Abstract
The hepatic falciform artery (HFA) may be found in 68 % of subjects in post-mortem dissections. It is well known by interventional radiologists who perform selective hepatic angiography. The reason essentially results from the potential supraumbilical skin complications which may produce by the distribution of chemotherapeutic agents through the HFA after transcatheter chemoinfusion or chemoembolization for liver tumors. Nevertheless, the spontaneous visualization of the HFA remains very unusual in current abdominal CT practice. We hereby report the demonstration of a patent HFA during conventional abdominal CT in two patients presenting without liver disease but in which very unusual variants of the gastrointestinal arteries were simultaneously found. The first patient had a common celiomesenteric trunk and the second had a severe compression of both the celiac trunk and superior mesenteric artery by the median arcuate ligament of the diaphragm. We shortly review the literature about these rare variants. We hypothesize that the HFA was spontaneously visible in our patients because of hypertrophy due supplying collateralization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hepatic falciform artery (HFA) is essentially known by interventional radiologists who perform selective hepatic angiography. They are aware of the potential supraumbilical skin complications which may produce by the inadvertent distribution of chemotherapeutic agents through this artery when they perform transcatheter chemoinfusion or chemoembolization for liver tumors. Otherwise the spontaneous visualization of the HFA is very uncommon in current abdominal CT practice. We recently visualized a patent HFA during conventional abdominal CT in two patients presenting without liver disease but presenting very unusual variants of their gastrointestinal arteries. One had a common celiomesenteric trunk and the other had a severe compression of both the celiac trunk and superior mesenteric artery by the median arcuate ligament of the diaphragm. We shortly review the literature about these rare variants and hypothesize that the HFA was spontaneously visible because of hypertrophy due supplying collateralization.
Report of cases
Case 1
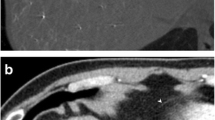
A 59-year-old man with a history of alcoholism and Korsakov syndrome presented with decrease of appetite and weight loss related to dysphagia for liquids and solids. Fibroscopy and Computed Tomography of the upper airways revealed an extremely invasive and circumferentially infiltrative tumoral process of the susglottic area. The tumor extended from the epiglotte to the hypopharynx. At admission, the patient already presented with episodes of false swallowing. Given to the risk of exacerbation of these episodes during radio-chemotherapy a preventive gastrostomy was proposed. Gastrostomy performed during gastroscopy was considered impracticable because of the high position of the stomach in this obese patient. Surgical gastrostomy was performed through sus-ombilical laparotomy. Twenty-four hours later the patient suddenly developed hypovolemic shock. Contrast enhanced emergency CT (Fig. 1) demonstrated massive hemoperitoneum. An active bleeding puddle was found just on the midline of the anterior epigastric area straddling the demarcation between properitoneal fat and the peritoneal cavity just under the level of the laparotomic scar. Post processing with maximal intensity projection (MIP) and volume rendering views (VR) (Fig. 2) diagnosed that bleeding site was feed by the hepatic falciform artery (HFA) clearly emerging from the proper hepatic artery. Emergency reintervention was performed.
