Abstract
Objective
To evaluate the feasibility, image quality, and radiation dose of automatic spectral imaging protocol selection (ASIS) and adaptive statistical iterative reconstruction (ASIR) with reduced contrast agent dose in abdominal multiphase CT.
Methods
One hundred and sixty patients were randomly divided into two scan protocols (n = 80 each; protocol A, 120 kVp/450 mgI/kg, filtered back projection algorithm (FBP); protocol B, spectral CT imaging with ASIS and 40 to 70 keV monochromatic images generated per 300 mgI/kg, ASIR algorithm. Quantitative parameters (image noise and contrast-to-noise ratios [CNRs]) and qualitative visual parameters (image noise, small structures, organ enhancement, and overall image quality) were compared.
Results
Monochromatic images at 50 keV and 60 keV provided similar or lower image noise, but higher contrast and overall image quality as compared with 120-kVp images. Despite the higher image noise, 40-keV images showed similar overall image quality compared to 120-kVp images. Radiation dose did not differ between the two protocols, while contrast agent dose in protocol B was reduced by 33 %.
Conclusion
Application of ASIR and ASIS to monochromatic imaging from 40 to 60 keV allowed contrast agent dose reduction with adequate image quality and without increasing radiation dose compared to 120 kVp with FBP.
Key Points
• Automatic spectral imaging protocol selection provides appropriate scan protocols.
• Abdominal CT is feasible using spectral imaging and 300 mgI/kg contrast agent.
• 50-keV monochromatic images with 50 % ASIR provide optimal image quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the dramatically increased clinical use of diagnostic CT imaging, attention has been focused on the potential risks of radiation exposure-induced carcinogenesis [1] and contrast agent-induced nephropathy [2]. Although the effect of contrast agent dose on nephropathy is not conclusive, reduced amounts of iodine can be beneficial in terms of patient safety [3, 4] and health care costs [4–7]. Scanning with low tube voltage has been the accepted method to reduce radiation dose and contrast medium volume [8–13] with iterative reconstruction algorithm used to reduce its quantum noise [14, 15]. However, the real-time variation in the tube voltage level in a single-energy CT examination for each patient is not time efficient and cannot provide more information than dual-energy CT [16].
Spectral CT, a dual-energy CT system with fast kilovoltage switching, allows the reconstruction of monochromatic images with selectable X-ray energy (40 to 140 keV) to optimize contrast and clinical applications [17]. The low-energy range has shown promise to reduce the amount of agent used because of the high contrast [18–20] and is expected to increase the image noise due to the reduced number of photons in the lower X-ray energy spectrum [17]. Adaptive iterative reconstruction algorithm (ASIR) thus has been incorporated into the monochromatic image sets to reduce image noise effectively and take full advantage of the increased contrast over the low-energy range [18, 21]. However, previous research about monochromatic energy level combined with ASIR was limited to fixed energy levels such as 60 keV [18] or 70 keV [21] without evaluating the effect on the lower-energy levels (40 to 60 keV), which show relatively higher iodine contrast [17] similar to the lower polychromatic X-ray energy in conventional CT [19]. Moreover, previously published spectral imaging scanning protocol settings were not based on the principle of individualization, displaying similar or even higher radiation dose as compared with conventional CT [16, 17, 22, 23]. The recently developed automatic spectral imaging protocol selection (ASIS) technique, a commercially available technique (GSI Assist, GE Healthcare), can individualize patient dose by providing assistance in the selection of the optimal preset based on the patient’s size and preferred scan parameters.
To the best of our knowledge, no study has been performed on liver spectral CT combining ASIS and low contrast agent volume. The present investigation was designed to investigate the image quality and radiation dose of contrast-enhanced abdominal spectral CT imaging using ASIS and ASIR with reduced contrast agent dose by using the conventional 120-kVp CT with filtered back projection algorithm(FBP)and standard contrast agent dose as a reference standard.
Methods
This prospective single-institution study was approved by the Human Research Committee of our institutional review board, and all patients provided written informed consent.
