Abstract
Purpose
The purpose of the study was to determine if CT texture features of untreated hepatic metastatic colorectal cancer (CRC) relate to pathologic features and clinical outcomes.
Methods
Tumor texture analysis was performed on single hepatic metastatic lesions on pre-treatment contrast-enhanced CT scans in 77 pts (mean age 58, 34F/43M) using a novel tool. Measures of heterogeneity, including entropy, kurtosis, skewness, mean, mean positive pixels (MPP), and standard deviation (SD) of pixel distribution histogram were derived with filter values corresponding to fine (spatial scaling factor (ssf) 2), medium (ssf 3, 4), and coarse textures (ssf 5, 6). Texture parameters were correlated with tumor grade, baseline serum CEA, and KRAS mutation status. Overall survival was also correlated using Cox proportional hazards models. Single-slice 2D vs. whole-tumor volumetric 3D texture analysis was compared in a subcohort of 20 patients.
Results
Entropy, MPP, and SD at medium filtration levels were significantly associated with tumor grade (MPP ssf 3 P = 0.002, SD ssf 3 P = 0.004, entropy ssf 4 P = 0.007). Skewness was negatively associated KRAS mutation (P = 0.02). Entropy at coarse filtration levels was associated with survival (Hazard ratio (HR) for death 0.65, 95% CI 0.44–0.95, P = 0.03). Texture results for 2D and 3D analysis were similar.
Conclusions
CT texture features, particularly entropy, MPP, and SD, are significantly associated with tumor grade in untreated CRC liver metastases. Tumor entropy at coarse filters correlates with overall survival. Single-slice 2D texture analysis appears to be adequate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States and one of the commonest cancers in the Western world [1–3]. Approximately 25% of patients present with metastatic disease at initial diagnosis and almost 50% of patients with CRC will develop metastases contributing to the high mortality rates reported for this cancer. However, a variety of potential treatment strategies are available in the setting of metastatic disease, and as these treatments continue to evolve and improve, patient outcomes continue to improve as well, with median survival now reaching nearly 30 months in some clinical trials. However, when discussing prognosis and treatment, better methods of tumor characterization and risk stratification are still needed and this has been highlighted as an important area of research by the American Society of Clinical Oncology [4].
Tumor heterogeneity on cross-sectional imaging is a recognized feature of malignancy that may represent regional differences in cell density, necrosis, and hemorrhage [5]. Texture analysis provides an assessment of tumor heterogeneity at imaging by analyzing the distribution and relationship of pixel or voxel-gray levels in the image [6]. Although direct pathologic correlates have not been determined, there is a suggestion that texture provides information on the tumor microenvironment [7–9]. CT tumor texture analysis has shown promise in a variety of tumor types in predicting pathologic features, overall survival, and response to therapy [5, 10–13]. Specifically in CRC, Ng et al. demonstrated that CT texture features of the primary tumor were independent predictors of 5 year survival [5] and Miles et al. demonstrated that whole liver CT texture analysis in patients with CRC during surveillance imaging may also be a predictor of survival [14]. Similarly, CT texture features taken in concert with other imaging features including 18F-FDG SUV max and CT perfusion characteristics have been found to correlate with Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation status and hypoxic or proliferative phenotypes [11]. However, there has been minimal investigation to date of the role of CT texture analysis in metastatic CRC. The purpose of this study was to correlate CT texture features of pre-treatment hepatic metastatic disease with pathologic features and clinical outcomes.
Materials and methods
This study was IRB-approved and HIPAA-compliant with a waiver of the need for informed consent.
