Abstract
Objectives
To evaluate CT aortography at reduced tube voltage and contrast medium dose while maintaining image quality through iterative reconstruction (IR).
Methods
The Institutional Review Board approved a prospective study of 48 patients who underwent follow-up CT aortography. We performed intra-individual comparisons of arterial phase images using 120 kVp (standard tube voltage) and 80 kVp (low tube voltage). Low-tube-voltage imaging was performed on a 320-detector CT with IR following injection of 40 ml of contrast medium. We assessed aortic attenuation, aortic attenuation gradient, image noise, contrast-to-noise ratio (CNR), volume CT dose index (CTDIvol), and figure of merit (FOM) of image noise and CNR. Two readers assessed images for diagnostic quality, image noise, and artefacts.
Results
The low-tube-voltage protocol showed 23–31 % higher mean aortic attenuation and image noise (both P < 0.01) than the standard-tube-voltage protocol, but no significant difference in the CNR and aortic attenuation gradients. The low-tube-voltage protocol showed a 48 % reduction in CTDIvol and an 80 % increase in FOM of CNR. Subjective diagnostic quality was similar for both protocols, but low-tube-voltage images showed greater image noise (P = 0.01).
Conclusions
Application of IR to an 80-kVp CT aortography protocol allows radiation dose and contrast medium reduction without affecting image quality.
Key Points
• CT aortography at 80 kVp allows a significant reduction in radiation dose.
• Addition of iterative reconstruction reduces image noise and improves image quality.
• The injected contrast medium dose can be substantially reduced at 80 kVp.
• Aortic enhancement is uniform despite a reduced volume of contrast medium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Computed tomography angiography (CTA) has benefited greatly from recent generations of multi-row detector CT. The greater number of detectors has advantages in vascular imaging, allowing faster imaging, improved spatial and temporal resolutions, and optimisations in contrast medium injection protocols [1]. Improvements in the areas of low-tube-voltage, high-output X-ray tubes [2], online tube current modulations [3], volume acquisitions [4], and, more recently, iterative reconstruction (IR) techniques [5] have enabled reductions in the radiation dose for CT equipment.
Low-tube-voltage CTA techniques allow a reduced volume of contrast medium by taking advantage of the greater attenuation of iodine at a lower tube voltage [6]. This lower quantity of contrast medium is particularly important because a recent meta-analysis showed a 6.4 % pooled incidence of contrast-induced nephropathy following CT [7]. However, low-tube-voltage CTA comes at the cost of greater image noise. Recent generations of CT systems have adopted X-ray tubes capable of high tube currents to counterbalance the higher image noise at a low tube voltage. Additionally IR has been introduced into clinical practice, and this serves as a method to further reduce image noise compared with filtered-back projection (FBP) [8].
The advent of wide detector configuration that provides 16-cm z-axis coverage has introduced non-helical volume acquisition modes. Volume acquisition is advantageous for whole organ coverage, particularly in brain and pancreas imaging, because it avoids the over-ranging and over-imaging caused by helical acquisition [4, 9]. Wide-volume acquisition is a method whereby multiple consecutive volumes (with a 2-cm overlap between volumes) are acquired and automatically stitched together to generate a single data set [10]. This volumetric data set can then be manipulated as 3D data and reconstructed with FBP or IR into various slice thicknesses and imaging planes. To the best of our knowledge, CT aortography with wide-volume acquisition has not been previously assessed. We hypothesise that wide-volume acquisition can rapidly cover the whole aorta at a low tube voltage using a reduced volume of contrast medium and at the same time maintain diagnostic image quality by applying IR.
This study presents an evaluation of CT aortography using a wide-volume acquisition method at a reduced tube voltage and contrast medium volume while maintaining comparable image quality by applying iterative reconstruction.
Materials and methods
This is a prospective single-centre study approved by the Institutional Review Board of Chang Gung Memorial Hospital. Informed consent was obtained. We compared CT aortography diagnostic quality and radiation dose after reducing the contrast medium volume from 100 ml 350 mg I/ml (35 g of iodine) at 120 kVp (standard tube voltage) to 40 ml 350 mg I/ml (14 g of iodine) at 80 kVp (low tube voltage) combined with adaptive iterative reconstructions. This adjustment was based on publications advocating CTA at a reduced radiation dose and contrast medium volume [11–13].
