Abstract
Objectives
To directly investigate the rapid respiratory effect of gadoxetate disodium in an experimental study using mice.
Methods
After confirming the steady respiratory state under general anaesthesia, eight mice were injected with all test agents in the following order: phosphate-buffered saline (A, control group), 1.25 mmol/kg of gadoteridol (B) or gadopentetate dimeglumine (C), or 0.31 mmol/kg of gadoxetate disodium (D, E). The experimenter was not blinded to the agents. The injection dose was fixed as 100 μL for Groups A-D and 50 μL for Group E. We continuously monitored and recorded respiratory rate (RR), peripheral oxygen saturation (SpO2), and heart rate. The time-series changes from 0 to 30 s were compared by the linear mixed method
Results
Groups D and E showed the largest RR increase (20.6 and 20.3 breaths/min, respectively) and were significantly larger compared to Group A (3.36 breaths/min, both P<0.001). RR change of Groups D and E did not differ. RR change of Groups B and C was smaller (0.72 and 12.4 breaths/min, respectively) and did not differ statistically with Group A. Significant bradycardia was observed only in Group C (P<0.001). SpO2 was constant in all groups.
Conclusions
Gadoxetate disodium causes a rapid tachypnoea without significant change of SpO2 and heart rate regardless of the dilution method.
Key Points
• Injection of gadoxetate disodium causes tachypnoea.
• Dilution method did not alter the rapid respiratory effect of gadoxetate disodium.
• The respiratory effect of gadoxetate disodium was larger than other contrast agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gadoxetate disodium is a liver-specific T1 contrast agent [1] used in the diagnosis of focal liver lesions. The main advantage of the agent is that it enables both dynamic contrast enhanced imaging and hepatobiliary phase imaging within a clinically feasible time. Thanks to this advantage, the agent provides additional differential diagnostic information comparable to that imparted when the usual extracellular gadolinium chelates are employed [2,3,4], so the agent is used worldwide for liver magnetic resonance imaging (MRI) [5, 6].
However, recent studies showed that suboptimal image quality is frequently observed in the arterial phase imaging with gadoxetate disodium [7,8,9]. The phenomenon was named “severe respiratory motion artefact”, and the cause of this artefact was first described as acute self-limiting dyspnoea from the idea that such respiratory effect of gadoxetate disodium deleteriously affects the arterial image quality [8]. Subsequently, many researchers assess the relation of this artefact and gadoxetate disodium, and severe respiratory motion artefact seems to occur most frequently in gadoxetate disodium-enhanced MRI, although one report claims that severe motion artefacts had a similar incidence using gadoxetate disodium and gadobutrol [10].
Two major hypotheses have been proposed as the cause of severe respiratory motion artefact. One is a truncation artefact. It is reported that either increasing the bolus volume by diluting the agent with saline or performing a slow injection minimised the artefact [11, 12]. Another theory is that gadoxetate disodium actually causes dyspnoea, which is a more resent and mainstream idea. This hypothesis is supported by the fact that breath-holding duration decreases after administration of gadoxetate disodium [13]. Some researchers have tried to assess the respiratory effect of gadoxetate disodium in humans, but by an indirect way using respiratory waveform analysis [14, 15].
In general, humans, as observation targets, are a highly inhomogeneous group. The biological effect of a drug may be largely affected by underlying factors such as age, sex, race, and underlying disease. It is also reported that severe respiratory motion artefact occurs more often in patients with chronic obstructive pulmonary disease [9], and there are some researchers who suspect that race has an effect on severe respiratory motion artefact [16].
Accordingly, the purpose of the present study was to assess the rapid respiratory effect of gadoxetate disodium directly by monitoring the vital signs of mice. We also assessed the respiratory effect of gadoteridol and gadopentetate dimeglumine to compare with gadoxetate disodium, since their effect is also unknown.
Materials and methods
All animal experiments were conducted in accordance with the guidelines of our institution and were approved by our animal research committee (study protocol ID: PA15-40).