Case 1: full coronal (a) and sagital medial (b) MPR views obtained during the arterial phase of contrast enhanced abdominal MDCT. A large amount of hemorragic ascitis (grey arrow) with massive fresh clots (white arrows) is found in the peritoneal cavity. An active bleeding puddle (small black arrows) is found just on the midline of the anterior epigastric area straddling the demarcation between properitoneal fat and the peritoneal cavity. It is situated just in front of the laparotomy site
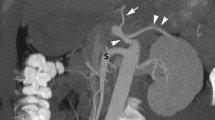
Case 1: sagital oblique maximal intensity projection (MIP) view (a), selective 3D volume rendering of the main hepatic artery (b) and of the common celiomesenteric trunk (c) and axial oblique MIP view of the hepatic artery (d) illustrate the hepatic falciform artery (HFA) (white arrows) feeding the bleeding puddle (black star on a, b) just in front of the laparotomy site (white star on a). This HFA emerges from the proper hepatic artery (small grey arrow). Black arrowhead (on b) = gastroduodenal artery. Grey arrowhead (on b, c) = gastroepiploic artery. White arrowhead (on b) = main common hepatic artery and grey arrow (on c) = short an thin superior mesenteric artery. Small white arrow (on b) = left hepatic artery
Case 2
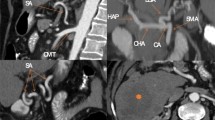
A 36-year female was referred to our department of medical imaging for Multidetector Angio-CT of the renal arteries in a context of arterial hypertension. The patient was absolutely free of any abdominal symptom. The renal arteries appeared normal but the attention was immediately focused by the convolutions of a very large and tortuous artery crisscrossing through the transverse mesocolon in front of the small intestine. Volume rendering reconstructions (Fig. 3) showed that it represented a major enlargement of the arcade of Riolan and of the inferior mesenteric artery (IMA). The reason of this hypertrophy was a major compression not only of the superior mesenteric artery (SMA) but also of the celiac trunk (CT) by the median arcuate ligament (MAL) of the diaphragm. The CT, SMA and the gastro-duodenal artery also had a rather small caliber and classical hypertrophy of the arterial pancreaticoduodenal arcades were also absent. Maximal intensity projection (MIP) (Fig. 4) and selective 3D volume rendering views of the hepatic artery (Fig. 3c, d) also illustrated the course of a long and tortuous hepatic falciform artery (HFA) anastomosing with the superior epigastric artery.
Case 2: anterior (a) and lateral (b) selective 3D volume rendering views of the gastrointestinal arteries. The celiac trunk (black arrow) and the superior mesenteric artery (short black arrow) appear of relatively small caliber and their emergences from the aorta are extremely constricted at the level of the arcuate ligament of the diaphragm (white arrowhead). The gastroduodenal artery also has a rather small caliber (white star). A long and large tortuous anastomosis from a large inferior mesenteric artery provides general substitution (white arrows). A small aneurysm is visible on the anastomosis of the gastroduodenal artery and the superior mesentery artery (in the white circle). Anterior (c) and lateral (d) selective 3D volume rendering views of the hepatic artery (black star) show the course of a long and tortuous anastomosis between the falciform (small black arrows) artery and the superior epigastric artery (long white arrows)
Case 2: a series of three axial MIP views obtained at the level of the falciform ligament (a–c) and another series of coronal MIP views at the same level (d–f) clearly illustrate the long tortuous anastomosis between the falciform (small white arrows) artery and the superior epigastric artery (small black arrow). The long and large tortuous anastomosis from the large inferior mesenteric artery (white arrows), the gastroduodenal artery (star) and the small caliber superior mesenteric artery (grey arrow) are also visible
Discussion
A perfect knowledge of the arterial hepatic vasculature is of primordial importance in several liver pathological conditions comprising orthotopic liver transplant, living donor liver transplantation and in the chemotherapy treatment of hepatic tumors. The presence of hepatic arterial variant is a condition with a high prevalence. Today multidetector computed tomography (MDCT) precisely analyses the hepatic arterial tree configuration. Recently Saba [15] observed anatomic variations in 38.73 % of patients in a large extensive MDCT study on 1910 patients.
Moreover, nonhepatic arteries (NHAs) commonly arise from the hepatic arteries. Song [16] found a prevalence of NHAs in 82 % of a series of 220 patients studied with digital subtraction celiac arteriography and selective left hepatic arteriography. NHAs emerge especially from the left hepatic artery (68 %) and from the proper hepatic artery. The most common NHA is the right gastric artery followed by the hepatic falciform artery (HFA). Accessory left gastric artery, posterior superior pancreatico duodenal artery and left inferior phrenic artery have a much lower prevalence. The most common origin of the right gastric artery and HFA are the proper hepatic artery and the segment 4 hepatic artery [1, 5, 16].
The falciform ligament is the embryologic remnant of the ventral mesentery and marks the separation of the left lobe of the liver into the medial and lateral segments. Arising most commonly as a small terminal branch of the proper or left hepatic artery, the HFA runs through this hepatic falciform ligament, distributes itself around the ombilicus and communicates with branches of the superior and inferior epigastric arteries [1, 5, 8].
