Abstract
Background and aim
To investigate the prevalence, anatomy and distribution of the hepatic falciform artery (HFA) and Sappey’s superior artery (SSA) using C-arm CT hepatic arteriography (C-arm CTHA).
Materials and methods
From January 2011 to December 2012, 220 patients who underwent C-arm CTHA during initial transarterial treatment for hepatocellular carcinoma were included in this retrospective study. The HFAs and SSAs prevalence and origin were evaluated using axial images of C-arm CTHA. A 5-point scale for HFAs and a 4-point scale for SSAs were used to designate the radiologically conspicuous arteries.
Results
The prevalences of the total HFAs and SSAs were 95 % (n=209) and 22 % (n=49), while those of radiologically conspicuous HFAs and SSAs were 62 % (n=137) and 10 % (n=22), respectively. Thirty HFAs (22 % of radiologically conspicuous HFAs and 14 % of the total study population) were distributed in the subcutaneous layer of the anterior abdominal wall, while the majority of SSAs ran through the superior part of the falciform ligament in the left-anterior direction and anastomosed with left inferior phrenic artery.
Conclusion
Our study using C-arm CTHA revealed that the prevalence of the HFA is higher than the existing knowledge and proved the existence of the SSA radiologically for the first time.
Key Points
• Prevalence of hepatic falciform artery is 95 %, higher than previously known.
• 22 % of conspicious hepatic falciform arteries distributed in subcutaneous tissue around umbilicus.
• The existence of Sappey’s superior artery was proved with a radiological method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transarterial liver-directed therapies, including chemoembolization (TACE) and radioembolization (TARE), have played important roles in the management of hepatocellular carcinoma (HCC) [1, 2]. The preprocedural identification of non-hepatic arteries arising from the hepatic arteries is important in order to reduce complications caused by inadvertent non-target infusion of toxic material [3, 4]. Knowledge about the parasitic arterial supply from non-hepatic arteries to the HCC is also important for maximizing the therapeutic efficacy [5–7].
The hepatic falciform ligament (HFL) is a remnant of the ventral mesentery of the fetus, attaching the liver to the anterior parietal and diaphragmatic peritoneum. Sappey described the veins of the falciform ligament and classified them into the superior and inferior groups [8, 9]. The inferior group is called the paraumbilical vein, which is dilated as a portosystemic shunt, especially in patients with portal hypertension [10, 11].
The hepatic falciform artery (HFA), an arterial counterpart to the paraumbilical vein, is an important non-hepatic artery that arises as a terminal branch of the left or middle hepatic artery and runs along the caudal part of the falciform ligament to terminate in the anterior body wall around the umbilicus [12, 13]. Skin complications after transarterial liver-directed therapies associated with the HFA have been reported [14–17]. While the anatomical prevalence of the HFA is 68 % [12, 13], the reported angiographic prevalence varies greatly, from 2 % to 52 % [13, 18–20].
To date, the superior groups of Sappey’s vessels have not been documented in detail. Ibukuro et al. showed the direct communication between the intrahepatic portal vein and the left internal mammary vein through the vessels in the falciform ligament, both radiologically and anatomically. They also asserted that its arterial counterpart, which we named Sappey’s superior artery (SSA) in our study, should be the main route of parasitic HCC supply from the internal mammary arteries or inferior phrenic arteries [10]. However, the SSA had never been identified in any previous radiological study.
The recent development of C-arm CT arteriography now allows the generation of high-resolution multiplanar reformation (MPR) CT and 3-D angiographic images, enabling precise visualization of fine arterial structures [21]. In this study, we investigated the prevalence, anatomy and destination of the HFA and SSA on C-arm CT hepatic arteriography (C-arm CTHA).
Materials and methods
Patient population
This retrospective study was approved by the institutional review board, and the requirement for informed consent waived. From January 2011 to December 2012, 802 consecutive patients underwent C-arm CTHA during an initial session of TACE or TARE. Among them, 220 patients were finally included after exclusion of any condition that might affect the prevalence or detection rate of the HFA or SSA according to the predetermined exclusion criteria, which included unavailable thin-section (3 mm or less) multidetector row CT (MDCT) (n=10), unavailable C-arm CTHA for the middle or left hepatic artery (n=9), poor quality of C-arm CTHA images (n=4), prior abdominal surgery (n=137), prior radiofrequency ablation or percutaneous ethanol injection (n=150), large tumour (>10 cm) or main portal vein invasion (n=51), and any tumour in the left or caudate lobes (n=221).