Animals
We used eight female C57BL6 mice purchased from Japan SLC (Hamamatsu, Japan) in the present study, and they were maintained in a specific pathogen-free facility. All mice weighed approximately 20 g and had ad libitum access to food and water. We shaved the backs of the mice for the sake of accurate monitoring of their vital signs.
We performed five experiments in all eight mice, as described in the following section.
Injection of contrast agent
We used four test agents in this study. Group A: phosphate-buffered saline (PBS, as control group), Group B: gadoteridol (ProHance; Bracco Eisai Co. Ltd., Tokyo, Japan), Group C: gadopentetate dimeglumine (Magnevist; Bayer Yakuhin Ltd, Osaka, Japan), and Groups D,E: gadoxetate disodium (Primovist; Bayer Yakuhin Ltd, Osaka, Japan), and the contrast agents were injected in this order. The experimenter was not blinded to the agents injected. Each experiment was performed under different anaesthesia sessions with an interval of 6 h at least. Only for Group E was the experiment performed after an interval of 24 h (i.e. performed on a different day) after the Group D experiment, taking the longer biological effect of gadoxetate disodium into account. We injected 1.25 mmol/kg of gadoteridol and gadopentetate dimeglumine and 0.31 mmol/kg of gadoxetate disodium. These doses are equivalent to clinically approved human dose (human equivalent dose, HED) after adjustment for body surface area, as recommended by the Food and Drug Administration [17]. All the gadolinium contrast agents were diluted with PBS, and the injection dose was fixed as 100 μL for Groups A-D. Only for gadoxetate disodium was an injection of 50 μL also performed without changing the total amount of gadoxetate disodium (Group E). All the test agents were injected manually via the retro-orbital injection method [18], and the injection duration was fixed at 10 s.
Monitoring the vital signs
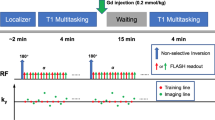
All the vital signs were continuously monitored and recorded by MouseOx Plus (Starr Life Sciences Corp, USA). The clip sensor was equipped onto the murine neck, and we monitored peripheral oxygen saturation (SpO2, %), heart rate (HR, beats/min), and respiratory rate (RR, breaths/min) every second. The mice were anaesthetised with 4% isoflurane in air, and the whole experiment was performed under anaesthesia. Before injecting the test agents, we tried to control the RR of mice in the range of 90 to 100 as much as possible by changing the concentration of isoflurane. We confirmed steady state for at least 2 min before the injection of the test agents. If RR control was difficult and not possible control in the range of 90 to 100 within 30 min, we injected the contrast agent after confirming a steady state at the amount of anaesthesia at 30 min. At the steady state, concentration of isoflurane was 0.75% to 2% isoflurane in air. We recorded the vital signs of the mice from 20 to 30 s before the injection of the test agents and until 60 s after the injection of the test agents. Schematic illustration of the study protocol is shown in Fig. 1.
Statistical analysis
All results are expressed as mean ± standard error of the mean. Time-series changes of each vital signs were compared by the linear mixed method with the assumption of the correlation structure of time points. To assume linearity of the time-series changes, we determined cut-off time by the median time point when the peak value was recorded for each vital sign and group. Thirty seconds was then determined as the cut-off time. This cut-off time was also determined so as to reflect the change in the timing of arterial phase imaging. For the correlation structure, AR(1) (autoregressive model 1) was selected. The initial value for each vital sign was determined by the mean of the measurements from -5 to 0 s. Multiplicity of testing was adjusted by Bonferroni’s method according to the number of testing for each analysis. We consider the differences were statistically significant if P < 0.05. All statistical analyses were performed using SPSS 23 (IBM Corp, Chicago, IL, USA).
Results
Variability of the baseline state was small in RR (95.0 ± 1.0 breaths/min), and SpO2 (98.3 ± 0.1%), and variability of the baseline state of HR was relatively large (366.1 ± 9.1 beats/min).