The presence of an angiographically identifiable HFA is reportedly found in about 2 % of cases. However, a patent falciform artery has been identified in as many as 68 % of postmortem dissections [16]. This high degree of discrepancies between angiography and dissection may be due to the slow velocity of the small terminal branches of the HFA, which prevents the vessel from being visualized during the early arterial angiographic phases [1, 5]. The HFA is more easily recognized in the capillary or venous phase of angiography because the contrast medium remains within this small vessel [5]. Anastomoses with the superior epigastric artery flowing toward the HFA may also washout the contrast within the HFA may be responsible of its non visualization [5].
The clinical significance of the HFA (essentially described during angiographic studies) has been discussed with reference to two main aspects [5]. First, rare tumors originating from the hepatic falciform ligament are fed by this artery. Second, regarding interventional radiology, supraumbilical skin complications after transcatheter chemoinfusion or chemoembolization for liver tumors have been shown after inappropriate ischemia and/or toxicity of injected anti-cancer or occlusion material within the anatomic territories disserved by the HFA [5, 7]. Fortunately adverse supraumbilical skin injury after hepatic transcatheter chemotherapy remains rare events in contrast with the high prevalence of the HFA [5]. The reason is the presence of constant anastomoses with the internal thoracic and superior epigastric arteries through the falciform ligament. Due to their preferential hepatopetal flow these anastomoses may protect the blood supply of the supraumbilical skin and thus prevent toxic effects of chemotherapeutic drugs [6].
Recently a propective study [19] demonstrated that the detection rates of the HFA with CT hepatic arteriography (thus dynamically performed through a catheter directly positioned in the hepatic artery) was far higher (77 %) than those obtained through conventional angiography and conventional dynamic CT (respectively, 37 and 10 % in this study). Song [16] also demonstrated the falciform artery in 51.6 % of patients using ultra selective arteriography.
In these reported studies, the rather great prevalence of the HFA may be due to the fact that the patients had pathologic livers. Indeed in patients with chronic liver disease, the hepatic arteries have been found being significantly enlarged compared with those of normal subjects [19]. Since the HFA is one of the terminal branches of the hepatic artery, it is reasonably estimable that this HFA can also be dilated as the hepatic artery expands.
Although, to our knowledge, there are no studies reporting the prevalence of the HFA detection during dynamic CT studies in healthy patients it is our opinion, based on our long personal experience with 64-row multidetector CT, that this prevalence remains extremely low.
In both reported cases it is likely that the HFA was visualized because it was enlarged by a compensatory phenomenon related to the critical state of digestive arteries of the patients. For the same reason it is also likely that blood flow in these HFAs was preferentially hepatopetal.
Variants of the celiac and hepatic arteries influence the prevalence and sites of origin of nonhepatic arteries [16]. In the first reported case the left hepatic artery emerged from the main hepatic artery proximally from the gastroduodenal artery and the bleeding HFA came from the right hepatic artery.
Moreover, in this first reported case these relatively atypical hepatic arterial anatomy was associated with the presence of a common celiomesenteric trunk (CMT), another very uncommon variant accounting for only 0.25–1 % of all celiac axis abnormalities in previous reports [11, 12] and found in 3.4 % of a very recent extensive multidetector computed tomographic (MDCT) study of 1500 patients [21].
During the embryologic development, abdominal arteries arise from the primitive dorsal abdominal aorta from four superposed roots: the left gastric artery, the hepatic artery, the splenic artery and finally superior mesenteric artery (SMA). These roots are joined together by a longitudinal ventral anastomose. Normally a cleft forms on this anastomose between the third and fourth roots. This cleft isolates the celiac trunk (CT) components (left gastric artery, hepatic artery and splenic artery) from the more distal SMA. As a result the orifice of the CT and of the SMA are normally classically separated. The persistence of the complete ventral anastomose gives the rare CMT [12].
A patient with CMT is potentially deprived of some of the protective benefits of dual origin vessels with multiple mutually supporting anastomoses. Occlusion or proximal stenosis affecting a common CMT can have serious ischemic consequences to the intestine because the classical redundancy between the celiac and superior mesenteric arterial circulation is absent [11]. Moreover any disorder involving the common CMT (dissection, thrombosis, emboly, and atheromatosis) or an extensive surgery (for example a Wipple’s procedure) may have dramatic consequences on the major abdominal viscera [12, 21].
The second reported case of spontaneous visualization of HFA was also associated with an exceptionally rare pattern of digestive arterial vessels. There was a simultaneous compression of the CT and of the SMA by the median arcuate ligament (MAL).
