Abstract
Objectives
To evaluate the diagnostic performance of lung biopsies performed immediately after radiofrequency ablation (RFA).
Methods
Twenty consecutive patients were treated with lung RFA. A biopsy was performed immediately after RFA, through the cannula used to insert the RFA probe to avoid hampering the RFA probe placement. Biopsies were analysed for diagnostic of malignancy and tumour morphological characteristics. Recurrence of RFA and procedure-related complications are reported.
Results
Mean tumour size was 17.3 mm (±6.2 mm). Ninety per cent (18/20) of biopsies were able to help diagnose malignancy. Cancer subtype and origin were determined in 70 % (14/20) of tumours, including 12 metastases and two primary lung cancers. During a median follow-up of 24 months, one tumour demonstrated local progression (5 %). The overall survival, lung disease-free survival and progression-free survival rates at 12 months were 100 %, 75 % and 65 %, respectively.
Adverse events of the procedure including RFA and biopsy were five pneumothoraces requiring chest tube placement (25 %), seven minor pneumothoraces (35 %) and one subsegmental intrapulmonary haemorrhage (5 %) not requiring any treatment.
Conclusions
A biopsy performed immediately after lung RFA allowed diagnosis of malignancy in 90 % of cases. This diagnosis is obtained without the need for additional puncture and does not hamper the accuracy of the initial RF probe placement.
Key Points
• Treatment and biopsy are feasible during the same procedure, avoiding multiple punctures.
• The best puncture path can be preserved to treat the lung tumour.
• Malignancy can be determined on a post-RFA biopsy in 90 % of cases.
• Cancer classification can be assessed in 70 % of cases after lung RFA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radiofrequency ablation (RFA) consists in delivering an alternating current of 400–500 kHz using specific probes to destroy a tumour and some surrounding parenchyma by thermal coagulation [1, 2]. RFA of lung tumours is a minimally invasive alternative to surgery for paucinodular metastases of less than 3 cm, especially in poor surgical candidates [3–6].
When there is high suspicion of malignancy, and when the clinical history is concordant with imaging, pathological confirmation is not obtained before treatment [7]. Biopsy obtained in a separate session can potentially induce pneumothorax, haemorrhage, gas embolism, cancer seeding along the biopsy puncture tract [8, 9] and will increase patient discomfort.
A lung biopsy performed immediately before RFA during the same procedure carries the risk of potential difficulties in tumour targeting due to post-biopsy alteration on imaging, with haemorrhage that can blur the tumour and alter the accuracy of RF probe placement, or biopsy-induced pneumothorax that renders the puncture more difficult.
Some preclinical and clinical data suggest that the morphological features of tumour cells are preserved after RF ablation, at least during the first month [10, 11]. As showed in a series [11] of 10 pulmonary malignancies treated with RFA and then resected surgically (at day 3), standard staining (haematoxylin and eosin) showed preserved tissue architecture with only few alterations suggestive of tumour regression when electron microscopy and transferase-mediated nick end-labelling (TUNEL) showed ultrastructurally ablated tumour cells (apoptotic bodies) and double-stranded DNA fragmentation, respectively. Morphologic alterations on post-ablation tumour cells become visible (using standard staining) after 4 weeks following the ablation [10]. Hence, diagnosis of malignancy and characterization of the tumour morphology could theoretically be feasible on post-ablation pathological examinations, thereby avoiding risk, discomfort of a biopsy in a separate session and the potential difficulty in ablation when performing a biopsy immediately before RFA.
The purpose of this study was to evaluate lung biopsies performed immediately after RFA of lung tumours in order to determinate the diagnostic yield of the biopsy to confirm diagnosis of the treated tumour afterwards.
Materials and methods
Study design
The institutional review board approved this single-centre study, and informed consent was obtained from all patients. The characteristics of the patients and related procedures were collected from a prospectively maintained database and analysed retrospectively.
From December 2011 to December 2012, 20 consecutive patients treated with RFA for a lung lesion by a single operator (TdB, with 15 years of experience in pulmonary RFA and 25 years of experience in interventional radiology) underwent a post-RFA lung biopsy.