MDCT technique
Contrast-medium-enhanced multiphase CT was performed in all patients within 30 days (mean = 15 days) before treatment with MDCT scanners, including an 8-detector row CT scanner (Lightspeed Ultra, GE Medical Systems, Milwaukee, WI, USA) (n=24), a 16-detector row CT scanner (Sensation 16, Siemens, Erlangen, Germany) (n=61), 64-detector row CT scanners (Brilliance 64, Philips Medical Systems, Eindhoven, The Netherlands; Somatom Definition, Siemens; Discovery CT750HD, GE Medical Systems) (n=65, n=26, and n=23, respectively), and a 320-detector row CT scanner (Aquilion One, Toshiba Medical Systems, Otawara, Japan) (n=21). Unenhanced images were first obtained in a craniocaudal direction. Dynamic images consisted of three phases (the hepatic arterial, portal venous and equilibrium phases). After acquisition of unenhanced liver images, contrast medium (Iopromide, Ultravist 370; Schering, Berlin, Germany) was administered, followed by a 30-ml sterile saline flush using a power injector. Contrast medium volumes (delivered at 2 ml per kg of body weight) varied from 100–150 ml. Hepatic arterial-phase scan delays were 11–17 s after descending aorta enhancement to 100 HU, measured using a bolus-tracking technique, and portal venous phase interscan delays were 20–30 s. The equilibrium phase was acquired 180 s after the completion of the administration of contrast medium.
Digital subtraction angiography and C-arm CTHA techniques
All digital subtraction angiography and C-arm CTHA images were acquired with an angiography system (AXIOM Artis dTA/VB30; Siemens) equipped with a 30 cm × 40 cm flat-panel detector. Before C-arm CTHA scanning, angiography of the celiac trunk and the superior mesenteric artery was performed using a 5-F catheter (RH catheter; Cook, Inc., Bloomington, IN, USA) to evaluate the vascular anatomy and blood flow. C-arm CTHA images of the hepatic artery were obtained using the following techniques. The arms of the patients were positioned above the head to avoid streak artefacts from the forearms. A series of three-dimensional rotational C-arm CTHA images were obtained during a single breath-hold over a 211° circular trajectory for 8 s. A 5-F catheter (RH catheter) or a microcatheter with a 2.7-F tip (Radio Star; Taewoong Medical Co., Gimpo, Republic of Korea) was used depending on the case. The catheter tip was located at the celiac trunk, common hepatic artery, or proper hepatic artery. Contrast medium (Pamiray 300; Dongkook Pharmaceutical, Seoul, Republic of Korea) without dilution was injected at a flow rate of 3–6 ml/s for 12 s after 4–6 s of x-ray delay using a power injector. The injection rate was determined by considering the blood flow of the artery being injected, the maximum flow rate of the catheters, and the total amount of contrast material needed. The acquired images were transferred to the affiliated workstation (X-Leonardo with DynaCT; Siemens). MPR images in all planes were reconstructed with a 0.4-mm slice thickness, and maximum-intensity projection images were obtained.
Definition of terms
-
1)
Hepatic falciform artery (HFA): an artery derived from a branch of the hepatic artery and running along the caudal part of the falciform ligament. It might terminate at any point during its course to the umbilicus or be distributed to the subcutaneous fat layer in the periumbilical skin. If there were multiple arteries satisfying the definition above, the most dominant one in diameter and length was regarded as the HFA and was then categorised into one of the following grades based on its diameter (D) on the axial images of C-arm CTHA: 0 = not present; 1 = hairy (D < 0.4 mm); 2 = thin (0.4 mm ≤ D < 0.6 mm); 3 = intermediate (0.6 mm ≤ D < 1.0 mm); and 4 = prominent (D ≥ 1.0 mm). The HFA was considered radiologically conspicuous if the grade was 2 or higher.
-
2)
Sappey’s superior artery (SSA): an artery traversing the cranial portion of the falciform or coronary ligaments (Fig. 1). It may connect the intrahepatic artery around the falciform ligament-attaching area with an artery of the diaphragm (a branch of any inferior phrenic artery) or the body wall (a branch of any internal mammary artery). It may terminate at any point during its course as seen on C-arm CTHA. The flow direction relative to the liver can be either hepatofugal or hepatopetal. If multiple arteries satisfied the definition above, the most dominant one in diameter and length was regarded as the SSA and was then categorised by the following grades: 0 = not present; 1 = suspicious; 2 = intermediate; or 3 = prominent. The SSA was considered radiologically conspicuous if the grade was 2 or higher.