Patient selection
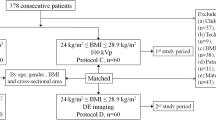
The inclusion criteria included patients with known or suspected liver lesions, chronic hepatitis, or liver cirrhosis based on a treatment history, prior imaging examination, or abnormally increased tumour marker (a-fetoprotein >11 ng/mL or carcinoembryonic antigen > 5 ng/mL) level and was scheduled for clinically indicated multiphase liver CT. Clinical exclusion criteria were patients younger than 18 years of age, pregnant or lactating, had history of an allergic reaction to iodinated contrast media, or compromised renal function (estimated glomerular filtration rate <30 mL/min/1.73 m2). Thirty-four (17.5 %) of the 194 patients were excluded for the following: 10 patients had advanced liver cirrhosis defined as Child-Pugh class C; there was a technical failure during contrast injection for six patients; 18 patients declined after reviewing the informed consent.
Finally, 160 patients were recruited between February and May 2015. Morphologic data (weight, height, and body mass index [BMI, calculated as the weight in kilograms divided by height in meters squared]) were recorded. Body weights in all patients prior to CT examination were measured to tailor the amount of contrast media of their individual needs.
Principle of automatic spectral imaging protocol selection
The ASIS technique (GSI Assist) is designed to recommend automatically the optimal spectral imaging preset that has the closest volume CT dose index (CTDIvol, mGy) to the target non-spectral imaging CTDIvol based on the target non-spectral imaging noise index at the same slice thickness, pitch and patient size (absorption path length) determined during the anteroposterior and lateral scout views. In the conventional, non-spectral CT imaging, the selection of noise index determines the required radiation dose (CTDIvol) using an automatic tube current modulation (x-y-z axis) mode such as AutomA/SmartmA techniques. In spectral CT imaging, a number of presets exist, each with fixed tube current during rotation. During spectral imaging, the same noise index as in the conventional non-spectral imaging is used and ASIS technique recommends a preset that has the closest CTDIvol to the conventional imaging mode.
CT examinations and image reconstruction
All patients underwent nonenhanced and two-phase contrast-enhanced CT examinations of the entire liver with a CT scanner (Discovery CT; GE Healthcare, Waukesha, WI, USA). Of the 160 enrolled patients, 80 were randomly assigned to the conventional 120-kVp protocol (protocol A) with automatic tube current selection and standard dose of contrast material (450 mg of iodine per kilogram of body weight[mgI/kg), routinely used in our hospital to obtain adequate liver enhancement of more than 50 HU to perform high diagnostic quality hepatic CT [24–26]. The other 80 patients were scanned in the spectral imaging protocol (protocol B) with ASIS technique (GSI Assist) for selecting spectral imaging preset and 33 % less contrast agent dose: 300 mgI/kg, based on the previous report [18] and our clinical experience that the contrast agent dose down to 300mgI/kg just can maintain the similar enhancement in the liver to that of 450mgI/kg through the use of optimal low-energy levels in spectral CT. The detailed scanning parameters of each protocol are shown in Table 1.
Iohexol (Omnipaque, 350 mgI/mL, GE Healthcare, Milwaukee, WI, USA) was injected with a power injector (Envision CT Injector, Medrad, GA, USA) through a 20-G catheter inserted into an antecubital vein, followed by 20 mL of saline. The total iodine dose and the injection rate were adjusted by the imaging technicians based on patient body weight [24, 27]. The arterial-phase (AP) and portal venous-phase (PVP) images were acquired 30 s and 60 s after the beginning of contrast agent administration based on the recommendation of Awai et al. [27] to achieve nearly constant degree of arterial enhancement using a fixed delay regardless of patient weight or injection rate.
Images in protocol A was reconstructed with the traditional filtered back projection algorithm to serve as the reference standard. In protocol B, monochromatic images with energy values ranging from 40 to 70 keV in 10-keV increments were reconstructed with 50 % ASIR blending based on the recommendations of the manufacturer, previous study [18], and our practical experience.
Image analysis
Quantitative analysis.
A radiologist (P.J.L., with 5 years of experience in abdominal CT) who was blinded to the image review results performed quantitative measurements at a commercially available workstation (Advantage Workstation 4.6; GE Healthcare, Milwaukee, WI, USA). Hounsfield units (HU) of attenuation of the liver, abdominal aorta, portal vein, and paraspinal muscle at the level of portal vein were measured in a circular region of interest (ROI), as described in previous reports [11, 28, 29]. Image noise was defined as the standard deviation of the HU in a homogeneous region of the subcutaneous fat on the anterior abdominal wall away from artefact or vessels [29]. For all measurements, the size, shape, and position of the ROI were kept consistent between the two phases and were performed three times at different image levels to calculate the average values.