Patient population
Contrast-enhanced CT studies from 77 patients (34F/43M, mean age 58 ± 11.4 years) with hepatic metastatic CRC were retrospectively reviewed. Patients were selected in a consecutive manner from a database of patients receiving chemotherapy for metastatic CRC at our institution between 2003 and 2013. Those without imaging around the time of diagnosis and treatment and those without hepatic metastatic disease were excluded. All patients in the cohort had surgical pathology data either from resection of their primary tumor, biopsy of metastatic disease, or metastasectomy. Pathologic data were obtained from any or all of these sources, although the primary tumor usually had the most detailed information about tumor grade and KRAS mutation status. Tumor grade, KRAS status if available, baseline serum carcinoembryonic antigen (CEA), and performance status were collected. Clinical and imaging follow-up was obtained on all patients through the picture archiving and communication system (PACS) and electronic medical record. Overall survival was calculated from the date of diagnosis to date of last follow-up or date of death. Patient status (alive vs. deceased) at last clinical/imaging follow-up was also recorded.
CT images and analysis
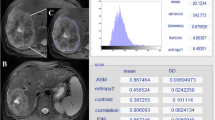
CT studies from the date closest to diagnosis of hepatic metastatic disease were selected for analysis. All studies were obtained within a mean of 25 days of patients beginning treatment for metastatic disease (median 17 days, range 0–367 days). All measurements were performed on contrast-enhanced portal venous phase exams. Given the time span, and referral of patients from different institutions, scans were obtained on a variety of scanners, but all with similar FOV and slice thickness (always 5 mm or less). Although the presence or absence of contrast does impact texture measures (and these studies were all in the portal venous phase), at least one study using CT texture analysis has suggested that the technique is resistant to other differences in image acquisition techniques [14]. Following review of the CT images on PACS (McKesson, San Francisco, CA), a single slice through the largest diameter of a single-index hepatic lesion was selected. All images were reviewed and appropriate slices were selected by an abdominal imaging attending radiologist (MGL 10 years experience). Single-slice images were then sent to a commercially available texture analysis program (TexRAD Ltd, Somerset, UK). Using the software, a region of interest was manually drawn around the outer margin tumor by a single reader (NS, medical student) under the supervision of the abdominal staff (Fig. 1). The software uses a filtration–histogram method in which an initial filtration step is performed that highlights image features of a specified size followed by histogram analysis of the filtered image. The initial filtration step uses a Laplacian of Gaussian (LoG) spatial band-pass filter to selectively extract features of different sizes and intensity variation [15]. This produces a series of derived images that show features ranging from fine (spatial scaling factor (ssf) 2), approximately 4 pixels in width, object radius approximately 2 mm) to coarse (ssf 5–6, approximately 12 pixels in width, object radius approximately 6 mm) texture (Fig. 1) [9, 16]. The software output includes standard histogram characteristics including mean pixel attenuation (average attenuation value), standard deviation of the pixel histogram (SD, variation or dispersion from the mean value), entropy (irregularity or complexity of pixel intensity), mean of the positive pixels (MPP, mean attenuation of pixels >0), skewness (asymmetry) of the pixel histogram, and kurtosis (peakness or pointedness) of pixel histogram at each spatial scaling factor. However, the filters are not independent, and texture features may track across filters to a certain extent. These values were recorded for each study and subsequently analyzed. Texture features were correlated with pathologic and clinical data including tumor grade, baseline CEA, KRAS mutation status, and overall survival.
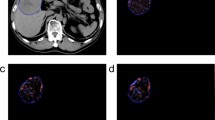
Texture analysis in two different patients with hepatic metastatic CRC. Contrast-enhanced CT image in the portal venous phase (A) with ROI drawn around an index metastatic lesion in the right hepatic lobe. Color texture output for this tumor is seen at fine (B), medium (C), and coarse (D) filter levels. Similar analysis is done on a CT image in a second pt (E arrow), with fine (F), medium (G), and coarse (H) texture output. Numeric outputs for a variety of texture parameter accompany the visual output (not shown).
A prior study by Ng et al. has suggested that single-slice assessment is sufficient, although volumetric tumor analysis may be more representative of tumor heterogeneity [17]. Nonetheless, on a subset of 20 patients in this cohort, both single-slice 2D and whole-lesion volumetric 3D assessments were obtained using the same lesions on Matlab software (Matlab R2011b, The Mathworks, Inc., Natick, MA) and compared.