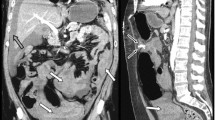
Fig. 1 A 71-year old male patient underwent C-arm CT hepatic arteriography (CTHA) before a chemoembolization procedure. (a) A Sappey’s superior artery (SSA; arrowhead) originated from the surface of the left hepatic lobe and distributed to the costal origin of the left diaphragm via the cranial portion of the falciform ligament and the anterior abdominal fat body which appeared as intraperitoneal fat space on this CT image. (b) The artery was seen merely as a vague linear structure (arrowhead) on the multidetector row CT image
-
3)
Anterior abdominal fat body: a component of the anterior abdominal wall that is found deep to the rectus sheath and immediately beneath the parietal peritoneum, where it composes the base of the falciform ligament (Fig. 2) [22]. The thickness and conspicuity of this entity is variable from person to person.
-
4)
Ensiform artery: an artery that usually runs a straight course, deep to the rectus sheath, as far down as the umbilicus [22]. Although it is generally known to originate from either the superior epigastric artery or the internal mammary arteries, we considered an artery running along the anterior abdominal fat body in a caudocranial direction to be the ensiform artery in this study, in which hepatic, not internal mammary, angiography was analysed. The ensiform artery may terminate at any point during its course on C-arm CTHA due to the blood-flow direction.
Image analysis
All C-arm CTHA images were analysed by two radiologists (J.W.C. and S.H., each with >5 years of experience) in consensus. Corresponding axial images of the contrast-enhanced CT were used as a reference to identify adjacent structures. The prevalence and anatomy of HFAs were analysed on axial images, which were displayed at a fixed window setting (window width: 2,500 Hounsfield units [HU]; window level: 500 HU) with reference to corresponding MDCT images, as needed.
The prevalences of the HFA, the SSA and the ensiform artery were determined. The prevalence, origin, diameter, course, destination and anastomosis with other arteries of radiologically conspicuous HFAs and SSAs were evaluated based on the axial C-arm CTHA images.
Results
Hepatic falciform artery
Prevalence and Diameter
HFAs were observed in 209 patients (95 %). Seventy-one patients (32 %) had a grade 1 HFA, 53 patients (24 %) had a grade 2 HFA, 41 patients (19 %) had a grade 3 HFA, and 43 patients (20 %) had a grade 4 HFA. The prevalence of radiologically conspicuous HFAs (grade 2 or higher) was 62 % (n=137) (Table 1). The mean diameter of the radiologically conspicuous HFAs was 0.82 mm (range, 0.4–1.5 mm).
Origin
Ninety-seven percent (n=133) of radiologically conspicuous HFAs (n=137) originated from the segment 3 or 4 hepatic arteries around the umbilical point (recessus umbilicalis) of the left portal vein (S3, 34 % [n=46]; S4, 54 % [n=74]; both, 9 % [n=13]). In four patients, the HFA had anomalous origins from the cystic artery (n=1) or the proximal left hepatic artery (n=3).
Course
Ninety-three percent (n=127) of the radiologically conspicuous HFAs entered the falciform ligament within the umbilical fossa and adjoined the HFL, while 7 % (n=10) arrived at the surface of segment 3 or 4, just as an isolated artery does, and then traveled along the hepatic surface to adjoin the HFL. After entering the falciform ligament, the HFA usually made an L-shaped turn to the left, coursing in the caudal and medial direction and running through the caudal part of the falciform ligament along with the ligamentum teres or paraumbilical veins, at variable distances from them.
Destination
Eighteen percent (n=24) of radiologically conspicuous HFAs that ran along the falciform ligament ended up within the anterior abdominal fat, while 20 % (n=27) passed through the fat body and reached the rectus abdominis muscle. Thirty of the HFAs, which equals 22 % among the radiologically conspicuous HFAs or 14 % among the whole study population, passed through both the fat body and the rectus abdominis muscle and were distributed to the subcutaneous fat layer around the umbilicus (Fig. 3). The distribution range could not be evaluated in four patients whose anterior abdominal walls were outside the field of view of C-arm CTHA as a consequence of large body habitus.