Contrast-to-noise ratios (CNRs) relative to muscle for the organ of interest (liver, aorta, and portal vein) were calculated by using the following formulas: CNRo = (ROIo – ROIm)/SDn, where ROIo is the mean attenuation of the organ of interest, ROIm is the mean attenuation of the paraspinal muscle, and SDn is the mean image noise.
Qualitative analysis.
Two radiologists (J.L. [observer 1] and J.B.G. [observer 2] with 15 and 33 years of clinical experience, respectively ) and one radiology chief resident (X.P.Y. [observer 3]) independently performed a blinded qualitative analysis of CT images at the same workstation. A total of 800 images (80 × 5 × 2), at the level of hepatic portal vein, comprising both 120 kVp images in protocol A and monochromatic images in the range of 40–70 keV in protocol B, were presented in a random order to each of the readers at each session. Patient demographics or CT parameters were removed from the images to ensure blinded evaluation. Although the images were initially presented on a preset soft-tissue window with width of 400 HU and level of 40 HU, the readers were allowed to change the window settings as necessary.
The three readers were asked to rank the image quality based on a previously reported three-point or five-point scoring scheme [11]. The assessment of image noise and overall image quality were according to a five-point scale (1, severe image noise and artefacts, unacceptable; 2, high image noise and artefacts, confidence in details and anatomical structure decreased, just acceptable; 3, moderate image noise and artefacts, decreased confidence in details, but anatomical structure still relatively clear; 4, mild image noise and artefacts, less clear anatomical structure and details; and 5, no obvious image noise and artefacts, sharp anatomical structure and satisfactory details) on both AP and PVP images, while evaluation of visibility of small vascular structures was based on a five-point scale (1, unacceptable visualization; 2, suboptimal visibility; 3, acceptable visibility; 4, above average visibility; and 5, excellent visualization) on AP images and abdominal organ contrast enhancement was done by using a three-point scale (1, unacceptable; 2, acceptable; and 3, excellent) on PVP images.
Lesion analysis
Twenty-five of the 160 patients (protocol A, n = 10; protocol B, n = 15) had solitary or multiple hypervascular hepatocellular carcinoma nodules. In total, 15 hypervascular hepatocellular carcinomas were identified in protocol A while 18 were identified in protocol B. Clinical diagnosis of hepatocellular carcinoma was confirmed by means of histological findings (n = 12) or typical imaging findings (n = 21) includes hypervascular lesions in the hepatic arterial phase with wash-out in the portal venous phase [23]. Only the three largest tumours were included if cases had more than three tumours.
An abdominal radiologist (Y.R.C.) with 3 years of experience measured the attenuations of the high-enhancing portions of the hypervascular liver lesions and adjacent non-tumorous liver parenchyma. The tumour-to-liver contrast ratio was calculated as the attenuation ratio between hypervascular liver lesions and adjacent hepatic parenchyma [18]. For the qualitative lesion analysis, three abdominal radiologists assessed the conspicuity of 33 hypervascular liver tumours on AP images, according to a five-point scale (score of 1, definitely an artefact mimicking a lesion; 2, probably an artefact mimicking a lesion; 3, subtle lesion; 4, well-seen lesion with poorly visualized margins; 5, well-seen lesion with visualized margins) [30].
Radiation dose
For each patient, the CTDIvol (mGy) and dose-length product (DLP, mGy · cm) values were recorded in protocols A and B on the AP images. Radiation dose for the PVP scan acquired during a single breath-hold by helical scan of the liver using the same scanning parameters as that for the AP scan, was not included in the analyses. The estimated effective radiation dose (mSv) was obtained by multiplying the DLP by a conversion factor of 0.015 for abdominal examination, as recommended by the International Commission on Radiological Protection (ICRP) publication 103[31]. To analyze effective radiation dose distribution according to patient sizes, four groups were categorized according to their body mass indices (BMI) (less than 18.5 kg/m2, underweight; between 18.5 and 23.9 kg/m2, normal BMI; between 24 and 28.9 kg/m2, overweight; and 29 kg/m2 or greater, obese)[32]. The correlations between BMI and recommended CTDIvol with ASIS technique were calculated.