Statistical analysis
Continuous variables were summarized with descriptive statistics. Frequency counts and percentages were obtained for categorical variables. To assess association between CT texture features and clinical data or outcomes, different methods were used depending on the type of response being considered. For association of texture parameters with numerical and ordinal variables (values such as tumor grade), ordinary least squares or simple linear regression analysis was utilized. Analysis was performed for each of the six parameters at each filter level, for a single phase of contrast (portal venous). Binary variables (such as KRAS mutation status) were analyzed using logistic regression. Odds ratios (OR) are reported with 95% confidence intervals (CIs). For survival or time-to-event responses, Cox proportional hazards regressions were fitted; hazard ratios and 95% CIs were obtained. Given that six main texture parameters were included, a Bonferroni correction was performed by dividing 0.05/6, using a P value of 0.0083 for statistical significance. Although each texture parameter was evaluated at multiple spatial scaling factors, these are not thought to be independent and therefore were not accounted for in our multiple testing correction.
Bland–Altman 95% limits of agreement and bias were computed to assess the agreement between 2D single slice and 3D volumetric texture measurements.
All statistical graphics and computations were obtained in R 3.1.0 (R Development Core Team 2014) [18].
Results
The demographic and tumor characteristics of the patient cohort are summarized in Table 1. All hepatic index tumors analyzed with texture tools were metastatic colorectal adenocarcinoma. There was a spectrum of tumor grade with 24 grade I tumors, 31 grade II tumors, and 10 grade III tumors. Twelve tumors did not have a tumor grade reported. KRAS mutation status was available in 36 patients (47%) with 15 mutated and 21 wild-type. Mean index lesion size was 2.9 cm ± 1.8 (range 0.9–9.5 cm). Mean overall survival was calculated at 19.9 months, median 14.9 months. 25 patients (32%) were alive at the date of last follow-up.
Entropy, mean of the positive pixels (MPP), and standard deviation of the pixel histogram (SD) at medium filter levels (ssf 3, 4) all showed negative association with tumor grade (eg., MPP ssf 3 intercept 2.2, slope −0.03, P = 0.002) (Fig. 2). No significant association was seen between baseline serum CEA and texture features, although similar features (MPP, entropy, SD) showed a trend toward significance. Although KRAS status was only available on about half the patients, there did seem to be a negative trend between KRAS mutation and skewness at medium texture filters (ssf 4, OR 0.15, 95% CI 0.025–0.92, P value 0.04). A trend toward significance was seen with kurtosis at coarse filters as well (ssf 6, OR 0.095, 0.008–1.1, P = 0.058) (Fig. 3; Table 2). Although MPP has been described as part of a multifunctional imaging signature for KRAS mutation previously [11], there was not a statistically significant association between KRAS mutation and MPP seen here (MPP ssf 5, OR 0.9, 0.79–1.0, P = 0.16).
Comparison of tumor grade and CT texture features. Regression plot comparing SD and tumor grade at ssf 3 (A) shows a statistically significant inverse association (P = 0.004). Similar plots are seen for tumor grade vs. entropy at ssf 4 (B) and MPP at ssf 3 (C), with significant inverse associations (P = 0.007 and P = 0.002, respectively).
Logistic regression plot comparing presence of KRAS mutation with skewness at SSF 4 (A) demonstrates an inverse association with an OR of 0.15 (95% CI 0.25–0.92), P = 0.04. Second plot comparing presence of KRAS mutation with kurtosis at ssf 6 (B) with similar relationship seen (OR 0.09, CI 0.008–1.1, P = 0.06).
Entropy at coarse filter levels (ssf 5) was negatively associated with survival (HR 0.65, 95% CI 0.44–0.95, P = 0.02), although with the Bonferroni correction, this association may not be statistically significant.
When comparing 2D vs. 3D assessment of lesions in a subset of 20 patients, overall results were fairly similar in Bland–Altman analysis. For example, for entropy, the limits of agreement were −0.0182, 0.029, bias 0.005 (Fig. 4).