All patients were eligible for RFA, without restriction in terms of the tumour location or patient history (previously known cancer, pathological subtype, appearance on pre-therapeutic imaging or previous anticancer treatments) for inclusion.
RFA procedure and post-RFA biopsy technical features
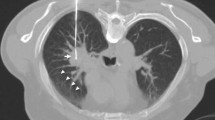
All RFA procedures (Fig. 1) were approved by a multidisciplinary board, attended by oncologists, radiation therapists, radiologists, thoracic surgeons, a pathologist and an interventional radiologist. RFA was preferred according to patient general status, number and location of tumours, or because of patient’s choice. All patients underwent a thoracic CT scan examination immediately before treatment in order to confirm the number and size of tumours. All procedures were done under general anaesthesia and CT guidance. The RFA protocol was the same as that described in previous studies [6]. Briefly, an expandable electrode made from 10 tines (LeVeen CoAccess; Boston Scientific, Natic, Massachusetts, US) with a 15-gauge coaxial system was used. RFA electrodes were 15 cm long with a 3-, 3.5- or 4-cm array diameter when expanded according to the targeted tumour diameter. Patients were placed in the prone, supine or lateral positions, whichever provided access to the best puncture pathway. The needle placement was performed under sequential scans (SmartStep, GE Medical Systems, Milwaukee, Wisconsin, US). Then multiplanar reconstructions from a helical CT volume were obtained after deployment of the electrodes in order to assess correct positioning.
Right upper lobe metastasis (22 mm) in a 60-year-old woman with breast cancer. a Pretreatment CT scan in an axial plane. b Puncture and needle placement. c RFA probe placement in the middle of the lesion. d Post-RFA biopsy, using the same coaxial system (without any additional puncture). e Control acquisition after RFA and post-RFA biopsy showing an ablation zone covering the initial lesion
A post-RFA biopsy of one tumour in each patient was obtained through the coaxial cannula left in place after retrieval of the RF probe. A 18-gauge semiautomatic core needle (Quick-core, Cook, Bloomington, Indiana, US) was used to obtain a sample for pathological analysis. After retrieval of the coaxial cannula, pneumothoraces were aspirated, if present, and a chest tube was inserted if the pneumothorax recurred after aspiration.
Data collection and analysis
RFA procedure
The technical parameters of the RFA procedure (maximum array diameter, number of RF energy deliveries, the duration of ablation and maximum power) were recorded. The correlation between the maximum power delivered during RFA and pathological changes was analysed. Adverse events are reported according to Society of Interventional Radiology (SIR) guidelines [12].
Pathological examination
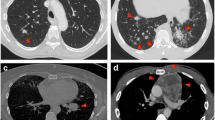
All the specimens were analysed by two pathologists (JA and VdM) who were blinded to patient clinical, imaging and pathological data. The sample size, tumour content, tumour type and histological RFA-induced coagulative necrosis were evaluated with haematoxylin–eosin–saffron (HES) staining (Fig. 2). Post-RF alterations were characterized according to the percentage of coagulation necrosis of the sample (mild, less than 33 %; moderate, at least 33 % and less than 66 %; and intense, at least 66 %).
Representative histological aspects after RFA (haematoxylin–eosin–saffron staining). Arrows indicate identifiable tumour glands within a fibrous stroma (adenocarcinoma) with mild (a) or moderate (b) tissue coagulation artefacts. In one case (c), important coagulation necrosis did not allow any histological diagnosis
Immunohistochemical staining was performed when needed (n = 6 cases), using a Benchmark XT platform (Ventana / Roche Diagnostics, Tucson, AZ) and TTF1 [8G7G3/1], cytokeratin 7 (CK7) [OV-TL 12/30] and cytokeratin 20 (CK20) [Ks20.8] clones (Dako, Golstrup, Denmark)
Outcome
Follow-up was based on clinical and CT scan evaluation at 1 month (baseline for further evaluation) and every 3 months for a total of 1-year of follow-up. After 1 year, follow-up was left to the discretion of the medical oncologist or the interventional radiologist in charge of the patient.