Statistical analysis
All statistical calculations were performed using SPSS software (SPSS, version 16, SPSS Inc., Chicago, IL, USA) with P < 0.05 indicating a statistically significant difference. All numeric values were reported as means ± standard deviation of the mean, and categorical variables were summarized by using counts and proportions. An independent sample t-test was used to investigate differences for CT attenuation, tumour-to-liver ratio, CNR for liver and vessels, CTDIvol, DLP and effective radiation dose between protocol A and protocol B. Mann–Whitney U test was used to analyze differences in categorical variables, qualitative parameters and effective radiation dose in BMI subgroup with non-normal distribution and heterogeneity of variance. Pearson correlation coefficient was calculated for the correlations between BMI and recommended CTDIvol with ASIS technique. Kappa analysis was used to evaluate the inter-reader agreement for qualitative parameters with κ statistics [17].
Results
Details of the baseline characteristics for all patients are shown in Table 2. No significant differences in age (P = 0.759), sex (P = 0.525), weight (P =0.768), BMI distribution (P =0.785) and the presence of underlying disease (P = 0.798) were observed between the two protocols.
Quantitative image analysis
Compared with protocol A, the mean CT numbers from 40 to 70 keV for the liver, abdominal aorta and portal vein in protocol B were significantly higher (P < 0.001 for each comparison) for the energy levels at 40 keV and 50 keV except for the liver during the AP at 50 keV (P =0.215), similar for the energy level at 60 keV (P =0.060 for AP and 0.184 for PVP) and lower at 70 keV (P < 0.001 for each comparison) (Table 3).
In protocol B, the mean image noise decreased as the monochromatic energy level increased from 40 to 70 keV. Mean image noise for protocol A was 17.00 HU ± 4.38 in AP and 17.29 HU ± 4.26 in PVP, respectively, significantly lower than those for the energy levels at 40 keV (23.37 HU ± 5.21 and 22.51HU ± 4.23), similar to those at 50 keV (16.98 HU ± 4.07, P =0.985 and 16.85 HU ± 3.84, P =0.078), higher than those at 60 keV (11.83 HU ± 2.98 and 12.06 HU ± 2.43) and 70 keV (11.25 HU ± 3.16 and 11.50 HU ± 2.97), an average increase of 44 % and 43 % for 60 keV, 51 % and 50 % for 70 keV, respectively (P < 0.001 for each comparison with statistical difference) (Fig. 1).
The CNR values for the liver, abdominal aorta and portal vein on monochromatic images in the range of 40–60 keV in protocol B were all significantly higher than those in protocol A (P < 0.001 for each comparison) except for the liver in AP (P = 0.127, 0.307, 0.121 for 40–60 keV, respectively) (Figs. 2 and 3), an average increase of 44–67 % in AP and 50–94 % in PVP, respectively. However, there were no significant differences in CNR of any ROI between protocol B at 70 keV and protocol A, except for the liver in PVP (P = 0.017) (Fig 2, Table 3).
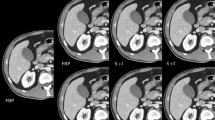
Transverse contrast-enhanced conventional CT images (a for AP, f for PVP) with 120 kVp, 90 mL contrast agent and FBP in a 57-year-old man with BMI of 25.71 kg/m2 and monochromatic images (b-e for AP and g-j for PVP) with spectral imaging, 43 mL contrast agent and ASIR in a 55-year-old man with BMI of 24.44 kg/m2. The overall image qualities for the images at 120 kVp were similar to that at 40 keV, lower than those at 50 keV and 60 keV, and higher than that at 70 keV
Qualitative image analysis
The image quality scores of the two protocols and the level of inter-observer agreement are shown in Table 4. All image quality scores, irrespective of the protocol, were equal to or greater than 2 which indicate an acceptable overall image quality. Compared with protocol A , the analyses of the subjective readers’ rankings were significantly higher in protocol B at 50 keV and 60 keV with regard to overall image quality (P < 0.001 for each comparison), similar at 50 keV, but lower at 60 keV with respect to image noise (P < 0.001), higher at 50 keV (P < 0.001), but similar at 60 keV with respect to small vascular structure visibility and abdominal organ enhancement. The energy level at 40 keV in protocol B showed higher small vascular structure visibility (P < 0.001), abdominal organ enhancement (P <0.001), and image noise (P =0.004 for AP; P <0.001 for PVP ), but similar overall image quality as compared with protocol A. Lower overall image qualities were observed for the energy level at 70 keV in protocol B than that in protocol A.
