorrhagic infarcts, early MVO, right ventricular edema, and LGE were found between MACE and no-MACE patients; however, MACE patients suffered from late MVO more frequently than no-MACE patients (n = 4, 50% vs. n = 11, 16%; p = 0.044) and showed larger late MVOs (2.30 ± 1.64 g vs. 0.18 ± 0.12 g; p = 0.000), as shown in Table 2. Receiver-operating characteristic (ROC) analysis identified an area under the curve of 0.895 (95% CI, 0.750–1.00; p = 0.000) (Fig. 2), and the Youden’s index (0.775) was maximized to determine a late MVO extent of 0.385 g as the best cut-off value for predicting the primary endpoint, with a sensitivity and a specificity of 87% and 90%, respectively. Kaplan-Meier curves showed that patients with late MVO size > 0.385 g had a higher likelihood (p = 0.000) of experiencing MACE at 5 years (events-free mean time 41.4 months; 95% CI, 29.9–52.9) compared to patients with late MVO extent < 0.385 g (events-free mean time 59.7 months; 95% CI, 59.2–60) (Fig. 3). In univariate Cox regression analysis, the extent of late MVO > 0.385 g was associated with the occurrence of the primary endpoint (HR = 2.24; 95% CI, 1.51–3.33; p = 0.000). Finally, multivariate analysis revealed creatinine (p = 0.002) to be an independent risk factor for MACE at 1 year.
Kaplan-Meier curves. Kaplan-Meier curves showing the time-to-first event for the primary composite endpoint (MACE) during follow-up according to the cut-off value of late microvascular obstruction (MVO) extent (> 0.385 g). The numbers of patients at risk at the start of each 10 months of follow-up are shown. The p value was calculated with the Mantel-Cox log-rank test. MACE, major adverse cardiovascular events
Discussion
In this study, we investigated the prognostic impact of early post-infarction CMR on long-term risk stratification of 77 successfully reperfused STEMI patients with preserved global systolic function (LVEF ≥ 50%). The main findings can be summarized as follow: (1) although a preserved systolic function is a favorable prognostic factor after AMI, a non-negligible number of patients (n = 8, 10.4%) experienced MACE over a 5 years of follow-up; (2) the presence and extent of late MVO are the only CMR tissue features which significantly differ between MACE and no-MACE patients; and (3) late MVO cut-off value > 0.385 g was a strong independent predictor of clinical outcome at long-term follow-up (Fig. 4).
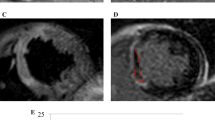
Fifty-year-old (a–e, patient 1) and 46-year-old (f–j, patient 2) men with anteroseptal acute myocardial infarction due to the occlusion of the proximal left anterior descending artery treated by PCI within 12 h after symptoms onset (7.4 h and 4.5 h, respectively). T2w-STIR short-axis views (a, f) revealed large areas-at-risk involving intraventricular septum and anterior wall, well-matching the gadolinium-enhanced areas on T1w-EGE (b, g) and T1w-LGE (c, h) images acquired on short-axis view. MVO occurrence was detected in both patients on both T1w-EGE and LGE images (late MVO size was 1.8 g for patient 1 and 0.16 g for patient 2). Corresponding cine-MR images acquired in end-diastolic (d, i) and end-systolic (e, j) phases; LVEF was 52% for patient 1 and 57% for patient 2. Patient 1 was re-admitted 8 weeks after discharge because of heart failure; over a 5-year follow-up, no MACE were experienced for patient 2
Currently, only very few studies have identified a set of clinical, laboratory, and functional factors that are correlated with MACE in STEMI patients with preserved ejection fraction [20, 21]. In a population-based cohort study that included 146 patients with prior MI and LVEF > 50%, Sacha Bathia et al reported that age, peripheral artery disease, systolic blood pressure, anemia, renal dysfunction, and respiratory rate were predictors of death at 1-year follow-up [20]. In the I-PRESERVE randomized control trial with 618 enrolled, post-ischemic HFPEF patients, Komajda et al observed that several clinical variables including NT-proBNP, age, atrial fibrillation, renal dysfunction, diabetes mellitus, 6-months history of HF hospitalization, and chronic lung disease were strong multivariable predictors of morbidity and mortality [21].