Study population
We enrolled 48 patients between June and December of 2011. Enrolled patients were referred for clinically indicated CT aortography following abdominal (n = 35) or thoracic (n = 4) endovascular aortic repair (EVAR), or serial follow-up of known thoracic (n = 3) or abdominal (n = 6) aortic aneurysm. Patients with previous CT examinations using a standard-tube-voltage protocol were included in this study and underwent low-tube-voltage volume CT with lower volume of contrast medium. The time interval between two CT examinations is 1 year for untreated aneurysms, 3 months for treated aneurysms in the first year, and annually thereafter. Patients with a history of aortic dissection were excluded. We measured the body weight and height of all patients before the low-tube-voltage CT examination.
CT protocol using standard-tube-voltage helical CT
In all patients, helical acquisitions covering the clavicles to the pubic symphysis were performed with either 320-detector-row CT (Aquilion ONE; Toshiba Medical Systems, Otawara, Japan; n = 26), 64-detector-row CT (Aquilion; Toshiba Medical Systems; n = 9), or 16-detector-row CT (Somatom Sensation 16; Siemens, Erlangen, Germany; n = 8; or GE Brightspeed; GE Healthcare, Milwaukee, WI, USA; n = 5). The contrast medium volume in all examinations was 100 ml 350 mg I/ml of non-ionic contrast medium (iohexol; Omnipaque 350; GE Healthcare, UK). The images from the Toshiba systems were reconstructed with noise filters to smooth out areas of uniform density (Quantum Denoising Software, QDS) and to reduce pattern noise and streak artefacts (Boost 3D) [14]. Reconstructed images of 5 mm thickness and interval were available for viewing on a picture-archiving and communication system (PACS).
Imaging and contrast medium injection protocol using low-tube-voltage volume CT
All patients were examined using a 320-detector-row volume CT system with maximum z-axis coverage of 16 cm. This protocol consists of a non-helical wide-volume acquisition during the arterial phase and covers the clavicles to the pubic symphysis in 5 to 8 volumes. Table 1 presents the detailed imaging parameters in the arterial phase for each protocol.
All patients had an 18-G intravenous catheter placed in an antecubital vein. Dual-head power injection (Mallinckrodt LF OptiVantage DH V8402, USA) of a fixed-dose injection of 40 ml of 350 mg I/ml non-ionic iodine contrast medium (iohexol) was followed by a 40-ml saline flush, both at a rate of 3 ml/s [12]. Real-time bolus tracking was started 15 s after the commencement of contrast medium injection. Imaging commenced automatically 4 s after contrast medium enhancement reached 120 HU in a region of interest (ROI) placed in the abdominal aorta between the T12 and L1 vertebra.
Image reconstruction of low-tube-voltage volume CT
Consecutive volume data acquisitions were automatically stitched together by a workstation into a single data set. This volume data set was then reconstructed using an IR algorithm (Adaptive Iterative Dose Reduction, AIDR) into axial slices of 5 mm at 5-mm intervals for online viewing with PACS. The AIDR algorithm reduces image noise through iteration loops in the image domain and by weighted summation of the original and iterative images [10].
Pilot study for the evaluation of volume FBP versus AIDR images
Few studies have investigated the performance of the AIDR algorithm in the setting of low tube voltage CT acquisitions. Five patients referred for follow-up of EVAR were included in a pilot study to undergo CT aortography using the low-tube-voltage protocol. The acquired volume data set was then reconstructed using FBP (with QDS and Boost 3D) and AIDR for each patient. The reconstructed FBP and AIDR images were compared quantitatively with circular ROI measurements as described below.
Radiation dose estimation
The volume CT dose index (CTDIvol) and dose-length product (DLP) obtained from the dose page provided by the CT systems for each patient and each protocol were recorded. Dose comparison was only made for the arterial phase. The estimated effective dose (ED) was obtained by multiplying the value of DLP by an ED/DLP conversion factor of 0.0186 mSv mG-1 cm-1 [15]. This conversion factor was based on International Commission on Radiological Protection publication 103 tissue-weighting factors [16].