Lesion analysis
The tumour-to-liver contrast ratio and lesion conspicuity are shown in Table 5. Among the energy levels from 40 to 70 keV in protocol B, only 40-keV and 50-keV images manifested both higher tumour-to-liver ratio and lesion conspicuity as compared to protocol A (P values ranging from < 0.003 to 0.033).
Radiation dose
There were no significant differences between the protocol A and protocol B in CTDIvol (12.52 ± 4.85 vs. 11.95 ± 4.21, P = 0.517), DLP (354.53 ± 124.27 vs. 332.70 ± 119.67, P = 0.363), and effective radiation dose (5.32 ± 1.86 vs. 4.99 ± 1.80, P = 0.363), irrespective of BMI (Fig. 4). The correlation between BMI and recommended CTDIvol with ASIS technique was positive (coefficient = 0.632, P < 0.001) .
Discussion
Previously published dose reports in rapid kV switching were inconclusive [16, 17, 22, 23] perhaps because of the failure of tube voltage-specific tube current modulation and photon starvation of 80-kVp images [16]. The recently introduced ASIS technique can individualize the spectral CTDIvol based on the target non-spectral imaging noise index to maintain similar dose as the conventional non-spectral imaging. Our results confirm that this principle can be applied to abdominal multiphase CT studies: based on the same noise index, the radiation dose in spectral CT with ASIS technique was almost the same as the 120-kVp CT, irrespective of BMI. It should be pointed out that for the patients with too low or too high BMI, the CTDIvol recommended in spectral CT may exceed the CTDIvol range allowed in the ASIS system, resulting in higher (for very small patients) or lower (for very large patients) dose than in 120-kVp CT.
Under the similar radiation dose as the conventional 120-kVp CT, reported by Matsumoto et al. [19] in vitro, the CT numbers of monochromatic spectral images at approximately 70 keV was equal to those of the 120-kVp CT images and increased as the X-ray energy decreased. Generally, the monochromatic energy is selectable with low energies yielding more contrast between materials [17]. The photoelectric effect in X-ray attenuation is increased at reduced X-ray energy, particularly in structures with a high effective atomic number, such as iodinated contrast material, providing iodine more attenuating at lower energies. Therefore, hypervascular hepatic lesions with more iodine are also more conspicuous at lower energies [17]. Findings of the our study revealed that low monochromatic energies (40–60 keV) maintain the same and even higher contrast by reducing 33 % contrast agent (300 vs. 450 mgI/kg); the tumour-to-liver contrast ratio that reflected the lesion detection capability gradually increased as the energy level decreased, significantly higher at 40 keV and 50 keV, similar to previous finding [17].
However, higher contrast in low-energy levels was often accompanied by higher image noise due to the lack of photon flux [16, 17, 19]. To compensate for the increased image noise, an ASIR algorithm can be incorporated [18, 21] to preserve the natural appearance of images [29]. Considering the process of customized ASIR for each energy level was not time efficient and would disrupt clinical workflow, we preset the energy levels with same ASIR percentage to choose the optimal level that can be sent directly to the picture communication systems. On the basis of the recommendations of the manufacturer, previous study [18], and our practical experience, the ASIR percentage was set at 50. Besides ASIR, the beam hardening artefacts are also less at the “virtual” monochromatic images than conventional 120-kVp CT images [17], favouring the reduction of image noise at all low-energy levels, particularly at 50 keV and 60 keV. These two energy levels manifested higher contrast and overall image quality. Previous study [18] of spectral imaging in vessels had also demonstrated that similar enhancement and image quality at 60 keV and 50 % ASIR with reduced contrast dose were obtained compared with conventional CT scan of 120 kVp.
Similar findings about the methods for reducing contrast agent and radiation dose were also reported by using low-kVp setting and iterative reconstruction algorithm, especially at 80 kVp [11, 33, 34]. Although the low-kVp CT scan may have the advantage of dose reduction over spectral CT, as a single-energy CT examination, its fixed energy level cannot optimize contrast and image noise for different patient sizes and clinical applications, and most importantly, cannot provide more information than dual-energy CT.