Recently, Symons et al investigated whether early post-infarction CMR parameters provide additional long-term prognostic value beyond traditional prognostic factors in STEMI patients, and it has been found that MVO is a strong prognostic factor in revascularized STEMI patients [22, 23].
However, to the best of our knowledge, this is the first study investigating the role of CMR in prognostic stratification of the AMI patient’s subgroup with preserved LV systolic function.
Myocardial no-reflow is a relevant phenomenon considering that the incidence of MVO in successfully reperfused infarcts ranges from 5 to 50%, according to the imaging time after contrast injection and the study group [24, 25]. De Waha et al studied 512 STEMI patients treated by PCI and found that the presence and extent of late MVO were independent predictors of cardiac death, HF, and reinfarction over a median follow-up of 19 months [18]. In a meta-analysis including more than 1000 patients with acute myocardial infarction, Van Kranenburg et al showed that MVO was an independent predictor of MACE at 2 years [26]. Despite the different populations examined, our results confirm, as reported by these and other studies [25, 27, 28], that CMR-assessed late MVO is a strong predictor of clinical outcome at 5-year follow-up also in STEMI patients with preserved LV function, regardless of whether it was considered as a continuous (late MVO extent) or cut-off (late MVO > 0.385 g) parameter. Moreover, in our cohort, the probability of experiencing MACE was approximately 2-fold higher in patients with late MVO > 0.385 g with respect to those with late MVO < 0.385 g.
Several studies have demonstrated a relationship between MVO and infarct size [29, 30], with the first related to time-to-reperfusion [31] and the latter being an important predictor of mortality [27, 32]. However, we did not observe any significant difference regarding LGE extent between MACE and no-MACE patients with LVEF > 50%, whereas late MVO predicted long-term clinical outcome independent of infarct size. The reason for the superior prognostic value of late MVO with respect to infarct size may be that, while contrast-enhanced myocardium encompasses different degrees of damage, late MVO represents the most severe grade of myocardial injury (profoundly disturbed subendocardial microcirculation surrounded by LGE-positive myocardium). This hypothesis is supported by previous studies showing that MVO predicts subsequent, post-infarction LV remodeling better than infarct size [33,34,35,36].
According to the most recent European Society of Cardiology guidelines on management of STEMI patients, LVEF measurement is recommended for immediate risk stratification of patients with AMI, considering those with preserved ejection fraction to be at low risk [37]. However, LVEF assessment represents the final result of the combination between the hypokinetic infarcted or stunned myocardium and the ability of the remote/healthy myocardium to compensate; therefore, it may not sufficiently reflect the severity of infarction [38]. In line with this concept, we observed eight adverse cardiac events among 77 STEMI patients with LVEF > 50%, and late MVO > 0.385 g was a stronger predictor of outcome compared to the traditional LV systolic function determinant.
In our cohort, the higher incidence of early MVO in MACE patients compared to no-MACE patients was not significant, even though not negligible (p = 0.061). Early MVO is identified by a prolonged perfusion defect in the core of the infarct on T1-weighted images obtained within the first minutes after contrast administration, and its prevalence is higher compared with late contrast-enhanced images [24, 39]. In our study, among the 17 out of 35 patients with early MVO who completely filled in the infarcted area during late enhanced phase filled, 15 showed a better prognosis (absence of MACE).
Furthermore, it should be noted that in our population of MACE patients, the right ventricle has been more frequently involved (RV LGE, 25% vs. 13%), which represents an independent factor affecting prognosis [11].
The cause of the adverse effects of late MVO remains speculative. Wu et al found that MVO was associated with MACE and LV remodeling (increased myocardial thinning and ventricular volumes at 6 months) [8]. Baks et al observed that dysfunctional cardiac segments without MVO had an increased end-diastolic wall thickness with respect to segments with MVO at follow-up [40]. Since ventricular remodeling is an important long-term prognostic parameter [41], these studies suggest that MVO may influence clinical outcome through its impact on subsequent LV remodeling predisposing to MACE.
