Abstract
Objectives
To determine whether image texture parameters analysed on pre-operative contrast-enhanced computed tomography (CT) can predict overall survival and recurrence-free survival in patients with hepatocellular carcinoma (HCC) treated by surgical resection.
Methods
We retrospectively included all patients operated for HCC who had liver contrast-enhanced CT within 3 months prior to treatment in our centre between 2010 and 2015. The following texture parameters were evaluated on late-arterial and portal-venous phases: mean grey-level, standard deviation, kurtosis, skewness and entropy. Measurements were made before and after spatial filtration at different anatomical scales (SSF) ranging from 2 (fine texture) to 6 (coarse texture). Lasso penalised Cox regression analyses were performed to identify independent predictors of overall survival and recurrence-free survival.
Results
Forty-seven patients were included. Median follow-up time was 345 days (interquartile range [IQR], 176–569). Nineteen patients had a recurrence at a median time of 190 days (IQR, 141–274) and 13 died at a median time of 274 days (IQR, 96–411). At arterial CT phase, kurtosis at SSF = 4 (hazard ratio [95% confidence interval] = 3.23 [1.35–7.71] p = 0.0084) was independent predictor of overall survival. At portal-venous phase, skewness without filtration (HR [CI 95%] = 353.44 [1.31–95102.23], p = 0.039), at SSF2 scale (HR [CI 95%] = 438.73 [2.44–78968.25], p = 0.022) and SSF3 (HR [CI 95%] = 14.43 [1.38–150.51], p = 0.026) were independently associated with overall survival. No textural feature was identified as predictor of recurrence-free survival.
Conclusions
In patients with resectable HCC, portal venous phase–derived CT skewness is significantly associated with overall survival and may potentially become a useful tool to select the best candidates for resection.
Key Points
• HCC heterogeneity as evaluated by texture analysis of contrast-enhanced CT images may predict overall survival in patients treated by surgical resection.
• Among texture parameters, skewness assessed at different anatomical scales at portal-venous phase CT is an independent predictor of overall survival after resection.
• In patients with HCC, CT texture analysis may have the potential to become a useful tool to select the best candidates for resection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) represents 70–90% of all cases of primary liver cancer, which account for about 745,000 deaths per year worldwide [1, 2]. Early diagnosis, which allows curative treatment such as surgical resection or liver transplantation, is made in only a minority of patients. However, in spite of patient selection, HCC recurrence rate after surgical resection remains high, reaching 60–70% at 5 years [3]. Identification of predictive factors of recurrence is crucial to select the best candidates for surgical resection. Preoperative evaluation is based mainly on macroscopic tumour extension and severity of underlying hepatopathy. There are other well-established predictive factors, but they are available only postoperatively at pathological examination of the surgical specimen; i.e. presence of satellite nodules, poor differentiation of the tumour and microvascular invasion (MVI) [4, 5].
In routine clinical setting, tumour imaging is currently used for diagnosis, staging and therapeutic response assessment but data are accumulating to indicate that image texture analysis, which reflects the degree of tumour heterogeneity, may provide predictive information on survival, response to treatment and risk of recurrence [6]. However, tumour heterogeneity is difficult to assess visually on computed tomography (CT) images because of photon noise and interobserver variability. CT texture analysis can overcome these drawbacks and quantify heterogeneity reflecting spatial differences in perfusion and proliferation inside the tumour [7, 8].
Tumour texture analysis has been reported as a useful tool for assessing survival in various types of cancers, such as oesophageal [9], colorectal [10], head and neck [11] and non-small cell lung [12] cancers, and for predicting response to treatment [13, 14]. However, most of these studies were single institution-based and conducted on small numbers of patients. They should be considered as exploratory studies. In liver diseases, the potential of texture analysis has been reported for quantifying hepatic fibrosis [15, 16], and predicting postoperative liver insufficiency [17]. In HCC patients, a recent study has reported the potential prognostic value of tumour texture features as analysed on preoperative non-contrast enhanced CT [18]. In addition, texture analysis could potentially be used as a decision-making strategy assistance, in order to choose between different types of treatment [19, 20]. The purpose of this retrospective study was to determine if texture analysis of preoperative contrast-enhanced CT images may predict overall survival (OS) and recurrence-free survival (RFS) in HCC patients treated by surgical resection.