The MAL is a archlike tendinous band connecting both diaphragmatic crura at the level of the aortic hiatus, posterior and superior to the origin of the CT at the level of the first lumbar vertebra [3, 10, 11, 13]. A low insertion of the MAL or a high origin of the visceral arteries may cause extrinsic compression of the CT, the SMA and sometimes of the renal arteries. Compression typically increases during expiration. The etiology of this type of compression is not clear. It has been suggested that these compressions are not congenital but may be favored by changes in the relationships between the aorta and musculoskeletal structures over time. An indentation or a compression of the CT can be found in 10–24 % of the population [11]. It is usually a fortuitous finding and most cases are asymptomatic. It is due to the rich collaterals between SMA and the CT, usually consisting of prominent and tortuous hypertrophy of the pancreatico duodenal arcades [11].
Thus the MAL syndrome—also called celiac artery compression syndrome or Dunbar syndrome—remains very debated and controversial [4, 9, 10, 13].
The major argument against MAL syndrome as a source of symptomatology is drawn from the observed high frequency of asymptomatic isolated celiac trunk compression and the idea that rich collateral circulation that exists in the splanchnic circulation prevents ischemia in the presence of single-vessel disease [4]. This controversed syndrome usually occurs in younger women and encompasses a constellation of clinical symptoms commonly comprising postprandial epigastric abdominal pain, weight loss, nausea and emesis [10, 17]. The diagnosis is difficult based on suggestive symptoms, typical imaging findings and complete exclusion of other cause. Consequently the surgical treatment of the MALs also remains controversial and questionable. Some vascular surgeons believe that two of the three mesenteric arteries must be occluded or severely stenotic to produce symptoms of chronic mesenteric ischemia, although this view has been refuted by others [10].
In severe CT compression, the hepatic arterial supply is provided by the SMA via the pancreatico duodenal arcades. The proper hepatic artery is then reverse vascularized by the gastroduodenal artery [3].
Curl et al. [2] reported one of the first cases of hemodynamically significant compression of both the SMA ant the CT by the MAL. Nevertheless, this type of double compression has only been infrequently reported. Only 4 of the 51 patients with MAL syndrome reported by Reilly had both CT and SMA compressions [14]. Kopecki [9] and Doyle [4] also reported other isolated cases.
This type of rare compression of both the TC and SMA by the MAL is clearly found in our second reported case. Moreover, this case also clearly illustrates very unusual findings that boost the controversial debate on the MAL syndrome.
First our patient was completely asymptomatic despite the major compression of two main vessels. Then the major compression of the TC in our case was not compensated by the usual hypertrophy of the pancreatico duodenal arcades. The reason was of course the simultaneous compression of the AMS. This particular situation could explain the presence of enlarged umbilical artery which undoubtedly supplied the hepatic artery by classical anastomose with the internal thoracic and superior epigastric arteries. Finally the complete main supply of the entire gastrointestinal tract was nearly exclusively ensured by a large and extremely tortuous and hypertrophic arcade of Riolan (classically described as connecting the middle colic branch of the SMA with the left colic branch of the inferior mesenteric artery). This unique supply is of considerable importance for the patient especially in case of eventual surgery of the aorta or in the territory of the inferior mesenteric artery [3].
Moreover, our patient also presented with a small aneurysm developed on the anastomose of the gastroduodenal artery with the SMA. CT stenosis or occlusion—and compression by the MAL is considered to be one of the common causes of CT stenosis—is known to be one of the main factors for increased collateral circulation and aneurysms, which account for about 50–60 % of all pancreatico duodenal artery aneurysms [18, 20]. Similarly, there have also been several reports regarding gastroduodenal artery aneurysm or jejunal artery aneurysm in association with celiac trunk axis stenosis as reported in our second case. Indeed altered hemodynamics changes in the vascular networks, particularly increased flow through the pancreaticoduodenal arcades, may be related to aneurysm formation. These hemodynamic changes may possibly affect the wall shear stress of the arteries and a close relationship between a high wall shear stress and the initiation of aneurysm formation has already been demonstrated in animal models [18, 20].
