Abstract
Objectives
Our aim was to retrospectively evaluate the occurrence of respiratory motion artefacts in patients undergoing dynamic liver magnetic resonance (MR) either with gadoxetate disodium or gadobutrol.
Methods
Two hundred and thirty liver MR studies (115 with gadobutrol, 115 with gadoxetate disodium) were analysed. Respiratory motion artefacts on dynamic 3D T1-weighted MR images (pre-contrast, arterial, venous, and late-dynamic phase) were assessed using a five-point rating scale. Severe motion was defined as a score ≥ 4. Mean motion scores were compared with the Mann-Whitney-U-test. The chi-squared-test was used for dichotomous comparisons.
Results
Mean motion scores for gadoxetate disodium and gadobutrol showed no relevant differences for each phase of the dynamic contrast series (pre-contrast: 1.85 ± 0.70 vs. 1.88 ± 0.57, arterial: 1.85 ± 0.81 vs. 1.87 ± 0.74, venous: 1.82 ± 0.67 vs. 1.74 ± 0.64, late-dynamic: 1.75 ± 0.62 vs. 1.79 ± 0.63; p = 0.469, 0.557, 0.382 and 0.843, respectively). Severe motion artefacts had a similar incidence using gadoxetate disodium and gadobutrol (11/460 [2.4 %] vs. 7/460 [1.5 %]; p = 0.341).
Conclusions
Gadoxetate disodium is associated with equivalent motion scores compared to gadobutrol in dynamic liver MRI. In addition, both contrast agents demonstrated a comparable and acceptable rate of severe respiratory motion artefacts.
Key Points
• Gadobutrol and gadoxetate disodium showed comparable motion scores in dynamic phase imaging.
• The incidence of severe motion artefacts was pronounced in arterial phase imaging.
• Adverse respiratory side effects were not recorded in 115 examinations with gadoxetate disodium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dynamic contrast-enhanced imaging is the essential part of a liver magnetic resonance imaging (MRI) examination. According to current guidelines, the diagnosis of hepatocellular carcinoma (HCC) in patients with chronic liver disease, e.g., cirrhosis, is based solely on early arterial phase enhancement of liver lesions followed by rapid washout in the portal and late venous phases [1–4]. For other lesions, including focal nodular hyperplasia (FNH) or haemangioma, high-quality multiphase dynamic imaging is of comparable importance in order to establish a diagnosis [5]. Although most guidelines addressing lesion-specific enhancement patterns have been established based on clinical experience using extracellular contrast agents (ECCA), gadolinium-based contrast media (GBCM) with hepatobiliary uptake and excretion have become widely used in clinical routine. These contrast agents allow for acquisition of an additional hepatobiliary phase, which has proven to be advantageous for the detection and characterization of liver lesions, including HCC, FNH, adenoma or metastases [6–11].
Currently, two hepatobiliary agents are approved for clinical use in Europe and in the United States: gadoxetate disodium (Primovist©/ Eovist©, Bayer Healthcare, Germany) and gadobenate dimeglumine (MultiHance©, Bracco Imaging, Italy). Similar to other GBCM, both agents behave like conventional ECCA during the first minutes after injection. Compared to gadobenate dimeglumine, gadoxetate disodium allows for acquisition of an "earlier" hepatobiliary phase, which represents a logistic advantage in clinical routine imaging.
Recently, the occurrence of an acute self-limiting dyspnoea has been described in relation to gadoxetate disodium administration, which causes a higher incidence of non-diagnostic hepatic arterial phase images [12]. In the context of contrast media application, dyspnoea or related symptoms are considered as adverse respiratory reactions and have to be taken seriously [13]. The incidence of severely degraded arterial phase images after application of gadoxetate disodium was found to range between 11 and 17 % [12, 14, 15]. However, these results are discordant with data provided by controlled clinical trials and clinical trial databases [16].
As gadoxetate disodium is an important contrast agent in clinical liver MRI and due to this contradictory data, we retrospectively compared the incidence of severely degraded dynamic contrast enhanced images after administration of gadoxetate disodium to that of the extracellular contrast agent gadobutrol. The null hypothesis of our study was that there would be no significant difference between the two GBCM. The study aim was to retrospectively evaluate the occurrence of respiratory motion artefacts in patients undergoing dynamic liver magnetic resonance (MR), either with gadoxetate disodium or gadobutrol.