Several potential limitations of our study merit consideration. Firstly, this study reflects our preliminary experience with a relatively small patient population. Secondly, the target spectral imaging noise index setting in the ASIS technique was not individualized in the current study, and was based on the fixed target non-spectral imaging noise index. Thirdly, considering the convenience of clinical operations, the ASIR percentage was only set at 50 instead of being adjusted according to the energy level. This may influence the study results. Fourthly, reconstructed data of 120 kVp with ASIR were not included for comparison and further study will be performed. Fifthly, the same contrast media dose given to all patients regardless of patient size might result in unnecessary higher doses in smaller size patients and too low doses in larger patients. Lastly, no comparison between GSI-IR technique and a low kVp-IR technique were performed. Further work along these lines will be the next step.
In conclusion, spectral CT using ASIS technique and ASIR algorithm in the range of 40–60 keV monochromatic energy levels can dramatically decrease contrast agent dose with adequate image quality for the hepatic dynamic CT without increasing radiation dose in most patients, compared to 120 kVp with FBP algorithm. The use of spectral images at the energy level of 50 keV in combination with 50 % ASIR algorithm provides optimal balance between image contrast and image noise in both AP and PVP images.
Abbreviations
- AP:
-
Arterial phase
- PVP:
-
Portal venous phase
- CNR:
-
Contrast-to-noise ratio
- kVp:
-
Peak kilovoltage
- keV:
-
Kiloelectron volts
- CTDIvol:
-
Volume computed tomography dose index
- ASIS:
-
Automatic spectral imaging protocol selection
- ASIR:
-
Adaptive statistical iterative reconstruction
- FBP:
-
Filtered back projection
- GSI:
-
Gemstone spectral imaging
References
Brenner DJ, Hall EJ (2007) Computed tomography--an increasing source of radiation exposure. N Engl J Med 357:2277–2284
Katzberg RW, Barrett BJ (2007) Risk of iodinated contrast material--induced nephropathy with intravenous administration. Radiology 243:622–628
McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW (1997) Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med 103:368–375
Parfrey PS, Griffiths SM, Barrett BJ et al (1989) Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med 320:143–149
Morcos SK, Thomsen HS, Webb JA (1999) Contrast-media-induced nephrotoxicity: a consensus report. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Eur Radiol 9:1602–1613
Newhouse JH, RoyChoudhury A (2013) Quantitating contrast medium-induced nephropathy: controlling the controls. Radiology 267:4–8
Faggioni L, Neri E, Sbragia P et al (2012) 80-kV pulmonary CT angiography with 40 mL of iodinated contrast material in lean patients: comparison of vascular enhancement with iodixanol (320 mg I/mL)and iomeprol (400 mg I/mL). AJR Am J Roentgenol 199:1220–1225
Nakayama Y, Awai K, Funama Y et al (2005) Abdominal CT with low tube voltage: preliminary observations about radiation dose, contrast enhancement, image quality, and noise. Radiology 237:945–951
Buls N, Van Gompel G, Van Cauteren T et al (2015) Contrast agent and radiation dose reduction in abdominal CT by a combination of low tube voltage and advanced image reconstruction algorithms. Eur Radiol 25:1023–1031
Sigal-Cinqualbre AB, Hennequin R, Abada HT, Chen X, Paul JF (2004) Low-kilovoltage multi-detector row chest CT in adults: feasibility and effect on image quality and iodine dose. Radiology 231:169–174
Nakaura T, Nakamura S, Maruyama N et al (2012) Low contrast agent and radiation dose protocol for hepatic dynamic CT of thin adults at 256-detector row CT: effect of low tube voltage and hybrid iterative reconstruction algorithm on image quality. Radiology 264:445–454
Costello JE, Cecava ND, Tucker JE, Bau JL (2013) CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol 201:1283–1290
Sodickson A (2012) Strategies for reducing radiation exposure in multi-detector row CT. Radiol Clin North Am 50:1–14
Singh S, Kalra MK, Hsieh J et al (2010) Abdominal CT: comparison of adaptive statistical iterative and filtered back projection reconstruction techniques. Radiology 257:373–383
Volders D, Bols A, Haspeslagh M, Coenegrachts K (2013) Model-based iterative reconstruction and adaptive statistical iterative reconstruction techniques in abdominal CT: comparison of image quality in the detection of colorectal liver metastases. ASIR 50%. Radiology 269:469–474
Simons D, Kachelriess M, Schlemmer HP (2014) Recent developments of dual-energy CT in oncology. Eur Radiol 24:930–939