Incomplete treatment was defined as an increase in the overall size of the ablation zone or a change in shape indicating enlargement of one part of the lesion on CT in comparison with the baseline CT (1 month after RFA). The overall survival rate (interval between the date of RFA and the date of death, whatever the cause, or the date of the last follow-up for patients who were still alive), progression-free survival and lung disease-free survival rates (time to death, incomplete local treatment, detection of a new lung tumour or last follow-up if none of the previous had occurred) were evaluated.
Statistical analysis
Descriptive statistics were used to report post-RFA biopsy diagnostic performance. Percentages with 95 % confidence intervals and means with standard deviations were reported.
Correlation between the maximum RFA power and coagulative necrosis was evaluated using the Spearman non-parametric coefficient.
The Kaplan–Meier method was used to estimate time to failure curves.
Statistical analysis was performed with GraphPad Prism software, version 5.0 (LA Jolla, CA).
Results
Patient and tumours
Patients had a personal history of malignancy including colorectal cancer (n = 9), lung adenocarcinoma (n = 2), renal cell cancer (n = 2), parathyroid carcinoma, melanoma, osteosarcoma, cholangiocarcinoma, pancreatic, ovarian and breast adenocarcinoma (Table 1). All 20 RF-targeted lung nodules were considered malignant by the multidisciplinary board because of known history of malignancy, increase in size, or appearance on follow-up CT scans, or positive PET CT scan. Thus, no pathological diagnosis was required before treatment, upon decision of the tumour board.
Targeted nodules for treatment measured 17.3 ± 6.2 mm. Four nodules were in contact with the pleura, eight peripherally (less than 2 cm from the pleura) and eight centrally located [13]. Emphysema was present along the puncture tract in 4/20 patients (20 %).
One tumour was treated in 13 patients, and at least two tumours were treated in the remaining seven patients.
RFA procedure and complications
Table 2 summarizes procedures characteristics and complications. Briefly, the mean number of RF location of energy deliveries was 1.5 ± 0.7 per tumour, and overall RF delivery time was 24.9 ± 11.9 min, with a mean maximum power of 93 ± 34.1 W. At least one impedance rise (so-called roll-off) was obtained for each treated lesion.
Twelve pneumothoraces occurred (60 %) with five patients (25 %) requiring chest tube. One patient required surgery for pleural fixation for an intractable pneumothorax, related to diffuse panlobular emphysema.
One limited alveolar subsegmental haemorrhage had a spontaneous favourable outcome without any specific treatment.
Pathological data
Sample sizes obtained at biopsy ranged from 4 to 20 mm in length with a tumour cellular content of 0 to 11 mm (Table 3). RF-induced coagulation was always present, scored as mild (35 %), moderate (45 %) or intense (20 %). Such coagulation was observed through 87.2 ± 19.6 % of the sample surface. There was no correlation between RF-induced coagulation and the maximum RF-power delivered (r = 0.22, p = 0.34)
Pathological diagnosis of malignancy was possible in 18/20 cases (90 %, 95 % CI 69–98 %). Histological subtyping of malignant tumour proliferation (adenocarcinoma, squamous cell carcinoma, osteosarcoma) was possible in 14 cases (70 %, 95 % CI 48–86 %).
Among the 20 patients suspected of having metastasis, two unexpected primary lung cancers were discovered in two patients with a previous history of renal and colorectal carcinomas.
Immunohistochemistry
In four cases, the biopsy sample also included coagulated normal lung parenchyma, and scoring of TTF1 and CK7 expression showed loss of TTF1 expression in all four samples of lung tissues and loss of CK7 in three out of four cases. Scoring of CK20 or CK7 expression in tumours was expected to be positive (n = 5) because of previous characterization on another tumour location that showed a loss of CK20 immunoreactivity in three colorectal cancers and of CK7 in a lung adenocarcinoma and a cholangiocarcinoma.
Outcome
After a median follow-up of 24 months [14–35 months], one of 20 (5 %) tumours showed local tumour progression at the ablation site of a recurrence of primary lung adenocarcinoma, which was already a local recurrence after surgery. Overall survival, lung disease-free survival and progression-free survival rates at 12 months were 100 %, 75 % and 65 %, respectively.