A 50-year-old male patient with an HCC in the right hepatic lobe. (a) A right antertior oblique volume rendering image shows a conspicuous hepatic falciform artery (HFA) distributed to the subcutaneous fat tissue around the umbilicus. (b) An arterial branch (arrow) is distributed to the anterior abdominal fat body on C-arm CT hepatic arteriography (CTHA). (c) The same branch (arrow) was recognised on the multidetector row CT
Sappey’s superior rtery
Prevalence
SSAs were observed in 49 patients (22 %). Twenty-seven patients (12 %) had a grade 1 SSA, 15 patients (7 %) had a grade 2 SSA and seven patients (3 %) had a grade 3 SSA. The prevalence of radiologically conspicuous SSAs (grade 2 or higher) was 10 % (n=22) (Table 1).
Origin and course
Radiologically conspicuous SSAs originated from the segment 2, 3 or 4 hepatic artery or from the proximal portion of the HFA and entered into the falciform ligament at the border between the left lateral and medial sections, where the falciform ligament attaches to the liver (S2, 9 % [n=2]; S3, 23 % [n=5]; S4, 50 % [n=11]; HFA, 14 % [n=3]). The other SSA arose from the left inferior phrenic artery (LIPA), which originated from an accessary left gastric artery, ran through the mid-portion of the falciform ligament, and became the ensiform artery to be distributed in the subcutaneous fat layer around the umbilicus (Fig. 4).
A 43-year-old male patient whose Sappey’s superior artery (SSA; arrowhead) arises from the LIPA, which originates from an accessary left gastric artery (ALG), then runs through the mid-portion of the falciform ligament, and becomes the ensiform artery (E). It continues to the subcutaneous fat tissue around the umbilicus (SQ)
Ninety-one percent (n=20) of the radiologically conspicuous SSAs headed in the left anterior direction on axial images. Six were shown to anastomose with the LIPA (Fig. 5), but the other 14 ended their course in the falciform ligament. Meanwhile, one SSA from the segment 4 hepatic artery headed in the anterior direction and anastomosed with the right inferior phrenic artery (RIPA) and another SSA from the segment 2 hepatic artery passed the left coronary ligament in a leftward direction and anastomosed with the LIPA.
A 56-year-old male patient. (a) A Sappey’s superior artery (SSA; arrowhead) heads in the left anterior direction on an axial image. (b) The SSA (arrowhead) anastomoses with the left inferior phrenic artery (LIPA), as well as with the right inferior phrenic artery (RIPA) on this volume rendering image. (c) A different patient from an anatomical study by Ibukuro in 2008 shows similar anastomosis among the SSA (white arrowhead), the LIPA (black arrow), the RIPA (white arrow) and the right internal mammary artery (RIMA; black arrowhead) [Image courtesy of Ibukoro and with permission of Surgical and Radiological Anatomy]
Ensiform artery
The ensiform artery was visualised in 29 patients on C-arm CTHA. Twenty-eight ensiform arteries branched from the distal portion of the HFA when it entered into the anterior abdominal fat body and ran along the fat body in an upward direction. Among these, only one ensiform artery was proved to be anastomosed with the right superior epigastric artery in the selective angiography of the HFA. There was an ensiform artery that originated from the SSA and coursed down to reach the umbilical area, which was already mentioned above, in Fig. 4.
Relationships between the falciform ligament arteries
By integrating all of the observed information in this study, we composed a schematic drawing to summarise the relationships among the HFA, SSA, ensiform artery, internal mammary arteries and inferior phrenic arteries (Fig. 6).
Schematic drawing describing the relationships among the arteries of the falciform ligament, diaphragm, anterior abdominal fat body and anterior chest wall. HFA = hepatic falciform artery, SSA = Sappey’s superior artery, RIMA = right internal mammary artery, RMPA = right musculophrenic artery, RSEA = right superior epigastric artery, LIPA = left inferior phrenic artery, RIPA = right inferior phrenic artery, S4 = segment 4 hepatic artery, (a),(b) = potential connections in the falciform ligament
Discussion
Radiologically conspicuous HFAs and SSAs were observed in 62 % and 10 % of the cases, respectively. Although we counted the prevalence of HFAs conservatively by excluding grade 0 or 1 HFAs, this rate is still higher than any of the previously reported values from radiologic studies, but is similar to that of the milestone anatomical study by Michel [12, 19, 20]. The SSA was visualised radiologically and is being reported in the scientific literature for the first time, to our knowledge.
Although skin complications from chemoembolization or radioembolization have been reported [2, 14–17], some researchers are still skeptical of the necessity of the prophylactic embolization of the HFA because they consider the skin complication rate to be very low or insignificant [23, 24]. We agree that skin complications are not likely to occur through the tiny, grade 0 (5 %) or grade 1 (32 %) HFAs in our study. However, 14 % of the population whose HFAs were distributed into the subcutaneous fat around the umbilicus should have a significant chance of experiencing skin complications. Thus, we believe that at least some proportion of large-diameter HFAs should be embolised prophylactically before the infusion of toxic material into the left or middle hepatic artery.