Discussion
Multiple tumor types demonstrate association between texture features at imaging and pathologic subtypes or even genetic profiles (e.g., KRAS, EGFR) of the tumor [11, 19, 20]. In addition, texture features in a variety of tumor types have been shown to correlate with clinical outcomes such as response to therapy and survival [5, 10, 12, 13, 21–24]. Our data show that there is association of CT texture features with pathologic features and clinical outcomes in hepatic metastatic CRC. This data suggest that tumors that are more homogeneous (less entropy, smaller SD, higher in attenuation/higher mean of positive pixels) are potentially more aggressive in their biology (higher tumor grade, poorer overall survival). In a study in which Ng et al. looked at 57 primary colorectal tumors and assessed texture features, similar trends were seen. For example, they found that patients with lower entropy (less than a threshold of 7.9) and lower standard deviation had a poorer long-term prognosis [5]. This is similar to our results, which show an inverse association with tumor grade and entropy, MPP, and SD (less tumor heterogeneity correlates with a higher grade tumor, and in Goh’s study, with a poorer prognosis). Similarly, with our data, entropy was inversely associated with survival (increasing entropy had a HR for death <1, P = 0.02). This inverse correlation could possibly relate to higher cellular density and higher cellular vascular permeability as posited by Ng et al. [5].
In prior studies, tumor heterogeneity at medium and coarse texture filter levels correlated with hypoxia and angiogenesis in primary non-small cell lung cancer and CRC (negative correlation between SD and angiogenesis in CRC KRAS wild-type, skewness positively associated with angiogenesis in KRAS mutated colon cancer) [8, 9, 25]. Prior studies with lung cancer demonstrated that uniformity (similar to entropy) negatively correlated with hypoxia and MPP negatively correlated with angiogenesis [8]. It is possible that these features (increased hypoxia, increased angiogenesis and in turn decreased entropy and MPP) may be also be associated with higher tumor grade and be reflective of the inverse associations between entropy and MPP and tumor grade, survival seen here.
Goh et al. also found that skewness and kurtosis in the primary tumor was of prognostic value, and we found that both showed a trend toward association with KRAS mutation status, which can impact chemosensitivity (particularly to agents that target the epidermal growth factor receptor, EGFR), and ultimately, clinical outcome [26, 27]. Miles et al. looked at a multifunctional imaging signature for KRAS mutations in CRC, which included 18F-FDG uptake, CT texture, and perfusion [11]. Although their model did include CT texture features, MPP was the main texture feature used in the molecular signature, which was not significantly associated with KRAS status in our study (it was associated with tumor grade). However, Miles et al. was performing CT texture analysis on non-contrast CT images obtained at the time of PET, and using coarse texture filters, which may reflect different features than post-contrast imaging and medium filtration as seen in our study, which may be confounding. Although imaging features will likely never supplant true pathologic analysis, they can be a useful adjunct given the inherent heterogeneity of many tumors, particularly with respect to features like KRAS mutation in CRCs. KRAS status is usually determined via histologic sampling of tumor tissue; however, this histologic evaluation can be limited in that there is intratumoral heterogeneity and also heterogeneity between tumor sites in KRAS status in up to 10% of cases [28–30].
Biomarker analysis has become a critical tool in decision making for treating CRC. Mutational analyses change prognosis and therapy options. The recently presented CALGB 80405 study demonstrated the importance of knowing the mutational profile of CRC to select the ideal first-line chemotherapy, and its effects on survival [26, 27]. Imaging analysis of favorable and unfavorable tumor characteristics offers a powerful tool for real-time reassessment of tumor biology to help with therapeutic decision making. As an example, a patient with metastatic disease displaying concerning pathologic and imaging (texture) features could be placed into clinical trials specifically tailored to more aggressive phenotypes and put on more intensive follow-up imaging surveillance. In addition, for patients with oligometastic disease, pathologic and imaging features could impact decisions about surgical metastasectomy vs. other emerging locoregional techniques such as ablation, and may change considerations regarding peri-operative chemotherapy post procedure [31].