Lv P, Lin XZ, Chen K, Gao J (2012) Spectral CT in patients with small HCC: investigation of image quality and diagnostic accuracy. Eur Radiol 22:2117–2124
Zhu Z, Zhao XM, Zhao YF, Wang XY, Zhou CW (2015) Feasibility Study of Using Gemstone Spectral Imaging (GSI) and Adaptive Statistical Iterative Reconstruction (ASIR) for Reducing Radiation and Iodine Contrast Dose in Abdominal CT Patients with High BMI Values. PLoS One 10, e0129201
Matsumoto K, Jinzaki M, Tanami Y, Ueno A, Yamada M, Kuribayashi S (2011) Virtual monochromatic spectral imaging with fast kilovoltage switching: improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology 259:257–262
He J, Wang Q, Ma X, Sun Z (2015) Dual-energy CT angiography of abdomen with routine concentration contrast agent in comparison with conventional single-energy CT with high concentration contrast agent. Eur J Radiol 84:221–227
Yamada Y, Jinzaki M, Hosokawa T, Tanami Y, Abe T, Kuribayashi S (2014) Abdominal CT: an intra-individual comparison between virtual monochromatic spectral and polychromatic 120-kVp images obtained during the same examination. Eur J Radiol 83:1715–1722
Pan Z, Pang L, Ding B et al (2013) Gastric cancer staging with dual energy spectral CT imaging. PLoS One 8, e53651
Lv P, Lin XZ, Li J, Li W, Chen K (2011) Differentiation of small hepatic hemangioma from small hepatocellular carcinoma: recently introduced spectral CT method. Radiology 259:720–729
Ichikawa T, Erturk SM, Araki T (2006) Multiphasic contrast-enhanced multidetector-row CT of liver: contrast-enhancement theory and practical scan protocol with a combination of fixed injection duration and patients' body-weight-tailored dose of contrast material. Eur J Radiol 58:165–176
Megibow AJ, Jacob G, Heiken JP et al (2001) Quantitative and qualitative evaluation of volume of low osmolality contrast medium needed for routine helical abdominal CT. AJR Am J Roentgenol 176:583–589
Yamashita Y, Komohara Y, Takahashi M et al (2000) Abdominal helical CT: evaluation of optimal doses of intravenous contrast material--a prospective randomized study. Radiology 216:718–723
Awai K, Hiraishi K, Hori S (2004) Effect of contrast material injection duration and rate on aortic peak time and peak enhancement at dynamic CT involving injection protocol with dose tailored to patient weight. Radiology 230:142–150
Awai K, Takada K, Onishi H, Hori S (2002) Aortic and hepatic enhancement and tumor-to-liver contrast: analysis of the effect of different concentrations of contrast material at multi-detector row helical CT. Radiology 224:757–763
Marin D, Nelson RC, Schindera ST et al (2010) Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm--initial clinical experience. Radiology 254:145–153
Lee KH, Lee JM, Moon SK et al (2012) Attenuation-based automatic tube voltage selection and tube current modulation for dose reduction at contrast-enhanced liver CT. Radiology 265:437–447
Christner JA, Kofler JM, McCollough CH (2010) Estimating effective dose for CT using dose-length product compared with using organ doses: consequences of adopting International Commission on Radiological Protection publication 103 or dual-energy scanning. AJR Am J Roentgenol 194:881–889
Who EC (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363:157–163
Chen CM, Chu SY, Hsu MY, Liao YL, Tsai HY (2014) Low-tube-voltage (80 kVp) CT aortography using 320-row volume CT with adaptive iterative reconstruction: lower contrast medium and radiation dose. Eur Radiol 24:460–468
Lu GM, Luo S, Meinel FG et al (2014) High-pitch computed tomography pulmonary angiography with iterative reconstruction at 80 kVp and 20 mL contrast agent volume. Eur Radiol 24:3260–3268
Acknowledgements
The scientific guarantor of this publication is Jianbo Gao. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This study has received funding by National Natural Science Fund of China (Grant No. 81301220 to P. J. L.) and General Project of Medical Science and Technique in Henan Province (Grant No. 201403016 to P. J. L.). No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Approval from the institutional animal care committee was not required because the study was only on human subjects. No study subjects or cohorts have been previously reported. Methodology: prospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lv, P., Liu, J., Chai, Y. et al. Automatic spectral imaging protocol selection and iterative reconstruction in abdominal CT with reduced contrast agent dose: initial experience. Eur Radiol 27, 374–383 (2017). https://doi.org/10.1007/s00330-016-4349-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4349-8