Discussion
One of the drawbacks of RFA is the common lack of histological proof of the ablated tumour. This applies to any organ and can be extended to other image-guided therapies such as stereotactic body radiation therapy. Histological proof means either an additional transthoracic needle procedure a few days before treatment or a biopsy performed immediately before RFA. When biopsy is performed as a separate procedure, the patient needs two visits at the hospital, and there are risk of additional complications and discomfort [13–15]. When biopsy is performed immediately before RFA, parenchymal bleeding or pneumothorax induced by the biopsy may hamper accuracy of subsequent RFA needle placement by blurring or displacing the tumour. Schneider et al. recently reported a series of biopsies performed immediately before RFA during the same procedure under general anaesthesia [16]. The biopsy was performed with an additional puncture because of the lack of guiding cannula and demonstrated a rate of 24 % of pulmonary haemorrhage and 12 % of pneumothorax occurring after the biopsy and hence before RFA.
An alternative technique could be to place the RF probe first and, when position is secured, then to perform biopsy through a second puncture. This does not obviate the need for two needle punctures likely increasing the risk of complication (bleeding, pneumothorax, seeding), and displacement of nodules by the pneumothorax could be a problem if expandable needles are not used. Indeed, the targeted tumour can slip away from the needle.
The single approach proposed in this study combines the best tumour targeting for the ablation, with a diagnostic yield of 90 % for malignancy with acceptable 70 % cancer subtyping. These results are in line with those reported for lung biopsy [17–19], confirming the observations made by Clasen et al. on surgical samples, suggesting that tissue architecture is preserved after RFA [11]. It is also interesting to note that the local efficacy of RFA does not seem to be altered by post-RFA biopsy. Indeed, we report a 95 % complete ablation rate in accordance with previously published series [5, 6, 20], while Schneider et al. had 77 % at 12 months when performing the biopsy immediately before RFA [16].
The main limitation of post-RFA biopsy diagnosis ability is rare major RF coagulation (5 %), which prevents the morphological diagnosis and immunostaining, and hence the possibility of differentiating between primary lung cancer and metastatic disease. Such lack of immunostaining can strongly limit the evaluation of diagnostic and potentially predictive factors. Consequently, post-RF lung biopsy should be considered when the above are not mandatory. Post-RFA biopsy can easily and safely be performed after any RFA treatment in order to confirm the nature of the ablated tumour in the same way a surgeon asks for a pathology study after resection.
Although the degree of RF-induced coagulation could be thought of as predictive of the success of ablation, various degrees of coagulation at pathology were neither correlated with the intensity of RFA applied nor correlated with local recurrence, and thus such a biopsy is useless for the evaluation of the treatment, unless specific analyses are made [21]
The limitations of this study are the small number of patients and its retrospective design, even though consecutive patients were included prospectively in the database, and the pathological analysis was blinded for the entire medical file.
We herein report a safe and efficient method for obtaining the pathological diagnosis at the time of RFA. Future prospects such as molecular analysis of samples looking for specific mutations [22] have to be evaluated, and could potentially help adapt treatments and trials towards personalized medicine [23]. Another prospect could be post-ablation quantification of tumour antigens and proteins that play an important role in tumour response [24] in order to predict the need for and role of adjuvant immunotherapy [25, 26], as antigenic exposure may be increased after RFA as a result of cellular damage.