Materials and methods
Study population and sample size estimation
Ethics commission approval was obtained for this retrospective study, and the requirement to obtain written informed consent was waived. The study was not supported by the industry.
The sample size was chosen to detect an odds ratio (OR) of 5, based on an event rate of at least 4 % (severely degraded arterial phase images) in one of the groups with a power of 80 %. Thus, a total of 230 liver MR studies (115 administrations of gadobutrol and 115 administrations of gadoxetate disodium) in 208 different patients were retrospectively analysed. Twenty-two of 208 (10.6 %) patients underwent both gadoxetate and gadobutrol-enhanced liver MR examination. All examinations were performed between March 2012 and October 2013. The study population consisted of both inpatients and outpatients. The relevant liver MR studies were retrieved using the in-house radiological documentation software (MEDOS 9.3.2285, NEXUS / DIS GmbH, Frankfurt, Germany). This software allows a keyword-based search. The keywords "MRI liver", "gadoxetate" and "gadobutrol" were used to identify relevant examinations. In a second step, two tables were created, one containing liver MR studies with gadoxetate disodium and one containing liver MR studies with gadobutrol. For further analysis, the four dynamic images were anonymized and randomly exported to a workstation using dedicated software (ViewForum R5.1V1L2 SP1, Philips Healthcare, Best, The Netherlands).
Patient characteristics
Patient characteristics (e.g., the presence of cirrhotic liver disease) were retrieved with the help of the clinical information management system of the relevant institution. The amount of ascites and pleural effusions were determined visually by analysing randomized and anonymized T2 turbo spin echo spectral attenuated inversion recovery (SPAIR) sequences included in the liver MR protocol. Ascites and pleural effusions were rated using a scale from 1 to 3 (score 1: trace; score 2: mild; score 3: severe). Rating was performed by a board-certified radiologist (G.M.K.).
Contrast media
The selection of either gadoxetate disodium or gadobutrol as contrast agent was made by one of five board-certified radiologists, depending on the main clinical indication. Gadobutrol was generally preferred when vessel visualization was required for further therapeutic approaches. Gadoxetate disodium was preferentially used when detection of additional liver lesions in patients scheduled for therapy and characterization of suspicious liver lesions was of primary importance. A single dose bolus of 0.1 mmol per kilogram of body weight gadobutrol or 0.025 mmol per kilogram of body weight gadoxetate disodium was administered intravenously at a flow rate of 1.5 ml/s for both contrast agents, followed by a 25 ml saline flush. Contrast agent injection was performed using an automatic power injector (Spectris, Medrad, Inc., Warrendale, USA) in all patients.
Image acquisition
All scans were obtained using a 1.5 Tesla (T) or 3 T whole body MRI system (Ingenia 1.5 T and 3 T, Philips Healthcare, Best, The Netherlands) equipped with a 16-channel phased array coil. Dynamic three-dimensional T1-weighted gradient echo sequences with spectral fat suppression were obtained by using a manual two-dimensional fluoroscopic triggering method. Detailed sequence parameters are given in Table 1. For hepatic arterial phase imaging, breath-holding instructions were given when the contrast bolus was detected at the bifurcation of the abdominal aorta on fluoroscopic images. Imaging of the portal venous phase was performed approximately 45 seconds after bolus detection, followed by late venous phase imaging approximately 120 seconds after bolus detection. All dynamic images were acquired during breath-hold in expiration.
Image analysis
Two hundred and thirty consecutive liver MR examinations (115 for gadoxetate disodium and gadobutrol each) in 208 patients were retrospectively analysed. Two readers (G.M.K., more than 8 years of experience, J.A.L., more than 2 years of experience in abdominal MRI) blinded to the used GBCM analysed the data sets. Respiratory artefacts were rated for each phase using a motion score from 1 to 5: score 1, none; score 2, minimal with no effect on diagnostic quality; score 3, moderate with some but no severe effect on diagnostic quality; score 4, severe, but images still interpretable; and score 5, extensive, images non-diagnostic (see Fig. 1). In case of discrepancy, a consensus reading was held together with a third reviewer (P.A.K., 3 years of experience in abdominal MRI). Severe motion was defined as a motion score ≥ 4. Based on this definition, two dichotomized groups (severe vs. non-severe motion scores) were created.