Finally, in our study, late MVO was present in approximately 20% of patients, each with an angiographic post-PCI TIMI flow grade of 3; therefore, there is the need of novel therapeutic strategies in addition to primary PCI. Our findings may become clinically relevant for identifying high-risk patients who may benefit from either more rigorous follow-up or adjunctive treatments, for example vasodilators, antiplatelet therapy, thrombolysis, and embolic protection devices [42], to promote the repair of infarcted myocardium.
Study limitations
Some limitations of our retrospective study need to be addressed. First, no long-term CMR follow-up studies were conducted after acute myocardial infarction; thus, we could not provide information on the evolution of late MVO and ventricular remodeling after the acute phase of ischemic event. Second, sample size is too limited for drawing definite conclusions on prognosis of STEMI patients with preserved ejection fraction and to investigate influence of gender differences in this specific clinical setting [43]. Third, a 5-year follow-up (median of 48 months) on the patient cohort is not long enough to reach the primary endpoint for most of the patients (only 10% of the patients reached the primary endpoint); therefore, the results of the present study require to be validated over a longer follow-up. Finally, various reports have demonstrated that mapping techniques are predictors of LV remodeling and MACE [44, 45]. However, these parameters were not measured and thus their significance cannot be assessed in the present study.
Conclusions
Cardiac magnetic resonance may become the imaging modality of choice for stratifying STEMI patients given its unique ability to globally assess ventricular structure and function along with qualitative and quantitative characterization of infarcted myocardium. CMR-based late MVO extent after primary PCI is a stronger predictor of MACE at 5-year follow-up with respect to the traditional post-infarction risk stratification markers (ejection fraction and infarct size) in patients with LVEF > 50%. Noticeably, late MVO extent > 0.385 g provided relevant prognostic insights leading to improved long-term risk stratification of STEMI patients with preserved ejection fraction.
Abbreviations
- AAR:
-
Area at risk
- ACE:
-
Angiotensin converting enzyme
- AMI:
-
Acute myocardial infarction
- ARB:
-
Angiotensin receptor blocker
- AUC:
-
Area under the curve
- CABG:
-
Coronary artery bypass graft
- CMR:
-
Cardiac magnetic resonance
- EDV:
-
End-diastolic volume
- ESV:
-
End-systolic volume
- HF:
-
Heart failure
- HFPEF:
-
Heart failure with preserved ejection fraction
- IQR:
-
Interquartile range
- LGE:
-
Late gadolinium enhancement
- LVEF:
-
Left ventricular ejection fraction
- LVSD:
-
Left ventricular systolic dysfunction
- MACE:
-
Major adverse cardiovascular events
- MVO:
-
Microvascular obstruction
- NSTEMI:
-
Non ST segment elevation myocardial infarction
- PCI:
-
Percutaneous coronary intervention
- ROC:
-
Receiver-operating characteristic
- RV:
-
Right ventricle
- STEMI:
-
ST segment elevation myocardial infarction
- STIR:
-
Short tau inversion recovery
- TE:
-
Echo time
- TI:
-
Inversion time
- TIMI:
-
Thrombolysis in myocardial infarction
- TR:
-
Repetition time
- TSE:
-
Turbo spin echo
- WMSI:
-
Wall motion score index
References
Ng VG, Lansky AJ, Meller S et al (2014) The prognostic importance of left ventricular function in patients with ST-segment elevation myocardial infarction: the HORIZONS-AMI trial. Eur Heart J Acute Cardiovasc Care 3:67–77
Perelshtein Brezinov O, Klempfner R, Zekry SB, Goldenberg I, Kuperstein R (2017) Prognostic value of ejection fraction in patients admitted with acute coronary syndrome: a real world study. Medicine (Baltimore) 96:e6226
Rouleau JL, Talajic M, Sussex B et al (1996) Myocardial infarction patients in the 1990s--their risk factors, stratification and survival in Canada: the Canadian assessment of myocardial infarction (CAMI) study. J Am Coll Cardiol 27:1119–1127
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–270