Quantitative image analysis
For the purposes of this study, only images acquired during the arterial phase were evaluated. The maximum arterial enhancement was measured by carefully placing a circular ROI at the following sites: ascending aorta at the level of the main pulmonary trunk (ROI 1), aortic arch (ROI 2), descending aorta at the level of the main pulmonary trunk (ROI 3), abdominal aorta at the level of coeliac artery (ROI 4), stent-graft main body (1 cm above the bifurcation) or abdominal aorta (1 cm above the bifurcation) in patients without stent placement (ROI 5), and left and right external iliac arteries (ROI 6). Each ROI was placed at the centre of the vessel and made as large as possible, taking care to avoid metallic stent or vessel calcification. ROIs were placed in the bilateral psoas muscles above the level of the iliac crest to define the beam background attenuation [12]. These ROIs were placed carefully to avoid containing fatty spots and did not include the surrounding bone. Image noise was defined as the standard deviation of attenuation value measured in the foam mattress of the CT table [12]. To minimise the bias from single measurements, we calculated the average of all measurements for ROIs placed at three consecutive slices. The images from AIDR and FBP reconstructions for the pilot study patients were evaluated in the same manner.
The aortic attenuation gradient was calculated to compare the enhancement variations within the aortic lumen. The gradient was represented by the absolute attenuation difference between ROI 6 and ROI 1. To an average observer, a minimum attenuation difference of approximately 50 HU is necessary to be perceptible [17]. Based on this finding, we calculated the proportions of gradients greater than and less than 50 HU. The contrast-to-noise ratio (CNR) at each level was calculated by the formula: CNR i = (CTN i − CTNpsoas)/noise, where CTN i represents the mean CT number of ROI 1 to ROI 6 and CTNpsoas the mean CT number of psoas muscle. The mean CNR for each patient was calculated from all six CNRs.
The image quality was normalised to ED to account for differences in image quality caused by changing the tube voltage and tube current time setting by using the figure of merit (FOM). Due to image noise they were inversed squarely proportional to the radiation dose [18] (1/noise)2 was sometimes considered a method to express image quality when calculating FOMN, as FOMN = (1/noise)2/ED [8]. The FOM enabled evaluation of the effect of varying tube voltage settings on image noise independent of both ED and tube current-time settings. Similarly, to establish an independent relationship of CNR, the FOM for CNR (FOMCNR) normalised to ED was computed as FOMCNR = CNR2/ED.
Qualitative image analysis
A radiologist (C.C.) not involved in qualitative image analysis preselected images for review. Because the images reconstructed with the AIDR algorithm were visually distinct when viewed consecutively, we preselected representative axial images to minimise viewing bias. From each patient the five axial images used for performing ROI quantification in standard and low-tube-voltage CT were selected. The window level and width were adjusted to maintain a consistent viewing condition; for low-tube-voltage CT, this was either 150/800 or 70/500, and for standard-tube-voltage, this was CT 150/800. Two radiologists (S.C. and M.H., with 10 and 4 years of experience, respectively) reviewed the selected images in consensus. The CT images were randomised, and the readers were blinded to the image acquisition parameters. The readers graded the images for diagnostic quality (1, non-diagnostic/unacceptable; 2, poor, diagnostic confidence significantly affected; 3, moderate; 4, good; 5, excellent), the image noise (1, minimal or no image noise; 2, mild to moderate; 3, moderate to substantial), and the image artefacts (1, no artefact; 2, minimal artefacts; 3, substantial artefacts).
Statistical analysis
Statistical analysis was performed using statistical software (Statistica, version 7.1; www.statsoft.com). This study presents continuous variables (i.e., the number of images, image length, CTDIvol, DLP, ED, attenuation, gradient, image noise, CNR, and FOM) as means ± standard deviations and compares them using a paired two-tailed Student’s t test for normally distributed data or a Wilcoxon signed-rank test for non-normally distributed data. The comparison of proportions of the aortic attenuation gradient was performed using a chi-squared test. Ordinal variables (i.e., diagnostic quality, subjective noise, and artefact) are presented as medians with 2.5 and 97.5 percentile ranges and are compared using the Wilcoxon signed-rank test. Comparing images reconstructed by FBP and AIDR involved using the Wilcoxon matched-pairs test. P < 0.05 indicated a significant difference.