Patients and methods
Patients
All consecutive patients who underwent a resection for HCC at the Reims University Hospital from 2010 to August 2015 were retrospectively selected from a computer database. Among them were included those who underwent liver contrast-enhanced CT scanner within 3 months before surgery or preoperative portal embolisation (PE) and/or transarterial chemoembolisation (TACE). Patients who had a metastatic disease, R2 resection, or surgery for a recurrent HCC were excluded. The following data were collected: age, gender, cause of underlying hepatopathy, Child-Pugh stage, serum alpha-fetoprotein (AFP), type of surgery (major or minor hepatectomy), preoperative treatment (PE, TACE), number of nodules, size of the largest nodule, degree of hepatic fibrosis, resection margins, satellite nodules, MVI and histological differentiation.
In accordance with French law, this retrospective study on medical records was authorised by the Commission Nationale Informatique et Libertés (authorisation number 1118523), allowing the computerised management of the medical data at the Reims University Hospital. The participants were informed of the possibility of using the information concerning them, for biomedical research purposes, and had a right of opposition.
Follow-up and endpoint
All patients had clinico-biological and radiological evaluation every 3 months during the first 2 years after surgery and then every 6 months. Survival time was defined as the time between surgery and death. Disease progression was defined as intrahepatic or extrahepatic recurrence. The primary endpoint was OS and secondary endpoint was RFS. For OS, patients alive at the end of follow-up were censored. For RFS, patients without recurrence at the end of follow-up were censored.
CT acquisition technique
All patients had a 64-section contrast-enhanced CT scanner (Discovery HD 750; GE Healthcare, Little Chalfont, UK). A volume of 2 mL/kg body weight of non-ionic contrast material (iomeprol, 350 mg iodine/mL) was injected through an intravenous antecubital cannula at a flow rate of 5 mL/s, followed by perfusion of 50 mL of saline solution at the same flow rate. Bolus tracking software (Smartprep; GE Healthcare,) was used and late-arterial and portal-venous phases were acquired respectively 20 s after the attenuation increase in abdominal aorta reached the predefined threshold of 80 HU and 70 s after contrast material administration, respectively. Acquisition parameters were as follows: tube voltage, 120 kVp; section collimation, 64 × 1.25 mm; helical pitch, 1.375; scan time per spiral, 0.7 s. Images were reconstructed with a section thickness of 2.5 mm by using 40% adaptive statistical reconstruction (ASiR; GE Healthcare). An automatic tube current modulation technique (Smart mA; GE Healthcare) was used. Mean effective tube current-time product was 336 mAs.
Texture analysis
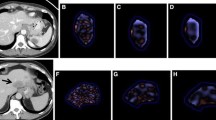
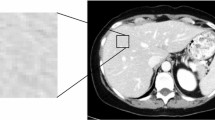
Tumour texture analysis was performed using the commercially available TexRAD software (TexRAD, Cambridge, UK), on both contrast-enhanced late-arterial and portal phase images of the latest pretreatment CT. Two different readers (reader 1 [S.M.], a radiologist with 8 years of experience, and reader 2 [L.B.D.], a gastroenterologist) independently selected the CT image demonstrating the largest cross-sectional area of the HCC lesion, in order to assess the inter-reader agreement. The region of interest (ROI) was manually delineated around the tumour. CT texture analysis was then performed in a two-step process including image filtration and statistical quantification. Spatial scale image filtration (SSF) selectively extracted features with different anatomic scales, corresponding to fine (SSF2, object radius of 2 mm), medium (SSF3-5, object radius of 3-5 mm) and coarse (SSF6, object radius of 6 mm) texture scales, by using a Laplacian of Gaussian special band pass filter (Fig. 1). The unfiltered images (SSF1) corresponded to conventional CT images. Tumour heterogeneity was then assessed by quantifying CT histogram parameters including mean grey-level intensity, standard deviation, kurtosis (measure of peakedness of the histogram distribution), skewness (symmetry of the histogram distribution) and by measuring entropy (irregularity of pixel intensities in space) [21]. In patients with several nodules, texture analysis was performed on the largest nodule. Texture analysis was performed using the results obtained by reader 1 (S.M.) as he was a radiologist (8-year experience in abdominal imaging), whereas reader 2 was a gastroenterologist.