References
Bhalani SM, Lewandowski RJ (2011) Radioembolization complicated by nontarget embolization to the falciform artery. Semin Intervent Radiol 28:234–239
Curl JH, Thopson NW, Stanley JC (1971) Median arcuate ligament compression of the celiac and superior mesenteric arteries. Ann Surg 173:314–320
Douard R, Chevallier JM, Delmas V, Cugnenc PH (2006) Clinical interest of digestive arterial trunk anastomoses. Surg Radiol Anat 28:219–227
Doyle AJ, Chandra A (2012) Chronic mesenteric ischemia in a 26-year-old man: multivessel median arcuate ligament compression syndrome. Ann Vasc Surg 26:108
Gibo M, Hasuo K, Inoue A, Miura N, Murata S (2001) Hepatic falciform artery: angiographic observations and significance. Abdom Imaging 26:515–519
Ibukuro K, Tsukiyama T, Mori K, Inoue Y (1998) Hepatic falciform ligament artery: angiographic anatomy and clinical importance. Surg Radiol Anat 20:367–371
Kanzaki H, Nouso K, Miyahara K, Kajikawa N, Kobayashi S et al (2009) A case of hepatocellular carcinoma with skin injury of the upper abdominal wall after transcatheter arterial chemoembolization: a case report. Cases J 2:7197
Kim DE, Yoon HK, Ko GY, Kwon JS, Song HY, Sung KB (1999) Hepatic falciform artery: is prophylactic embolization needed before short-term hepatic arterial chemoinfusion ? AJR 172:1597–1599
Kopecky KK, Stine SB, Dalsing MC, Gottlieb K (1997) Median arcuate ligament syndrome with multivessel involvement: diagnosis with spiral CT angiography. Abdom Imaging 22:318–320
Lamba R, Tanner DT, Sekhon S, McGahan JP, Corwin MT, Lall CG (2014) Multidetector CT of vascular compression syndromes in the abdomen and pelvis. Radiographics 34:93–115
Lee V, Alvarez MD, Bhatt S, Dogra VS (2011) Median arcuate ligament compression of the celiomesenteric trunk. J Clin Imaging Sci 1:8
Lin J (2005) Celiomesenteric trunk demonstrated by 3-dimensional contrast-enhanced magnetic resonance angiography. Hepatobiliary Pancreat Dis Int 4:472–474
Ozbülbül NI (2011) CT angiography of the celiac trunk: anatomy, variants and pathologic findings. Diagn Interv Radiol 17:150–157
Reilly LM, Ammar AD, Stoney RJ, Ehrenfeld WK (1985) Late results following operative repair for celiac artery compression syndrome. J Vasc Surg 2:79–91
Saba L, Mallarini G (2011) Anatomic variations of arterial liver vascularization: an analysis by using MDCTA. Surg Radiol Anat 33:559–568
Song SY, Chung JW, Lim HG, Park JH (2006) Nonhepatic arteries originating from the hepatic arteries: angiographic analysis in 250 patients. J Vasc Interv Radiol 17:461–469
Stein JJ, Costanza MJ, Rivero M, Gahtan V, Amankwah KS (2011) External compression of the superior mesenteric artery by the median arcuate ligament. Vasc Endovascular Surg 45:565–567
Sugiyama K, Takehara Y (2007) Analysis of five cases of splanchnic artery aneurysm associated with coeliac artery stenosis due to compression by the median arcuate ligament. Clin Radiol 62:688–693
Tajima T, Yoshimitsu K, Irie H, Nishie A, Hirakawa M et al (2009) Hepatic falciform ligament artery in patients with chronic liver diseases: detection on computed tomography hepatic arteriography. Acta Radiol 50:743–751
Tsukioka K, Nobara H, Nishimura K (2010) A case of inferior mesenteric artery aneurysm with an occlusive disease in superior mesenteric artery and the celiac artery. Ann Vasc Dis 3:160–163
Wang Y, Cheng C, Wang L, Li R, Chen JH, Gong SG (2014) Anatomical variations in the origins of the celiac axis and the superior mesenteric artery: MDCT angiographic findings and their probable embryological mechanisms. Eur Radiol 24:1777–1784
Conflict of interest
The author declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coulier, B. Uncommon CT imaging of the hepatic falciform artery in patients presenting with very unusual variants of gastrointestinal arteries: report of two cases. Surg Radiol Anat 37, 527–533 (2015). https://doi.org/10.1007/s00276-015-1461-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-015-1461-4