Results
All 48 patients (43 male, 5 female) were included for analysis. Table 2 shows the mean age, weight, height, and body mass index of the patients. There were no significant differences between the demographics of the pilot study population and the main study population.
Pilot study for the evaluation of volume FBP versus AIDR images
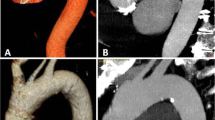
The mean aortic attenuations of FBP and AIDR images were not significantly different from each other. However, there was a 23 % reduction in image noise and a 28 % increase in CNR of the AIDR images compared with FBP images (Table 3). Figure 1 shows an example of image noise between FBP and AIDR images and the corresponding standard-tube-voltage image for comparison.
CT aortography of a 79-year-old male patient (weight 70 kg, BMI 27 kg/m2) after abdominal endovascular aortic repair. The first column shows axial images from the thorax, upper abdomen, and abdomen through an aortic stent at standard tube voltage (120 kVp, 100 ml contrast medium), the second column at low tube voltage using filtered-back projection (FBP) reconstruction (80 kVp, 40 ml contrast medium), and the third column at low tube voltage using adaptive iterative dose reduction (AIDR) reconstruction. The window level, 150, and window width, 800, were kept constant. The AIDR images showed less noise, fewer streak artefacts, and smoother texture compared with FBP images
Radiation dose
Table 4 shows the number of volumes, image length, CTDIvol, DLP, and ED. The mean CTDIvol and DLP were significantly greater in the standard-tube-voltage protocol (P < 0.01). The low-tube-voltage protocol showed a mean dose reduction in CTDIvol, DLP, and ED of 48 %, 37 %, and 37 %, respectively (P < 0.01).
Quantitative image analysis
The low-tube-voltage protocol showed a 23–31 % higher mean aortic segmental attenuation (Table 5). Figure 2 shows that the attenuation profile of the low-tube-voltage protocol fluctuates at the same attenuation level, whereas the profile of the standard-tube-voltage protocol shows a drop-off at the level of the iliac arteries (ROI 6). The mean aortic attenuation gradient for the low-tube-voltage protocol was smaller than that of the standard-tube-voltage protocol (58 ± 70 HU vs. 47 ± 50 HU, respectively, P = 0.34). The proportion of aortic attenuation gradients less than 50 HU for the low-tube-voltage protocol (69 %) was slightly higher than those of the standard-tube-voltage protocol (60 %), with no significant difference (P = 0.48). Although the low-tube-voltage protocol showed a 36 % higher mean image noise (P < 0.01), the CNR values in the two protocols were similar, with no significant difference (P = 0.40–0.86).
Graph demonstrating mean aortic attenuations along the z-axis (ROI 1 = ascending aorta at the level of the main pulmonary trunk; ROI 2 = aortic arch; ROI 3 = descending aorta at the level of the main pulmonary trunk; ROI 4 = abdominal aorta at the level of the coeliac artery; ROI 5 = stent-graft main body (1 cm above bifurcation) or abdominal aorta (1 cm above bifurcation) in patients without stent placement; ROI 6 = mean of left and right external iliac arteries)
The FOM results showed the image quality levels when the ED was kept constant. The FOMN using the low-tube-voltage protocol showed a slight 20 % decrease compared with the standard-tube-voltage protocol (P < 0.01; Fig. 3). FOMCNR showed an 81 % increase, but this was not significant (P = 0.06) when the low-tube-voltage protocol was compared with the standard-tube-voltage protocol (Fig. 4).