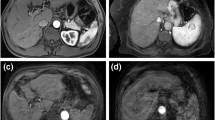
Transverse 3D-T1-weighted fat suppressed dynamic phase images showing different degrees of respiratory motion artefacts in a 54-year-old man (contrast agent: gadoxetate disodium). A: Pre-contrast phase showing moderate respiratory artefacts (motion score of 3). B: Arterial phase with severe respiratory artefacts (motion score of 4). C: Venous phase with only minimal respiratory artefacts (motion score of 2). D: Late-dynamic phase with no respiratory motion artefacts (motion score of 1)
In addition, all examinations were evaluated with regard to side effects like (pseudo-) allergic reactions, claustrophobia, tachycardia, bronchospasm and dyspnoea, based on documentation of the in-house radiology information system (RIS).
Statistical analysis
Statistical analysis was performed using SPSS version 17.0 (IBM, Armonk, USA). In order to consider the dependencies due to the measurement of 22 patients under both conditions, a mixed linear model was applied to compare mean respiratory motion scores between the gadoxetate disodium and the gadobutrol group. Patient characteristics are given as mean ± standard deviation (SD) or as absolute frequency. Continuous variables were tested for normal contribution. The independent two-sample Student’s t-test or the Mann-Whitney U test was used for comparison of continuous variables (e.g. mean motion scores, and age) between two different groups. Dichotomous variables (e.g. severe vs. non-severe motion scores, and male vs. female) were compared using the Chi-squared test (with a cell count > 5) or Fisher exact test (with a cell count ≤ 5). A p value lower than 0.05 was considered statistically significant.
Results
Ninety-seven of 230 examinations (42.2 %) were performed at 3 T and 133 of 230 examinations (57.8 %) at the 1.5 T MR system. Consensus motion score reading was necessary in 58/920 (6.3 %) of all image series. Mean age in the gadobutrol group was 57.5 ± 14.4 years (58.8 ± 12.6 in men [range: 26–86], 55.9 ± 16.1 in women [range: 18–86]; p = 0.278). Mean age in the gadoxetate disodium group was 58.1 ± 12.9 years (58.8 ± 12.9 in men [range: 29–82], 56.8 ± 12.6 years in women [range: 31–80]; p = 0.421). Age (p = 0.765), sex (p = 0.178) and body mass index (p = 0.292) did not differ significantly between both groups. There was a higher incidence of HCC in the gadoxetate disodium group (25/115 patients [21.7 %] vs. 13/115 patients [11.3 %], p = 0.033). A more detailed overview of the patient characteristics are given in Table 2. A total of three mild pseudo-allergic reactions (nausea after contrast administration in all cases) were recorded (1/115 [0.9 %] with gadobutrol and 2/115 [1.7 %] with gadoxetate disodium). Acute adverse or severe reactions were not recorded for any of the 230 examinations. In none of the reported cases was further treatment required.
Respiratory motion scores showed no relevant differences for the gadoxetate disodium group and the gadobutrol group. Mean motion scores were 1.85 ± 0.70 and 1.88 ± 0.57 for the pre-contrast phase, 1.85 ± 0.81 and 1.87 ± 0.74 for the arterial phase, 1.82 ± 0.67 and 1.74 ± 0.64 for the venous phase and 1.75 ± 0.62 and 1.79 ± 0.63 for the late-dynamic phase (p = 0.469, 0.557, 0.382 and 0.843, respectively; see Fig. 2). Severe respiratory motion artefacts (motion score ≥ 4) occurred at similar rates in the gadoxetate disodium and gadobutrol group for any of the pre-contrast and post-contrast series (see Table 3). After gadoxetate disodium administration, 11/460 (2.4 %) dynamic image series were severely degraded by respiratory artefacts. After application of gadobutrol, 7/460 (1.5 %) dynamic phases showed severe artefacts (p = 0.341). Arterial phase images were severely degraded by motion artefacts in 7/115 (6.1 %) in the gadoxetate disodium group and 4/115 (3.5 %) in the gadobutrol group (p = 0.539; see Table 3).