Statistical analysis
Quantitative variables were described as means with standard deviation or medians with minimum-maximum and categorical variables as percentages. The inter-reader agreement was assessed using intraclass correlation coefficients (ICC) classified as follows: no agreement, 0–0.20; weak agreement, 0.21–0.40; moderate agreement, 0.41–0.60; good agreement, 0.61–0.80; excellent agreement, 0.81–1. Multivariate analysis was performed to identify independent predictors of OS and RFS among HCC texture parameters. To take into account the correlation between the estimates of each texture parameter from the different filter values as well as the small number of events compared with the number of included covariates, a multivariate L1 (least absolute shrinkage and selection operator [Lasso]) penalised Cox regression model was built in order to select texture parameters [22]. The amount of sparsity of the resulting model is related to the so-called regularisation parameter, which was determined via tenfold cross-validation. The Lasso method allows variables selection by shrinking down to zero coefficients weights for variables non-related to outcome. Moreover, the Lasso method can handle collinearity issues. Variables with non-zero coefficients were selected as potential predictors of outcome and integrated into a multivariable Cox regression analysis, with clinico-biological and histopathological variables as covariates, in order to estimate associated hazard ratios (HR) and their 95% confidence intervals (CI 95%).
Additionally, univariate Kaplan-Meier analyses were performed to identify associated optimal thresholds separating patients with good and poor prognosis, using non-parametric log-rank test. Significance level was set at p < 0.05. Analyses were performed using R software (v.3.0.1).
Results
Patient characteristics
Eighty-six HCC patients treated by surgical resection were identified from our computer database. Fifty-eight of them had undergone liver contrast-enhanced CT within 3 months before treatment. Eleven were excluded for the following reasons: surgery for a recurrent HCC (n = 4), R2 resection (n = 2), metastatic disease (n = 1) and other pathological types of tumour (n=4). Forty-seven patients were finally included: 37 men and 10 women (Fig. 2). Among them, 41 and 43 had available arterial phase CT and portal phase CT, respectively. Thirty-seven patients had both. The demographic and tumour characteristics are summarised in Table 1. Median follow-up time was 345 days (IQR, 176–569). Thirteen patients died during the follow-up (median time, 274 days; IQR, 96–411). Nineteen had a recurrence, local in 13 and metastatic in 9 (median time, 190 days; IQR, 141–274).
Texture analysis
Overall survival analysis
Lasso penalised Cox regression analysis of textural features at arterial phase CT
The Lasso penalised Cox regression analysis identified medium texture (SSF = 4) kurtosis as a potential predictor of OS (coefficient weight, 0.20). Among other variables, MVI (coefficient weight, 0.90), presence of satellite nodules (coefficient weight, 0.88) and number of nodules (coefficient weight, 0.19) were identified as potential predictors of OS. Multivariate Cox regression analysis confirmed kurtosis at SSF4 (HR [CI 95%] = 3.23 [1.35–7.71], p = 0.0084), MVI (HR [CI 95%] = 20.51 [2.88, 145.94], p = 0.0026), presence of satellite nodules (HR [CI 95%] = 15.70 [2.28–108.13], p = 0.0052) and number of nodules (HR [CI 95%] = 1.96 [1.21–3.18], p = 0.0064) as independent predictors of OS (Table 2).
When dichotomised at the optimal threshold identified in Kaplan-Meier univariate analysis, arterial medium texture kurtosis above 0.33 at SSF = 4 was significantly associated with lower survival time after HCC surgical resection (Supplementary Fig. S1).