Danchin N, Vaur L, Genès N et al (1997) Management of acute myocardial infarction in intensive care units in 1995: a nationwide French survey of practice and early hospital results. J Am Coll Cardiol 30:1598–1605
Hanania G, Cambou JP, Guéret P et al (2004) Management and in-hospital outcome of patients with acute myocardial infarction admitted to intensive care units at the turn of the century: results from the French nationwide USIC 2000 registry. Heart 90:1404–1410
Bruder O, Breuckmann F, Jensen C et al (2008) Prognostic impact of contrast-enhanced CMR early after acute ST segment elevation myocardial infarction (STEMI) in a regional STEMI network: results of the "Herzinfarktverbund Essen". Herz 33:136–142
Wu KC, Zerhouni EA, Judd RM et al (1998) Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 97:765–772
Masci PG, Ganame J, Strata E et al (2010) Myocardial salvage by CMR correlates with LV remodeling and early ST-segment resolution in acute myocardial infarction. JACC Cardiovasc Imaging 3:45–51
Ganame J, Messalli G, Dymarkowski S et al (2009) Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur Heart J 30:1440–1449
Masci PG, Francone M, Desmet W et al (2010) Right ventricular ischemic injury in patients with acute ST-segment elevation myocardial infarction: characterization with cardiovascular magnetic resonance. Circulation 122:1405–1412
Van de Werf F, Bax J, Betriu A et al (2008) Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the task force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. Eur Heart J 29:2909–2945
Francone M, Carbone I, Agati L et al (2011) Utility of T2-weighted short-tau inversion recovery (STIR) sequences in cardiac MRI: an overview of clinical applications in ischaemic and non-ischaemic heart disease. Radiol Med 116:32–46
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on clinical cardiology of the American Heart Association. Circulation 105:539–542
Abdel-Aty H, Zagrosek A, Schulz-Menger J et al (2004) Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation 109:2411–2416
Bondarenko O, Beek AM, Hofman MB et al (2005) Standardizing the definition of hyperenhancement in the quantitative assessment of infarct size and myocardial viability using delayed contrast-enhanced CMR. J Cardiovasc Magn Reson 7:481–485
Lima JA, Judd RM, Bazille A, Schulman SP, Atalar E, Zerhouni EA (1995) Regional heterogeneity of human myocardial infarcts demonstrated by contrast-enhanced MRI. Potential mechanisms. Circulation 92:1117–1125
de Waha S, Desch S, Eitel I et al (2010) Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long-term outcome after ST-elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J 31:2660–2668
Basso C, Corbetti F, Silva C et al (2007) Morphologic validation of reperfused hemorrhagic myocardial infarction by cardiovascular magnetic resonance. Am J Cardiol 100:1322–1327
Bhatia RS, Tu JV, Lee DS et al (2006) Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 355:260–269
Komajda M, Carson PE, Hetzel S et al (2011) Factors associated with outcome in heart failure with preserved ejection fraction: findings from the irbesartan in heart failure with preserved ejection fraction study (I-PRESERVE). Circ Heart Fail 4:27–35
Symons R, Pontone G, Schwitter J et al (2017) Long-term incremental prognostic value of cardiovascular magnetic resonance after ST-segment elevation myocardial infarction: a study of the collaborative registry on CMR in STEMI. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2017.05.023
Symons R, Masci PG, Francone M et al (2016) Impact of active smoking on myocardial infarction severity in reperfused ST-segment elevation myocardial infarction patients: the smoker’s paradox revisited. Eur Heart J 37:2756–2764
Bekkers SC, Backes WH, Kim RJ et al (2009) Detection and characteristics of microvascular obstruction in reperfused acute myocardial infarction using an optimized protocol for contrast-enhanced cardiovascular magnetic resonance imaging. Eur Radiol 19:2904–2912
Niccoli G, Burzotta F, Galiuto L, Crea F (2009) Myocardial no-reflow in humans. J Am Coll Cardiol 54:281–292
van Kranenburg M, Magro M, Thiele H et al (2014) Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging 7:930–939