Within the mixed linear model, the mean differences for motion scores between the gadoxetate disodium group and the gadobutrol group were: -0.086 [95 % confidence interval -0.296, 0.124] for the pre-contrast phase, -0.045 [-0.268, 0.178] for the arterial phase, -0.129 [-0.381, 0.124] for the venous phase, and -0.209 [-0.459, 0.041] for the late-dynamic phase (p = 0.420, 0.691, 0.365, and 0.101, respectively). The odds ratio for severe motion artefacts between both contrast agents was 0.747 [95 % confidence interval 0.262, 2.129] (p = 0.584).
Across both contrast media, cirrhotic patients (61/230 [26.5 %]) did not show higher mean respiratory motion scores than non-cirrhotic patients (169/230 [73.5 %]) for each of the dynamic contrast series (pre-contrast phase: 1.90 ± 0.71 vs. 1.86 ± 0.62, p = 0.690; arterial phase: 1.88 ± 0.83 vs. 1.86 ± 0.76, p = 0.854; venous phase: 1.76 ± 0.65 vs. 1.78 ± 0.66, p = 0.832; late-dynamic phase: 1.69 ± 0.59 vs. 1.80 ± 0.67, p = 0.284). Older patients (median age of the whole study population: 57.5 years) had significantly higher mean respiratory motion scores compared to younger patients: pre-contrast phase: 1.98 ± 0.64 vs. 1.76 ± 0.63, arterial phase: 2.00 ± 0.81 vs. 1.73 ± 0.73, venous phase: 1.88 ± 0.69 vs. 1.68 ± 0.60, late-dynamic phase 1.82 ± 0.68 vs. 1.72 ± 0.63 (p = 0.007, 0.008, 0.020 and 0.228, respectively). The presence of mild or severe ascites had no impact on respiratory motion scores in arterial phase (1.82 ± 0.80 vs. 1.87 ± 0.78, p = 0.736). Mild or severe pleural effusions were also not associated with higher respiratory motion scores on arterial phase imaging (1.90 ± 0.79 vs. 1.86 ± 0.77, p = 0.835). The total number of severe motion scores was higher during arterial phase imaging (11/230 [4.8 %]) when compared to pre-contrast (2/230 [0.9 %]), venous (2/230 [0.9 %]), and late-dynamic phase imaging (3/230 [1.3 %]) (p = 0.002, 0.006, and 0.094, respectively).
Discussion
The present study demonstrates that respiratory motion scores in liver MRI and the incidence of severely degraded images did not differ relevantly from each other between the gadoxetate disodium group and the gadobutrol group.
We found 6.1 % of arterial phase liver examinations using gadoxetate disodium to be degraded by severe motion artefacts. In contrast to our results, several other studies reported rates of up to 17 % severely motion degraded arterial phase images in a comparable number of patients [12, 14, 15]. These studies compared the occurrence of artefacts between the liver-specific contrast agents gadoxetate disodium and gadobenate dimeglumine. Gadobenate dimeglumine was always administered based on weight (0.1 mmol per kilogram of body weight [on label]), whereas gadoxetate disodium was typically administered at a fixed dose of either 10 ml or 20 ml (off label dosage) [12, 14, 15, 17]. Interestingly, a recently published study on 559 liver MRI studies with gadoxetate disodium reported severe respiratory motion artefacts as significantly more common after administration of 20 ml compared to 10 ml [17]. In our study, gadoxetate disodium was administered weight-adjusted with a mean dose of 7.7 ml (approved dosage). Our results and the results of the previously mentioned studies therefore indicate that occurrence of motion artefacts after application of gadoxetate disodium may be related to the applied dose. Another recently published study reported that patients showing severe respiratory artefacts in arterial phase imaging have a higher risk to again show those artefacts in a subsequent investigation [18]. In addition to dosage-related effects, individual factors such as lung disease may influence the occurrence of transient dyspnoea. In our study, many factors potentially influencing respiratory motion, i.e., concomitant lung disease, ascites or pleural effusion, were comparable between both groups. However, the general susceptibility to higher motion scores in older patients found in our study indicates that patient age may influence the image quality regardless of the contrast medium used.