The time-series changes of each vital signs from the baseline state are shown in Fig. 2. Regardless of the dilution methods used for gadoxetate disodium, Groups D and E showed a similar change in vital signs. They demonstrated strong tachypnoea toward 30 s, and a slight decrease of HR between 10 to 20 s. In Group C, moderate tachypnoea toward 30 s and moderate bradycardia toward 30 s were observed. These fluctuations of vital signs in Groups C-E gradually returned to the original state after 30 s. For Group B, a slight elevation of RR was observed between 30 to 40 s, and the RR remained constant at 40 to 60 s. Also, slight bradycardia toward 30 s was observed in Group B. For SpO2, the fluctuation was within 1% throughout the whole study time in all groups.
The time-series changes of RR (a), SpO2 (b), and HR (c). The data shown at each time are average including data of 1 s before and after of each time. Error bars represent standard errors (n=8 mice for each group). Both Groups D and E showed an elevation of approximately 20 breaths/min within 30 s from the start of injection without significant change in SpO2 and HR.
The result of the statistical analysis is shown in Tables 1, 2 and 3. In Group B, although a slight decrease in HR was observed, no significant difference was observed in all three vital signs compared to Group A. Group C showed a significant decrease of HR (P < 0.001) compared to Group A and a moderate increase of RR, but the latter increase was not statistically significant (P = 0.145). Groups D and E demonstrated a significant increase of RR compared to Group A (both P < 0.001), and no significant difference was observed in SpO2 and HR compared to Group A. Also, no significant difference between Groups D and E was observed; namely, the dilution methods used for gadoxetate disodium did not alter their effect on respiration.
Discussion
In our study, gadoxetate disodium increased the RR rapidly, and the effect on respiration tended to be larger than gadoteridol and gadopentetate dimeglumine. Also, no significant change was observed in SpO2 and HR. All these data were obtained directly by using mice.
Recently, several researchers have tried to evaluate the respiratory effect of gadoxetate disodium in humans. McClellan et al. reported that maximum breath-holding duration is decreased by the injection of gadoxetate disodium compared to saline and gadoterate meglumine [13]. Recent studies using respiratory waveform analysis showed that standard deviation of respiratory waveform correlates with the overall image quality of hepatic arterial phase [14] and aberrant respiratory waveform peaks in the arterial phase are usually associated with transient tachypnoea [15]. Our result also shows that gadoxetate disodium increase the RR during the timing of arterial phase imaging, and the effect on respiration tended to be larger than gadoteridol and gadopentetate dimeglumine. We believe that our result is strong supporting evidence of these former reports.
Hayashi et al. showed that the severe artefact group and the non-severe artefact group showed a similar and insignificant change in SpO2 during the hepatic arterial phase and concluded that intravenous gadoxetate disodium does not cause changes in SpO2 and HR that lead to image quality degradation [19]. No significant SpO2 change was also observed in mice injected with gadoxetate disodium in our study, but significant RR increase was also observed at the same time. Thus, we think that our result matches with the result of Hayashi et al., although we believe that their result is not an enough evidence to conclude that gadoxetate disodium is not necessarily related to severe artefact during the arterial phase with transient dyspnoea.
In our study, Groups D and E showed similar change in vital signs. This means that, including respiration, dilution methods used for gadoxetate disodium did not alter their effect on these vital signs. Since the injection duration is fixed in our study, total dose of contrast agent injected per second is constant regardless of the dilution methods. Thus, the two injection methods of gadoxetate disodium in our study imitate the study by Kim et al. that compared the image quality of the arterial phase of gadoxetic acid-enhanced MR imaging between the injection of undiluted gadoxetic acid at 1.0 mL/s vs. the injection of twofold diluted gadoxetic acid at 2.0 mL/s [20]. They reported that injection of twofold diluted gadoxetic acid at 2.0 mL/s significantly reduced the artefact of the arterial phase imaging. Since the expected bolus shape is unchanged in the faster injection of twofold diluted gadoxetic acid, it is difficult to understand why just adding more saline should have any effect on physiology. Furthermore, our study clearly demonstrated that the dilution method does not cause any physiological changes.