The majority of radiologically conspicuous SSAs were seen as linear enhancing structures in the left anterior direction, arising from the superior convexity of the left hepatic lobe where the falciform ligament attaches. They usually originated from segment 3 or 4 of the hepatic artery. The origin and course of the SSA correspond well with the anatomy of the HFL, which attaches posteriorly to the border between the left lateral and medial segments of the liver and anteriorly to the midline of the diaphragm and the body wall. They also correlate well with the description by Sappey: “The superior group drains the median part of the diaphragm and traverses the upper part of the falciform ligament to reach the convex surface of the liver, where they enter the sublobular divisions of the portal vein” [8].
Ibukuro observed anastomosis between Sappey’s superior vein and the bilateral internal mammary and inferior phrenic veins in the region where the anterior body wall and diaphragm meet [10]. We confirmed this anastomosis between the SSA and the inferior phrenic and internal mammary arteries (Fig. 5), which means that SSAs can become important collateral pathways to HCCs, especially those located in the peripheral border zone between segments 3 and 4, where the falciform ligament attaches. Ibukuro et al. asserted that the phrenic branch of RIMA, which was described by Kim et al. as a collateral arterial pathway from RIMA to an HCC in the left lobe should be an artery running through the falciform ligament rather than through the right part of the diaphragm [6, 10]. We agree with this supposition because the liver and diaphragm are separated by two layers of peritoneum, as long as there is no post-inflammatory adhesion or direct tumour invasion. This collateral supply can be explained by the anastomosis between the phrenic branch of RIMA and the SSA as illustrated in Fig. 6. In the same manner, we think that the anteromedial limb of the LIPA, which was described by Kim et al. as the most frequent parasitic supplier of HCCs arising from the LIPA, is not the LIPA itself but of the SSA in the falciform ligament that is connected to the LIPA [25]. When we compared the representative figures of the anteromedial limb with our figures of the SSA, the origins and courses were nearly identical and were far from the phrenic muscle. Further studies on C-arm CT angiography of the inferior phrenic or internal mammary arteries might provide more evidence to support our hypothesis.
The suggested schematic drawing in Fig. 6 also enables the easy interpretation of complex cases. For example, the route of the contrast agent travelling in the complex case shown in Fig. 4, in which the subcutaneous fat tissue around the umbilicus receives blood flow from the hepatic artery, can be interpreted as common hepatic artery→left hepatic artery→accessory left gastric artery→LIPA→SSA→(a)→ ensiform artery→umbilical skin. The concept of anastomosis among the arteries of the anterior body wall, diaphragm and liver can help in forecasting and searching for extrahepatic collateral arteries that supply liver tumours located near the falciform ligament attachment to the liver.
Our study has several limitations. First, the decision regarding the presence or radiological conspicuity of an HFA or SSA was not clear-cut each time because their imaging features varied from hairy or barely identifiable structures to definite arteries. We used a grading system for this reason, but the question about which grade should be used as the cut-off value for the determination of the prevalence or radiological conspicuity still remained, aside from the reliability of the grading system. Second, the full evaluation of the distribution of HFAs or SSAs was not always possible because of the small field of view and z-axis scan coverage of C-arm CTHA in this retrospective study of examinations performed during routine transarterial treatments. Third, the prevalence of SSAs and ensiform arteries might be underestimated because lack of visualization of an SSA or ensiform artery on C-arm CTHA does not mean that these do not exist, but that the contrast agent injected into the common hepatic artery did not fill their structures.
In conclusion, C-arm CTHA was able to visualise the small arteries in the falciform ligament. The prevalence of HFAs was higher than reported in previous radiological studies and the presence of SSAs was proved for the first time with a radiological method.