In contrast to previous publications, we performed contrast-enhanced dynamic imaging during expiration. It has been shown that diaphragmatic positional variation and displacement during suspension of respiration at end expiration is significantly smaller than during end inspiration [19]. The reason for this observation is physiologic: With suspended breathing at end inspiration, the diaphragm progressively relaxes and starts moving upward. During breath holding in end expiration, the movement of the diaphragm is slower because inspiratory muscles are already relaxed. This acquisition technique might have contributed to the overall lower rates of motion artefacts observed in our data sets compared to the higher rates reported in previous studies [12].
The acquisition time (i.e., breath-hold time) for each of the dynamic contrast series ranged between 14 and 15 seconds. Previous studies reported acquisition times ranging from 18 to 22 seconds [12]. The shorter breath-hold time might also have contributed to the reduced incidence and severity of motion artefacts in our study.
In total, three adverse reactions were recorded in 230 examinations. All reactions were considered as mild (pseudo-) allergic reactions by the responsible radiologist and required no further treatment. In this respect, our findings are concordant with previously published data [20].
We found arterial phase imaging using both contrast agents to be significantly more often degraded by respiratory motion artefacts than non-contrast and contrast-enhanced venous phase imaging. This finding reflects a generally increased susceptibility of arterial phase liver MRI to motion artefacts and is of potential importance for the diagnosis of focal liver lesions in the context of underlying liver disease. Current literature recommends using a bolus timing technique for appropriate arterial phase imaging [21–23]. However, the time window between bolus detection and arterial phase imaging is usually very short. Therefore, during arterial phase imaging the patient is confronted with the sensation of contrast agent injection followed by immediate instruction to hold the breath in a potentially uncomfortable environment. As dynamic arterial phase imaging cannot be repeated without a second contrast agent injection, upcoming techniques compensating respiratory motion by means of higher temporal resolution are potentially useful, especially in debilitated in-hospital patients who are unable to hold their breath adequately [24]. A recently published study showed that multiple arterial phase acquisitions during a single-breath-hold recovered most arterial phases that would otherwise have been compromised by motion artefacts [15].
The key finding in this study is a lack of difference in arterial phase motion artefacts between the gadobutrol and gadoxetate disodium group. Two conclusions can be drawn from these results. First, the impact of regularly dosed gadoxetate disodium on respiratory motion artefacts is substantially lower than in earlier reported cohorts, in which mainly off-label dosed gadoxetate disodium was used [12, 14, 15]. Second, even if gadoxetate disodium would show a significantly higher motion rate compared to gadobutrol in a larger study cohort, the clinical impact of such a finding would be negligible.
Our study has several limitations. Due to the retrospective study design, the choice of the contrast agent was exclusively influenced by the main clinical question, which might have led to an unbalanced patient distribution between the two groups. However, the fact that patient characteristics—except for the prevalence of HCC (which was higher in patients receiving gadoxetate disodium)—did not differ significantly between both groups at least partially compensates for this limitation. Another limitation due to the retrospective design of the study is that pre-contrast images with severe motion might have been repeated by the responsible technical assistant, and therefore may not have been included into image analysis. However, this issue relates to both contrast groups. Another limitation is that adverse reactions (e.g., acute dyspnea) were not monitored using a dedicated questionnaire. Therefore, we admit that mild adverse reactions like cool sensations or a sense of metallic taste during contrast injection might not have been recorded. Finally, the number of patients included in our analysis was limited to 230 and our results should be confirmed or disproved in further studies. However, our results have sufficient statistical power to show that the rate of motion artefacts related to the administration of on label dosed gadoxetate disodium is much lower than reported in previous studies.
In conclusion, we showed that dynamic liver MRI using either gadoxetate disodium or gadobutrol is associated with a comparable and acceptable rate of respiratory motion artefacts. Arterial phase imaging is significantly more often degraded by severe motion than pre-contrast or venous phase imaging in general.