In our study, we also checked the respiratory effect of gadoteridol and gadopentetate dimeglumine. While other contrast agents demonstrated peak RR increase around 30 s, and then falling until 60 s, gadoteridol demonstrated the smallest increase of RR after 30 s lasting to 60 s. Also, a slight decrease of HR was observed, which was not significant against PBS. It is formerly reported that an injection of 0.6 mmol/kg gadoteridol (approximately 3.3 times that of HED) causes similar hypotension and bradycardia with gadopentetate dimeglumine, but the changes lasted longer [21]. Thus, considering our result, gadoteridol seem to cause weak cardiovascular and pulmonary effects compared to other contrast agents in a dose-dependent fashion.
Gadopentetate dimeglumine showed a relatively strong decrease of HR and a moderate increase of RR. Li et al. reported that gadopentetate dimeglumine caused significant deleterious hemodynamic effects in a dose-dependent fashion in rats with acute myocardial infarction. They observed an approximate 10% heart rate decrease from the baseline by 0.5 mmol/kg injection (approximately 0.8 times that of HED) of gadopentetate dimeglumine [22]. Also, Wible et al. observed an approximate 13% heart rate decrease from the baseline by 0.6 mmol/kg injection (approximately 3.3 times that of HED) of gadopentetate dimeglumine in the anaesthetised dog [21]. We observed an approximate 12% decrease in heart rate in our study, and this finding is consistent with these former studies. The discrepancy with effect on HR of gadoxetate disodium (which is the same ionic linear contrast agent) also needs further investigation.
Our study has some limitations. First, the number of mice in each group is limited, and also we did not perform a power analysis. However, we believe that our experimental results were unequivocal that this limited number of mice was sufficient enough to show significant change of the vital signs among groups. Second, the information of the agents was not blinded to the experimenter. Although this might raise the possibility of bias, we believe that the possibility of bias is minimised by performing the experiment strictly following the study protocol that we made. Indeed, the variabilities of baseline vital signs were small in our experiment. Third, although the total amount of contrast agent is equivalent to human dose and the injection duration is also similar, amount of liquid (50 or 100 μL) per weight is larger compared to human. Although we compared with the same amount of phosphate-buffered saline, the biological effect of the contrast agents we found in our experiment might be exaggerated. Finally, we injected each test agent via retro-orbital injection, which is not a direct venous injection. Therefore, there is a concern if the contrast bolus curve is constant. However, the retro-orbital and tail vein routes afforded similar results in terms of the kinetics of the contrast agent [18], we believe the bolus curve after the injection of agents were constant in this study.
In conclusion, injection of gadoxetate disodium caused a stronger increase of RR compared to gadoteridol and gadopentetate dimeglumine, in the meantime did not cause any change in SpO2 and HR. Dilution methods used for gadoxetate disodium did not alter the vital signs in our study.