Texture analysis is easy to retrospectively perform on data that is routinely acquired during standard CT imaging protocols. It seems robust and not significantly impacted by technical parameters of CT scanning in some prior studies [14]. This helps to maximize the information that can be derived from these studies that are already being obtained as part of routine clinical care.
There are limitations to this study. Only a single hepatic metastatic index lesion was selected for evaluation in each patient, and only a single slice of that lesion was assessed. This may not adequately represent what may be heterogeneous disease characteristics, although comparison of 2D vs. 3D measurements of single lesions showed fairly comparable results as also seen in prior studies [17]. Only patients with hepatic metastatic disease were assessed. Our reasoning is that this is the most common site of metastatic disease in colon cancer; however, it is possible different disease sites may show different features and this may warrant additional study. The factors that impact CT texture measurements are not well delineated, although all studies were obtained with intravenous contrast and in the portal venous phase to try to minimize differences in contrast technique. Filtration was also used to try to limit the impact of differences in image noise. However, there was heterogeneity in the CT acquisition parameters/technique given the timespan and the scanning of patients performed at local sites. Further, biologic and pathologic correlates for texture features at different filter levels are still being delineated and many still need to be confirmed in histologically validated studies. Finally, with texture analysis, a large number of features are generated, which leads to testing of a large number of hypotheses that could lead to an increase in Type I error, however, we attempted to offset this using a Bonferroni correction, which is known to be conservative.
In summary, certain CT texture features of hepatic metastatic CRC (entropy, MPP, SD) are significantly associated with tumor grade and show a trend toward association with overall survival. Further investigation into the underlying causes and clinical implications is warranted.
References
Sargent D, Shi Q, Yothers G, et al. (2011) Two or three year disease-free survival (DFS) as a primary end-point in stage III adjuvant colon cancer trials with fluoropyrimidines with or without oxaliplatin or irinotecan: data from 12,676 patients from MOSAIC, X-ACT, PETACC-3, C-06, C-07 and C89803. Eur J Cancer 47(7):990–996
Van Cutsem E, Cervantes A, Nordlinger B, Arnold D (2014) Metastatic colorectal cancer: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(Suppl 3):iii1–iii9
Ferlay J, Soerjomataram I, Dikshit R, et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Desch CE, Benson AB 3rd, Somerfield MR, et al. (2005) Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol 23(33):8512–8519
Ng F, Ganeshan B, Kozarski R, Miles KA, Goh V (2013) Assessment of primary colorectal cancer heterogeneity by using whole-tumor texture analysis: contrast-enhanced CT texture as a biomarker of 5-year survival. Radiology 266(1):177–184
Ganeshan B, Miles KA (2013) Quantifying tumour heterogeneity with CT. Cancer Imaging 13:140–149
Ganeshan B, Abaleke S, Young RC, Chatwin CR, Miles KA (2010) Texture analysis of non-small cell lung cancer on unenhanced computed tomography: initial evidence for a relationship with tumour glucose metabolism and stage. Cancer Imaging 10:137–143
Ganeshan B, Goh V, Mandeville HC, et al. (2013) Non-small cell lung cancer: histopathologic correlates for texture parameters at CT. Radiology 266(1):326–336
Miles KA, Ganeshan B, Hayball MP (2013) CT texture analysis using the filtration-histogram method: what do the measurements mean? Cancer Imaging 13(3):400–406
Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K (2012) Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol 22(4):796–802
Miles KA, Ganeshan B, Rodriguez-Justo M, et al. (2014) Multifunctional imaging signature for V-KI-RAS2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations in colorectal cancer. J Nucl Med 55(3):386–391