Reference
Bruix J, Sherman M (2005) Management of hepatocellular carcinoma. Hepatology 42:1208–1236
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022
Willatt JM, Hussain HK, Adusumilli S, Marrero JA (2008) MR Imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology 247:311–330
Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A (2012) Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 13:e11–e22
Albiin N (2012) MRI of Focal Liver Lesions. Curr Med Imaging Rev 8:107–116
Grazioli L, Morana G, Kirchin MA, Schneider G (2005) Accurate differentiation of focal nodular hyperplasia from hepatic adenoma at gadobenate dimeglumine-enhanced MR imaging: prospective study. Radiology 236:166–177
Hammerstingl R, Huppertz A, Breuer J et al (2008) Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol 18:457–467
Huppertz A, Haraida S, Kraus A et al (2005) Enhancement of focal liver lesions at gadoxetic acid-enhanced MR imaging: correlation with histopathologic findings and spiral CT–initial observations. Radiology 234:468–478
Raman SS, Leary C, Bluemke DA et al (2010) Improved characterization of focal liver lesions with liver-specific gadoxetic acid disodium-enhanced magnetic resonance imaging: a multicenter phase 3 clinical trial. J Comput Assist Tomogr 34:163–172
Denecke T, Steffen IG, Agarwal S et al (2012) Appearance of hepatocellular adenomas on gadoxetic acid-enhanced MRI. Eur Radiol 22:1769–1775
Fujita N, Nishie A, Kubo Y et al (2014) Hepatocellular carcinoma: clinical significance of signal heterogeneity in the hepatobiliary phase of gadoxetic acid-enhanced MR imaging. Eur Radiol. doi:10.1007/s00330-014-3349-9
Davenport MS, Viglianti BL, Al-Hawary MM et al (2013) Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology 266:452–461
Stenver D (2009) Pharmacovigilance: when to report adverse reactions. In: Thomsen H, Webb J (eds) Contrast media safety issues and ESUR guidelines. Springer, Berlin
Davenport MS, Caoili EM, Kaza RK, Hussain HK (2014) Matched within-patient cohort study of transient arterial phase respiratory motion-related artifact in MR imaging of the liver: gadoxetate disodium versus gadobenate dimeglumine. Radiology. doi:10.1148/radiol.14132269:132269
Pietryga JA, Burke LM, Marin D, Jaffe TA, Bashir MR (2014) Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology 271:426–434
Bergmann K, Agris J, Balzer T (2013) Does intravenous administration of gadoxetate disodium have any effect on breath-hold times. Radiology 268:926–927
Davenport MS, Bashir MR, Pietryga JA, Weber JT, Khalatbari S, Hussain HK (2014) Dose-toxicity relationship of gadoxetate disodium and transient severe respiratory motion artifact. AJR Am J Roentgenol 203:796–802
Bashir MR, Castelli P, Davenport MS et al (2014) Respiratory motion artifact affecting hepatic arterial phase mr imaging with gadoxetate disodium is more common in patients with a prior episode of arterial phase motion associated with gadoxetate disodium. Radiology. doi:10.1148/radiol.14140386:140386
Holland AE, Goldfarb JW, Edelman RR (1998) Diaphragmatic and cardiac motion during suspended breathing: preliminary experience and implications for breath-hold MR imaging. Radiology 209:483–489
Huppertz A, Balzer T, Blakeborough A et al (2004) Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology 230:266–275
Tanimoto A, Lee JM, Murakami T, Huppertz A, Kudo M, Grazioli L (2009) Consensus report of the 2nd International Forum for Liver MRI. Eur Radiol 19(Suppl 5):S975–S989
Haradome H, Grazioli L, Tsunoo M et al (2010) Can MR fluoroscopic triggering technique and slow rate injection provide appropriate arterial phase images with reducing artefacts on gadoxetic acid-DTPA (Gd-EOB-DTPA)-enhanced hepatic MR imaging? J Magn Reson Imaging 32:334–340
Ringe KI, Husarik DB, Sirlin CB, Merkle EM (2010) Gadoxetate disodium-enhanced MRI of the liver: part 1, protocol optimization and lesion appearance in the noncirrhotic liver. AJR Am J Roentgenol 195:13–28
Agrawal MD, Spincemaille P, Mennitt KW et al (2013) Improved hepatic arterial phase MRI with 3-second temporal resolution. J Magn Reson Imaging 37:1129–1136
Acknowledgments
The scientific guarantor of this publication is Dr. Guido M. Kukuk. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luetkens, J.A., Kupczyk, P.A., Doerner, J. et al. Respiratory motion artefacts in dynamic liver MRI: a comparison using gadoxetate disodium and gadobutrol. Eur Radiol 25, 3207–3213 (2015). https://doi.org/10.1007/s00330-015-3736-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3736-x