Lasso penalised Cox regression analysis of textural features at portal phase CT
The Lasso penalised multivariate Cox regression analysis identified skewness as a potential predictor of OS without filtration (coefficient weight, 0.33), at fine (SSF = 2) texture scale (coefficient weight, 0.60), and at medium (SSF = 3) texture scale (coefficient weight, 0.13). Age (coefficient weight, -0.0085), MVI (coefficient weight, 1.17), number of nodules (coefficient weight, 0.45) and presence of satellite nodules (coefficient weight, 1.20) were also identified as potential predictors of OS. Multivariate Cox regression analysis confirmed skewness without filtration (HR [CI 95%] = 353.44 [1.31–95102.23], p = 0.039), at fine (SSF = 2) texture scale (HR [CI 95%] = 438.73 [2.44–78968.25], p = 0.022) and at medium (SSF = 3) texture scale (HR [CI 95%] = 14.43 [1.38–150.51], p = 0.026) as independent predictors of OS (Table 3).
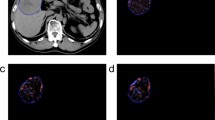
When dichotomised at the optimal threshold identified in Kaplan-Meier univariate analysis, portal skewness above -0.285, -0.005 and 0.01 without filtration, at fine (SSF = 2) and at medium (SSF = 3) texture scale respectively, were associated with lower survival time after HCC surgical resection (Fig. 3).
Kaplan-Meier survival curves according to skewness at (a) no filtration, (b) fine (SSF = 2), (c) medium (SSF = 3) texture filter, at portal phase CT. Patients with skewness above optimal thresholds: -0.285 (log-rank test, p = 0.045), -0.005 (log-rank test, p = 0.0001) and 0.01 (log-rank test, p = 0.01) at no filtration, fine SSF = 2 and medium SSF = 3 texture filter respectively, show significantly poorer survival
Recurrence-free survival analysis
Lasso penalised Cox regression analysis of textural features at arterial phase CT
No arterial textural feature was identified as a potential predictor of RFS by using the Lasso penalised Cox regression analysis. Among other variables, the presence of satellite nodules and the MVI were identified as predictors of RFS.
Lasso penalised Cox regression analysis of textural features at portal phase CT
No portal textural feature was identified as a potential predictor of RFS by using the Lasso penalised Cox regression analysis. Among other variables, the presence of satellite nodules and the degree of differentiation were identified as predictors of RFS.
Inter-reader agreement
The texture features calculated from two sets of ROIs showed an ICC value of 0.34–0.81 at arterial phase CT and 0.63–0.94 at portal phase CT. In particular, inter-reader agreement was weak for skewness and kurtosis assessment at arterial phase (ICC values, 0.34 and 0.44 respectively), whereas it was good at portal phase (ICC values, 0.63 and 0.67 respectively).
Discussion
Our study suggests that HCC heterogeneity as evaluated by texture analysis of contrast-enhanced CT images is an independent predictor of OS in patients treated by surgical resection. After adjusting for clinico-biological variables and taking into account collinearity between texture parameters estimated at different spatial scales, pretreatment HCC skewness, derived from the pixel distribution histogram, was significantly associated with OS when analysed at the portal phase, without filtration and at fine and medium texture scales. At the arterial phase, HCC kurtosis was an independent predictor of OS, with medium texture scale. As inter-reader agreement for measurements of texture features was weak at arterial phase and good to excellent at portal phase, texture analysis of portal enhanced CT images appears as the most appropriate method for predicting OS preoperatively in HCC patients.
Tumour biological heterogeneity is a well-known property of malignancy [6]. The tumour phenotypic variability is a result of genetic/epigenetic heterogeneity and morphological plasticity [6]. Morphological plasticity refers to phenotypic changes due to interactions between cancer cells and their microenvironment in particular the neoformed vascular network. The heterogeneity of blood supply inside the tumour translates in areas of hypoxia which may play a major role in the selection of more aggressive tumour clones [7, 8]. Whereas preoperative biopsy is subject to a sampling effect, tumour texture image analysis can provide quantitative information on global tumour heterogeneity [8]. A correlation between CT texture parameters and histopathological markers of hypoxia and/or angiogenesis has been reported in patients with non-small-cell lung cancer [23] and soft tissue sarcoma [24].