Eitel I, de Waha S, Wöhrle J et al (2014) Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol 64:1217–1226
Ndrepepa G, Tiroch K, Fusaro M et al (2010) 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol 55:2383–2389
Ndrepepa G, Tiroch K, Keta D et al (2010) Predictive factors and impact of no reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Circ Cardiovasc Interv 3:27–33
Wu E, Ortiz JT, Tejedor P et al (2008) Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart 94:730–736
Arcari L, Cimino S, De Luca L et al (2015) Impact of heart rate on myocardial salvage in timely reperfused patients with ST-segment elevation myocardial infarction: new insights from cardiovascular magnetic resonance. PLoS One 10:e0145495
Di Bella G, Siciliano V, Aquaro GD et al (2013) Scar extent, left ventricular end-diastolic volume, and wall motion abnormalities identify high-risk patients with previous myocardial infarction: a multiparametric approach for prognostic stratification. Eur Heart J 34:104–111
Hamirani YS, Wong A, Kramer CM, Salerno M (2014) Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: a systematic review and meta-analysis. JACC Cardiovasc Imaging 7:940–952
Nijveldt R, Beek AM, Hirsch A et al (2008) Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol 52:181–189
Ørn S, Manhenke C, Greve OJ et al (2009) Microvascular obstruction is a major determinant of infarct healing and subsequent left ventricular remodelling following primary percutaneous coronary intervention. Eur Heart J 30:1978–1985
Francone M, Bucciarelli-Ducci C, Carbone I et al (2009) Impact of primary coronary angioplasty delay on myocardial salvage, infarct size, and microvascular damage in patients with ST-segment elevation myocardial infarction: insight from cardiovascular magnetic resonance. J Am Coll Cardiol 54:2145–2153
Steg PG, James SK, Atar D et al (2012) ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 33:2569–2619
Califf RM, Harrelson-Woodlief L, Topol EJ (1990) Left ventricular ejection fraction may not be useful as an end point of thrombolytic therapy comparative trials. Circulation 82:1847–1853
Cochet AA, Lorgis L, Lalande A et al (2009) Major prognostic impact of persistent microvascular obstruction as assessed by contrast-enhanced cardiac magnetic resonance in reperfused acute myocardial infarction. Eur Radiol 19:2117–2126
Baks T, van Geuns RJ, Biagini E et al (2006) Effects of primary angioplasty for acute myocardial infarction on early and late infarct size and left ventricular wall characteristics. J Am Coll Cardiol 47:40–44
Ndrepepa G, Mehilli J, Martinoff S, Schwaiger M, Schömig A, Kastrati A (2007) Evolution of left ventricular ejection fraction and its relationship to infarct size after acute myocardial infarction. J Am Coll Cardiol 50:149–156
Jaffe R, Dick A, Strauss BH (2010) Prevention and treatment of microvascular obstruction-related myocardial injury and coronary no-reflow following percutaneous coronary intervention: a systematic approach. JACC Cardiovasc Interv 3:695–704
Canali E, Masci P, Bogaert J et al (2012) Impact of gender differences on myocardial salvage and post-ischaemic left ventricular remodelling after primary coronary angioplasty: new insights from cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging 13:948–953
Blume U, Lockie T, Stehning C et al (2009) Interleaved T(1) and T(2) relaxation time mapping for cardiac applications. J Magn Reson Imaging 29:480–487
Carrick D, Haig C, Rauhalammi S et al (2015) Pathophysiology of LV remodeling in survivors of STEMI: inflammation, remote myocardium, and Prognosis. JACC Cardiovasc Imaging 8:779–789
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Marco Francone, MD PhD, EBCR.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all patients in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Rights and permissions
About this article
Cite this article
Galea, N., Dacquino, G.M., Ammendola, R.M. et al. Microvascular obstruction extent predicts major adverse cardiovascular events in patients with acute myocardial infarction and preserved ejection fraction. Eur Radiol 29, 2369–2377 (2019). https://doi.org/10.1007/s00330-018-5895-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5895-z