References
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022
Salem R, Lewandowski RJ, Mulcahy MF et al (2010) Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 138:52–64
Chung JW, Park JH, Han JK et al (1996) Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology 198:33–40
Hoven AF, Braat MN, Prince JF et al (2016) Liver CT for vascular mapping during radioembolisation workup: comparison of an early and late arterial phase protocol. Eur Radiol 26:1–9
Kim HC, Chung JW, Lee W, Jae HJ, Park JH (2005) Recognizing Extrahepatic Collateral Vessels That Supply Hepatocellular Carcinoma to Avoid Complications of Transcatheter Arterial Chemoembolization. Radiographics 25:S25–S39
Kim HC, Chung JW, Choi SH, Jae HJ, Lee W, Park JH (2007) Internal Mammary Arteries Supplying Hepatocellular Carcinoma: Vascular Anatomy at Digital Subtraction Angiography in 97 Patients. Radiology 242:925–932
Hur S, Kim HC, Chung JW et al (2011) Hepatocellular carcinomas smaller than 4 cm supplied by the intercostal artery: can we predict which intercostal artery supplies the tumor? Korean J Radiol 12:693–699
Sappey MC (1883) Memoire sur les veines portes accessoires. J Anat Phys Norm Pathol Homme Anim 19:517–525
Martin B, Tudor R (1980) The umbilical and paraumbilical veins of man. J Anat 130:305
Ibukuro K, Tanaka R, Fukuda H, Abe S, Tobe K (2008) The superior group of vessels in the falciform ligament: anatomical and radiological correlation. Surg Radiol Anat 30:311–315
Morin C, Lafortune M, Pomier G, Robin M, Breton G (1992) Patent paraumbilical vein: anatomic and hemodynamic variants and their clinical importance. Radiology 185:253–256
Michels NA (1955) Blood supply and anatomy of the upper abdominal organs with a descriptive atlas. B Lippincott Company, Philadelphia, p 7
Williams DM, Cho K, Ensminger W, Ziessman H, Gyves J (1985) Hepatic falciform artery: anatomy, angiographic appearance, and clinical significance. Radiology 156:339–340
Bhalani SM, Lewandowski RJ (2011) Radioembolization complicated by nontarget embolization to the falciform artery. Semin Intervent Radiol 28:234–239
Chuang W-L, Lin C-C, Wu D-K, Shih PM-C, Liu G-C (2004) Supraumbilical skin rash and fat necrosis after transcatheter arterial chemoembolization: a case report. Kaohsiung J Med Sci 20:36–39
Kanzaki H, Nouso K, Miyahara K et al (2009) A case of hepatocellular carcinoma with skin injury of the upper abdominal wall after transcatheter arterial chemoembolization: a case report. Cases J 2:7197
Leong QM, Lai HK, Lo RG, Teo TK, Goh A, Chow PK (2009) Radiation dermatitis following radioembolization for hepatocellular carcinoma: a case for prophylactic embolization of a patent falciform artery. J Vasc Interv Radiol 20:833–836
Baba Y, Miyazono N, Ueno K et al (2000) Hepatic falciform artery: angiographic findings in 25 patients. Acta Radiologica 41:329–333
Gibo M, Hasuo K, Inoue A, Miura N, Murata S (2001) Hepatic falciform artery: angiographic observations and significance. Abdom Imaging 26:515–519
Song SY, Chung JW, Lim HG, Park JH (2006) Nonhepatic arteries originating from the hepatic arteries: angiographic analysis in 250 patients. J Vasc Interv Radiol 17:461–469
Wallace MJ, Kuo MD, Glaiberman C, Binkert CA, Orth RC, Soulez G (2008) Three-dimensional C-arm cone-beam CT: applications in the interventional suite. J Vasc Interv Radiol 19:799–813
Merklin RJ (1971) The anterior abdominal fat body. Am J Anat 132:33–43
Kim GM, Kim HC, Chung JW et al (2012) Chemoembolization for hepatocellular carcinoma supplied exclusively by the hepatic falciform artery. Cardiovasc Intervent Radiol 35:845–851
Kim DE, Yoon H, Ko G, Kwon J, Song H, Sung K (1999) Hepatic falciform artery: is prophylactic embolization needed before short-term hepatic arterial chemoinfusion? AJR Am J Roentgenol 172:1597–1599
Kim HC, Chung JW, An S et al (2009) Left inferior phrenic artery feeding hepatocellular carcinoma: angiographic anatomy using C-arm CT. AJR Am J Roentgenol 193:W288–W294
Acknowledgements
The scientific guarantor of this publication is Jin Wook Chung. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This research was supported by a grant (No. HI15C1532) of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare and by a grant (No. 10051357) of the Industrial Strategic Technology Development Program funded by the Ministry of Trade, Industry and Energy of Republic of Korea. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: Retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hur, S., Chung, J.W., Zhou, C.G. et al. Arteries of the falciform ligament on C-arm CT hepatic arteriography: The hepatic falciform artery and the Sappey’s superior artery. Eur Radiol 27, 1440–1447 (2017). https://doi.org/10.1007/s00330-016-4523-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4523-z