In our study, preoperative high values of skewness and kurtosis at different texture scales were able to identify HCC patients who were less likely to benefit from surgical resection of their tumour. The hypothesis of an association between histogram skewness and OS has been recently evaluated in several types of cancer. In patients with locally advanced squamous cell carcinoma of the head and neck treated with chemotherapy, higher values of skewness as measured on pretreatment contrast-enhanced CT images at fine texture scale were associated with reduced OS [11]. Similarly, higher skewness values were shown to be independent predictors for decreased 5-year OS in colorectal cancer [10] and decreased 3-year OS in non-small cell lung cancer treated by chemoradiotherapy [25]. In addition, in patients with pancreatic neuroendocrine tumours, higher skewness measured on arterial phase of CT scan images has been shown to independently predict histopathological grade 2/3, which is associated with poor prognosis [26].
In the filtration-histogram approach for texture analysis, skewness reflects the measure of the asymmetry of the histogram corresponding to the grey-level values within a predefined ROI. A predominantly bright texture leads to a positive skewness, whereas predominantly dark texture generates negative skewness [21].
Other studies have reported an association between histogram kurtosis and OS. In a pilot study performed in patients with Hodgkin’s and non-Hodgkin’s lymphomas, pretreatment higher kurtosis values measured on non-contrast-enhanced CT images at medium texture scale were associated with lower RFS and provided prognostic information complementary to positron emission tomography [27]. In non-small-cell lung cancer, tumour CT-based density histogram analysis showed that higher kurtosis value at medium texture scale seemed to increase the risk for nodal metastases [28].
In the filtration-histogram approach for texture analysis, kurtosis is a measure of the tailedness of the histogram. Kurtosis value is inversely related to the number of objects highlighted (whether bright or dark) and its value increased by intensity variations in highlighted objects [21]. In our study, the reason for the greater kurtosis value at arterial phase in HCC with poorer survival can only be speculative, as biological correlates remain hypothetic due to the lack of data. We suggest that hyperenhancement at the arterial phase in a highly vascularised tumour may result in mostly similar high-density values translating in a steep peak in the density histogram. In accordance with this hypothesis and our results, it has been reported that hypervascular HCC as diagnosed by contrast-enhanced CT scan or ultrasonography prior to radiofrequency ablation had significantly more risk of recurrence than less hypervascular or non-hypervascular HCC [29, 30].
In this study, multivariate analysis of HCC texture features was adjusted for clinico-biological variables and the independent predictive values of MVI and satellite nodules for OS and RFS and of histological differentiation for RFS were confirmed as previously reported [4, 5].
Our results confirm the potential of tumour texture analysis as a prognostic tool in patients with resectable HCC as has been recently reported [18]. However, this latter study has been performed on non-contrast-enhanced CT images which may lead to less reproducible delineation of the ROI. Although questionable, inter-observer reproducibility was not evaluated in this study [18]. In our work, a good reproducibility was demonstrated when texture analysis was performed on portal phase contrast-enhanced CT. Our study has several limitations. First, it was a retrospective monocentric study with a relatively small number of patients. This should be considered as an exploratory study and more comprehensive studies on larger number of patients are needed to validate our results. Second, CT texture analysis was performed in the largest cross-sectional area of HCC and not on the whole tumour, which is probably more representative of the tumour heterogeneity [31]. Third, inter-observer agreement was weak for measurements of texture features at arterial phase. This may be due to the fact that some advanced HCCs, especially infiltrative HCCs, may appear non-hypervascular [32], which may render more difficult and less reproductible the delineation of the ROI at arterial phase. Therefore, for potential clinical application, texture analysis should better be performed on portal enhanced CT images. Fourth, as an automatic tube current modulation technique was used, effective tube current-time product values and also image noise varied from a patient to another. However, it has previously been shown that texture parameters are relatively insensitive to the different tube voltages and currents used, and that variations in tube current were found to cause less effect on texture parameters than variations in tube voltage [33]. Fifth, the influence of image slice thickness on the quality of texture parameters was not evaluated. In our retrospective series, all abdominal CT images were reconstructed with a section thickness of 2.5 mm, which is the usual procedure in the clinical routine of our institution. It has been previously reported that slice thickness did not significantly influence the stability of texture parameters derived from liver CT images [34]. Finally, our results were obtained using a proprietary texture analysis software, the most widely used in the recent literature, and we cannot extend our conclusions to other software programs. In conclusion, our study suggests that in patients with resectable HCC, pretreatment portal phase-derived CT histogram skewness is significantly associated with OS and may have the potential to become a useful tool to select the best candidates for resection.