Yip C, Landau D, Kozarski R, et al. (2014) Primary esophageal cancer: heterogeneity as potential prognostic biomarker in patients treated with definitive chemotherapy and radiation therapy. Radiology 270(1):141–148
Zhang H, Graham CM, Elci O, et al. (2013) Locally advanced squamous cell carcinoma of the head and neck: CT texture and histogram analysis allow independent prediction of overall survival in patients treated with induction chemotherapy. Radiology 269(3):801–809
Miles KA, Ganeshan B, Griffiths MR, Young RC, Chatwin CR (2009) Colorectal cancer: texture analysis of portal phase hepatic CT images as a potential marker of survival. Radiology 250(2):444–452
Goh V, Ganeshan B, Nathan P, et al. (2011) Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology 261(1):165–171
Ganeshan B, Miles KA, Young RC, Chatwin CR (2009) Texture analysis in non-contrast enhanced CT: impact of malignancy on texture in apparently disease-free areas of the liver. Eur J Radiol 70(1):101–110
Ng F, Kozarski R, Ganeshan B, Goh V (2013) Assessment of tumor heterogeneity by CT texture analysis: can the largest cross-sectional area be used as an alternative to whole tumor analysis? Eur J Radiol 82(2):342–348
Team RDC (2014) R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing
Ba-Ssalamah A, Muin D, Schernthaner R, et al. (2013) Texture-based classification of different gastric tumors at contrast-enhanced CT. Eur J Radiol 82(10):e537–e543
Weiss GJ, Ganeshan B, Miles KA, et al. (2014) Noninvasive image texture analysis differentiates K-ras mutation from pan-wildtype NSCLC and is prognostic. PLoS ONE 9(7):e100244
Chong Y, Kim JH, Lee HY, et al. (2014) Quantitative CT variables enabling response prediction in neoadjuvant therapy with EGFR-TKIs: are they different from those in neoadjuvant concurrent chemoradiotherapy? PLoS ONE 9(2):e88598
Ganeshan B, Skogen K, Pressney I, Coutroubis D, Miles K (2012) Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: preliminary evidence of an association with tumour metabolism, stage, and survival. Clin Radiol 67(2):157–164
Mattonen SA, Palma DA, Haasbeek CJ, Senan S, Ward AD (2014) Early prediction of tumor recurrence based on CT texture changes after stereotactic ablative radiotherapy (SABR) for lung cancer. Med Phys 41(3):033502
Yip C, Davnall F, Kozarski R, et al. (2014) Assessment of changes in tumor heterogeneity following neoadjuvant chemotherapy in primary esophageal cancer. Dis Esophagus. doi:10.1111/dote.12170
Ganeshan B, Ziauddin Z, Goh VJ, et al. (2012) Quantitative imaging biomarkers from PET-CT as potential correlates for angiogenesis and hypoxia in colorectal cancer. In: Eurpoean Congress of Radiology Conference, Vienna, Austria
Venook AP, Niedzwiecki D, Lenz H-J, et al. (2014) CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC) 2014 ASCO Annual Meeting. J Clin Oncol 32:3
Heinemann V, von Weikersthal LF, Decker T, et al. (2014) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 15(10):1065–1075
Richman SD, Chambers P, Seymour MT, et al. (2011) Intra-tumoral heterogeneity of KRAS and BRAF mutation status in patients with advanced colorectal cancer (aCRC) and cost-effectiveness of multiple sample testing. Anal Cell Pathol 34(1–2):61–66
Bossard C, Kury S, Jamet P, et al. (2012) Delineation of the infrequent mosaicism of KRAS mutational status in metastatic colorectal adenocarcinomas. J Clin Pathol 65(5):466–469
Watanabe T, Kobunai T, Yamamoto Y, et al. (2011) Heterogeneity of KRAS status may explain the subset of discordant KRAS status between primary and metastatic colorectal cancer. Dis Colon Rectum 54(9):1170–1178
Nordlinger B, Sorbye H, Glimelius B, et al. (2013) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 14(12):1208–1215
Disclosures
Dr. Pickhardt co-founded VirtuoCTC, is a shareholder in Cellectar Biosciences, and is a consultant for Check-Cap.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lubner, M.G., Stabo, N., Lubner, S.J. et al. CT textural analysis of hepatic metastatic colorectal cancer: pre-treatment tumor heterogeneity correlates with pathology and clinical outcomes. Abdom Imaging 40, 2331–2337 (2015). https://doi.org/10.1007/s00261-015-0438-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-015-0438-4