References
Rossi S, Di Stasi M, Buscarini E et al (1995) Percutaneous radiofrequency interstitial thermal ablation in the treatment of small hepatocellular carcinoma. Cancer J Sci Am 1:73–81
de Baere T (2011) Lung tumor radiofrequency ablation: where do we stand? Cardiovasc Intervent Radiol 34:241–251
Yasui K, Kanazawa S, Sano Y et al (2004) Thoracic tumors treated with CT-guided radiofrequency ablation: initial experience. Radiology 231:850–857
Dupuy DE (2011) Image-guided thermal ablation of lung malignancies. Radiology 260:633–655
Simon CJ, Dupuy DE, DiPetrillo TA et al (2007) Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology 243:268–275
de Baere T, Palussiere J, Auperin A et al (2006) Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology 240:587–596
Manhire A, Charig M, Clelland C et al (2003) Guidelines for radiologically guided lung biopsy. Thorax 58:920–936
Hiraki T, Mimura H, Gobara H et al (2009) Two cases of needle-tract seeding after percutaneous radiofrequency ablation for lung cancer. J Vasc Interv Radiol 20:415–418
Yamakado K, Akeboshi M, Nakatsuka A et al (2005) Tumor seeding following lung radiofrequency ablation: a case report. Cardiovasc Intervent Radiol 28:530–532
Wang Q, Huang J, Ma K et al (2012) Evaluation of ghost cell survival in the area of radiofrequency ablation. PLoS One 7, e53158
Clasen S, Krober SM, Kosan B et al (2008) Pathomorphologic evaluation of pulmonary radiofrequency ablation: proof of cell death is characterized by DNA fragmentation and apoptotic bodies. Cancer 113:3121–3129
Sacks D, McClenny TE, Cardella JF, Lewis CA (2003) Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202
Yeow KM, Su IH, Pan KT et al (2004) Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 126:748–754
Ko JP, Shepard JO, Drucker EA et al (2001) Factors influencing pneumothorax rate at lung biopsy: are dwell time and angle of pleural puncture contributing factors? Radiology 218:491–496
Khan MF, Straub R, Moghaddam SR et al (2008) Variables affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiol 18:1356–1363
Schneider T, Puderbach M, Kunz J et al (2012) Simultaneous computed tomography-guided biopsy and radiofrequency ablation of solitary pulmonary malignancy in high-risk patients. Respiration 84:501–508
Montaudon M, Latrabe V, Pariente A, Corneloup O, Begueret H, Laurent F (2004) Factors influencing accuracy of CT-guided percutaneous biopsies of pulmonary lesions. Eur Radiol 14:1234–1240
Lucidarme O, Howarth N, Finet JF, Grenier PA (1998) Intrapulmonary lesions: percutaneous automated biopsy with a detachable, 18-gauge, coaxial cutting needle. Radiology 207:759–765
Gupta S, Wallace MJ, Cardella JF, Kundu S, Miller DL, Rose SC (2010) Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol 21:969–975
Lencioni R, Crocetti L, Cioni R et al (2008) Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 9:621–628
Sofocleous CT, Garg S, Petrovic LM et al (2012) Ki-67 is a prognostic biomarker of survival after radiofrequency ablation of liver malignancies. Ann Surg Oncol 19:4262–4269
Yoon HJ, Lee HY, Lee KS et al (2012) Repeat biopsy for mutational analysis of non-small cell lung cancers resistant to previous chemotherapy: adequacy and complications. Radiology 265:939–948
Arnedos M, Vielh P, Soria JC, Andre F (2013) The genetic complexity of common cancers and the promise of personalized medicine: is there any hope? J Pathol. doi:10.1002/path.4276
Chu KF, Dupuy DE (2014) Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 14:199–208
Hamamoto S, Okuma T, Yamamoto A et al (2013) Radiofrequency ablation and immunostimulant OK-432: combination therapy enhances systemic antitumor immunity for treatment of VX2 lung tumors in rabbits. Radiology 267:405–413
Kageyama K, Yamamoto A, Okuma T et al (2013) Radiofrequency ablation of liver tumors in combination with local OK-432 injection prolongs survival and suppresses distant tumor growth in the rabbit model with intra- and extrahepatic VX2 tumors. Cardiovasc Intervent Radiol 36:1383–1392
Acknowledgments
The authors would like to thank Lorna Saint-Ange for editing. The scientific guarantor of this publication is Dr Lambros Tselikas. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
The authors state that this work has not received any funding. One of the authors has significant statistical expertise and no complex statistical methods were necessary for this paper.
Institutional review board approval was obtained. Written informed consent was waived by the institutional review board. Methodology: retrospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tselikas, L., de Baere, T., Deschamps, F. et al. Diagnostic yield of a biopsy performed immediately after lung radiofrequency ablation. Eur Radiol 27, 1211–1217 (2017). https://doi.org/10.1007/s00330-016-4447-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4447-7