Abbreviations
- AFP:
-
Alpha-fetoprotein
- HCC:
-
Hepatocellular carcinoma
- MVI:
-
Microvascular invasion
- NASH:
-
Non-alcoholic steatohepatitis
- OS:
-
Overall survival
- PE:
-
Portal embolisation
- RFS:
-
Recurrence-free survival
- SSF:
-
Spatial scale image filtration
- TACE:
-
Transcatheter arterial chemoembolisation
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Lim KC, Chow PK, Allen JC, Siddiqui FJ, Chan ES, Tan SB (2012) Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg 99:1622–1629
Kluger MD, Salceda JA, Laurent A et al (2015) Liver resection for hepatocellular carcinoma in 313 Western patients: tumor biology and underlying liver rather than tumor size drive prognosis. J Hepatol 62:1131–1140
Imamura H, Matsuyama Y, Tanaka E et al (2003) Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 38:200–207
Marusyk A, Almendro V, Polyak K (2012) Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer 12:323–334
Davnall F, Yip CS, Ljungqvist G et al (2012) Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3:573–589
Ganeshan B, Miles KA (2013) Quantifying tumour heterogeneity with CT. Cancer Imaging 13:140–149
Ganeshan B, Skogen K, Pressney I, Coutroubis D, Miles K (2012) Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: preliminary evidence of an association with tumour metabolism, stage, and survival. Clin Radiol 67:157–164
Ng F, Ganeshan B, Kozarski R, Miles KA, Goh V (2013) Assessment of primary colorectal cancer heterogeneity by using whole-tumor texture analysis: contrast-enhanced CT texture as a biomarker of 5-year survival. Radiology 266:177–184
Zhang H, Graham CM, Elci O et al (2013) Locally advanced squamous cell carcinoma of the head and neck: CT texture and histogram analysis allow independent prediction of overall survival in patients treated with induction chemotherapy. Radiology 269:801–809
Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K (2012) Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol 22:796–802
Ravanelli M, Farina D, Morassi M et al (2013) Texture analysis of advanced non-small cell lung cancer (NSCLC) on contrast-enhanced computed tomography: prediction of the response to the first-line chemotherapy. Eur Radiol 23:3450–3455
Tian F, Hayano K, Kambadakone AR, Sahani DV (2015) Response assessment to neoadjuvant therapy in soft tissue sarcomas: using CT texture analysis in comparison to tumor size, density, and perfusion. Abdom Imaging 40:1705–1712
Barry B, Buch K, Soto JA, Jara H, Nakhmani A, Anderson SW (2014) Quantifying liver fibrosis through the application of texture analysis to diffusion weighted imaging. Magn Reson Imaging 32:84–90
Lubner MG, Malecki K, Kloke J, Ganeshan B, Pickhardt PJ (2017) Texture analysis of the liver at MDCT for assessing hepatic fibrosis. Abdom Radiol (NY) 42:2069–2078
Simpson AL, Adams LB, Allen PJ et al (2015) Texture analysis of preoperative CT images for prediction of postoperative hepatic insufficiency: a preliminary study. J Am Coll Surg 220:339–346
Kiryu S, Akai H, Nojima M et al (2017) Impact of hepatocellular carcinoma heterogeneity on computed tomography as a prognostic indicator. Sci Rep 7:12689
Li M, Fu S, Zhu Y et al (2016) Computed tomography texture analysis to facilitate therapeutic decision making in hepatocellular carcinoma. Oncotarget 7:13248–13259
Fu S, Chen S, Liang C et al (2017) Texture analysis of intermediate-advanced hepatocellular carcinoma: prognosis and patients’ selection of transcatheter arterial chemoembolization and sorafenib. Oncotarget 8:37855–33765
Miles KA, Ganeshan B, Hayball MP (2013) CT texture analysis using the filtration-histogram method: what do the measurements mean? Cancer Imaging 13:400–406
Simon N, Friedman J, Hastie T, Tibshirani R (2011) Regularization paths for Cox's proportional hazards model via coordinate descent. J Stat Softw 39:1–13
Ganeshan B, Goh V, Mandeville HC, Ng QS, Hoskin PJ, Miles KA (2013) Non-small cell lung cancer: histopathologic correlates for texture parameters at CT. Radiology 266:326–336