Abbreviations
- HR:
-
Heart rate
- RR:
-
Respiratory rate
- SpO2:
-
Peripheral oxygen saturation
References
Weinmann HJ, Schuhmann-Giampieri G, Schmitt-Willich H, Vogler H, Frenzel T, Gries H (1991) A new lipophilic gadolinium chelate as a tissue-specific contrast medium for MRI. Magn Reson Med 22:233–237
Huppertz A, Balzer T, Blakeborough A et al (2004) Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology 230:266–275
Akai H, Kiryu S, Matsuda I et al (2011) Detection of hepatocellular carcinoma by Gd-EOB-DTPA-enhanced liver MRI: comparison with triple phase 64 detector row helical CT. Eur J Radiol 80:310–315
Vogl TJ, Kümmel S, Hammerstingl R et al (1996) Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology 200:59–67
Chen L, Zhang J, Zhang L et al (2012) Meta-analysis of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging for the detection of liver metastases. PLoS One 7:e48681
Bashir MR, Gupta RT, Davenport MS et al (2013) Hepatocellular carcinoma in a North American population: does hepatobiliary MR imaging with Gd-EOB-DTPA improve sensitivity and confidence for diagnosis? J Magn Reson Imaging 37:398–406
Davenport MS, Viglianti BL, Al-Hawary MM et al (2013) Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology 266:452–461
Pietryga JA, Burke LM, Marin D, Jaffe TA, Bashir MR (2014) Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology 271:426–434
Davenport MS, Bashir MR, Pietryga JA, Weber JT, Khalatbari S, Hussain HK (2014) Dose-toxicity relationship of gadoxetate disodium and transient severe respiratory motion artifact. AJR Am J Roentgenol 203:796–802
Luetkens JA, Kupczyk PA, Doerner J et al (2015) Respiratory motion artefacts in dynamic liver MRI: a comparison using gadoxetate disodium and gadobutrol. Eur Radiol 25:3207–3213
Motosugi U, Ichikawa T, Sou H et al (2009) Dilution method of gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI). J Magn Reson Imaging 30:849–854
Tanimono A, Hibuchi N, Ueno A (2012) Reduction of ringing artifacts in the arterial phase of gadoxetic acid-enhanced dynamic MR imaging. Magn Reson Med Sci 11:91–97
McClellan TR, Motosugi U, Middleton MS et al (2017) Intravenous gadoxetate disodium administration reduces breath-holding capacity in the hepatic arterial phase: a multi-center randomized placebo-controlled trial. Radiology. 282:361–368
Park YS, Lee CH, Yoo JL et al (2016) Hepatic arterial phase in gadoxetic acid-enhanced liver magnetic resonance imaging: analysis of respiratory patterns and their effect on image quality. Invest Radiol 51:127–133
Davenport MS, Malyarenko DI, Pang Y, Hussain HK, Chenevert TL (2017) Effect of gadoxetate disodium on arterial phase respiratory waveforms using a quantitative fast fourier transformation-based analysis. AJR Am J Roentgenol 208:328–336
Motosugi U (2015) Gadoxetic acid-induced acute transient dyspnea: the perspective of Japanese radiologists. Magn Reson Med Sci 14:163–164
Guidance for industry estimating the maximum safe starting dose in Initial clinical trials for therapeutics in adult healthy volunteers. Food and Drug Administration. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078932.pdf., Published July 6 2005. Accessed September 1, 2015.
Wang F, Nojima M, Inoue Y, Ohtomo K, Kiryu S (2015) Assessment of MRI contrast agent kinetics via retro-orbital injection in mice: comparison with tail vein injection. PLoS One 10:e0129326
Hayashi T, Saitoh S, Tsuji Y et al (2015) Influence of gadoxetate disodium on oxygen saturation and heart rate during dynamic contrast-enhanced MR Imaging. Radiology 276:756–765
Kim YK, Lin WC, Sung K et al (2016) Reducing artifacts during arterial phase of gadoxetate disodium-enhanced MR imaging: dilution method versus reduced injection rate. Radiology 15:160241
Wible JH Jr, Galen KP, Wojdyla JK (2001) Cardiovascular effects caused by rapid administration of gadoversetamide injection in anesthetized dogs. Invest Radiol 36:292–298
Li HT, Saeed M, Wendland MF, Higgins CB (1993) Cardiovascular responses after ionic and nonionic magnetic resonance contrast media in rats with acute myocardial infarction. Invest Radiol. 28:11–19
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Shigeru Kiryu.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Dr. Masanori Nojima) is Master of Public Health, and has significant statistical expertise.
Ethical approval
Approval from the institutional animal care committee was obtained.
Methodology
• experimental
• performed at one institution
Rights and permissions
About this article
Cite this article
Akai, H., Yasaka, K., Nojima, M. et al. Gadoxetate disodium-induced tachypnoea and the effect of dilution method: a proof-of-concept study in mice. Eur Radiol 28, 692–697 (2018). https://doi.org/10.1007/s00330-017-5037-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5037-z