Hayano K, Tian F, Kambadakone AR et al (2015) Texture analysis of non-contrast-enhanced computed tomography for assessing angiogenesis and survival of soft tissue sarcoma. J Comput Assist Tomogr 39:607–612
Ahn SY, Park CM, Park SJ et al (2015) Prognostic value of computed tomography texture features in non-small cell lung cancers treated with definitive concomitant chemoradiotherapy. Invest Radiol 50:719–725
Choi TW, Kim JH, Yu MH, Park SJ, Han JK (2018) Pancreatic neuroendocrine tumor: prediction of the tumor grade using CT findings and computerized texture analysis. Acta Radiol 59:383–392
Ganeshan B, Miles KA, Babikir S et al (2017) CT-based texture analysis potentially provides prognostic information complementary to interim FDG-PET for patients with Hodgkin’s and aggressive non-Hodgkin’s lymphomas. Eur Radiol 27:1012–1020
Craigie M, Squires J, Miles K (2017) Can CT measures of tumour heterogeneity stratify risk for nodal metastasis in patients with non-small cell lung cancer? Clin Radiol 72:899.e1–899.e7
Park Y, Kim YS, Rhim H, Lim HK, Choi D, Lee WJ (2009) Arterial enhancement of hepatocellular carcinoma before radiofrequency ablation as a predictor of postablation local tumor progression. AJR Am J Roentgenol 193:757–763
Ishii T, Numata K, Hao Y et al (2017) Evaluation of hepatocellular carcinoma tumor vascularity using contrast-enhanced ultrasonography as a predictor for local recurrence following radiofrequency ablation. Eur J Radiol 89:234–241
Ng F, Kozarski R, Ganeshan B, Goh V (2013) Assessment of tumor heterogeneity by CT texture analysis: can the largest cross-sectional area be used as an alternative to whole tumor analysis? Eur J Radiol 82:342–348
Choi JY, Lee JM, Sirlin CB (2014) CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology 272(3):635–654
Miles KA, Ganeshan B, Griffiths MR, Young RC, Chatwin CR (2009) Colorectal cancer: texture analysis of portal phase hepatic CT images as a potential marker of survival. Radiology 250:444–452
Duda D, Kretowski M, Bezy-Wendling J (2013) Effect of slice thickness on texture-based classification of liver dynamic CT scans. In: Saeed K, Chaki R, Cortesi A, Wierzchoń S (eds) Computer information systems and industrial management. CSIM 2013. Lecture Notes in Computer Science, vol 8104. Springer, Berlin Heidelberg
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. G Thiéfin, Service d’Hépato-Gastroentérologie et de Cancérologie Digestive, Centre Hospitalier Universitaire de Reims, France
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Two of the authors (A. Tenenhaus, PhD and S. Mulé, MD, PhD) have statistical expertise.
Informed consent
Written informed consent was not required for this study. In accordance with French law, this retrospective study on medical records has been authorised by the Commission Nationale Informatique et Libertés (authorisation number 111 85 23), allowing the computerised management of the medical data at the Reims University Hospital. The participants were informed of the possibility of using the information concerning them, for biomedical research purposes, and had a right of opposition.
Ethical approval
Institutional Review Board approval was not required. In accordance with French law, this retrospective study on medical records has been authorised by the Commission Nationale Informatique et Libertés (authorisation number 111 85 23), allowing the computerised management of the medical data at the Reims University Hospital. The participants were informed of the possibility of using the information concerning them, for biomedical research purposes, and had a right of opposition.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Electronic supplementary material
ESM 1
(DOCX 79 kb)
Rights and permissions
About this article
Cite this article
Brenet Defour, L., Mulé, S., Tenenhaus, A. et al. Hepatocellular carcinoma: CT texture analysis as a predictor of survival after surgical resection. Eur Radiol 29, 1231–1239 (2019). https://doi.org/10.1007/s00330-018-5679-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5679-5