Box and whisker graph plots show median, interquartile range, and extreme cases of figure of merit of image noise FOMN=(1/noise)2/ED. Figure of merit of image noise decreased by 20 % for low tube voltage (60 % reduced volume of contrast medium ) compared with the standard-tube-voltage protocol (standard volume of contrast medium ). The data of the median, first quartile, and third quartile are 82.7 × 10-5, 50.9 × 10-5, and 132 × 10-5 for the standard-tube-voltage protocol; 66.3 × 10-5, 51.3 × 10-5, and 92.3 × 10-5 for the low-tube-voltage protocol, P < 0.01. O = outlier
Box and whisker graph plots show median, interquartile range, and extreme cases of figure of merit of contrast-to-noise ratio (CNR) [FOMCNR = CNR2/ED]. Figure of merit of CNR slightly increased by 81 % for low-tube-voltage (60 % reduced contrast medium dose) compared with that for the standard-tube-voltage protocol (standard volume of contrast medium ). The data of the median, first quartile, and third quartile are 38.4, 22.5, and 62.1 for the standard-tube-voltage protocol; 69.6 47.7 and 92.3 for the low-tube-voltage protocol, P = 0.06. O = outlier
Qualitative image analysis
Table 6 shows the subjective image quality scores, indicating moderate-to-good diagnostic quality for the standard and low-tube-voltage protocols. The modal scores for diagnostic quality were 4 (good quality). There was no significant difference in the diagnostic quality score between the two protocols (P = 0.74). Scores assigned for image noise were significantly greater for the low-tube-voltage protocol (P = 0.01). The modal score for image noise using the low-tube-voltage protocol was 2 (mild to moderate). There was no significant difference in the scores for artefacts between the two protocols (P = 0.37). Most images had no artefacts.
Discussion
A contrast medium dose of 45 ml (370 mg I/ml) was sufficient for thoraco-abdominal CTA at 80 kVp [12]. Faster CT systems can reduce the required duration of aortic peak enhancement and by extension the required contrast medium volume [19]. In this study, we used a fixed volume of contrast medium (40 ml) administered at a fixed rate (3 ml/s) for the low-tube-voltage CT protocol. The attenuation of iodine was 7.58 cm2/g at 80 kV (~60 keV) compared with 3.51 cm2/g at 120 kVp (~80 keV), increasing by a factor of 2.12; hence, a contrast medium volume reduction of a factor of 2.5 was selected. Using this injection protocol, the mean aortic attenuation was 364 ± 46 HU, with no values falling below the 200-HU threshold. Although the image noise increased slightly in the low-tube-voltage protocol (11.6 ± 2.7 HU) compared to the standard-tube-voltage protocol (8.5 ± 2.4 HU), the 200-HU threshold is adequate for CT angiographic images [17]. With a 40-ml volume of contrast medium, 69 % of patients showed an absolute aortic attenuation gradient of less than 50 HU, suggesting uniform contrast medium enhancement. Using a 40-ml saline flush extended the duration of aortic enhancement and contributed to a lower attenuation gradient, despite a low volume of contrast medium [20].
This study shows a mean dose saving based on a CTDIvol of 48 % for a reduction of 120 to 80 kVp. Low-tube-voltage CTA can result in mean dose savings based on CTDIvol of 24 % (140 vs. 90 kVp) [11], 28 % (100 vs. 80 kVp) [12], and 37 % (120 vs. 100 kVp) [21]. However, discrepancies in reported dose reductions achievable must be evaluated in the context of subjective acceptability of the image noise, contrast medium injection protocol, reconstruction kernels, and tube current-time settings. CTA studies may yield incidental findings in the chest, abdomen, and pelvis. Despite low tube voltages, every effort to reduce image noise should be made as image noise influences the overall image quality and diagnostic accuracy of the examination [6].
Previous experience with IR in abdominal CT has shown mean image noise reductions of approximately 20–37 % compared with FBP [22–24]. Recently published reports on the use of AIDR in wide-volume acquisition lumbar spine CT and volume coronary CTA have shown a noise reduction of 42 % and 31 %, respectively, compared with FBP [10, 25]. Based on the results of the pilot study group, the AIDR algorithm contributed to a 30 % reduction in image noise and a 53 % increase in CNR compared with FBP. In the main study group, the low-tube-voltage protocol showed a similar mean CNR compared with the standard-tube-voltage protocol, despite a 36 % higher mean image noise. However, when normalised to the ED, the FOM of image noise for the low-tube-voltage protocol showed a slight decrease (20 %) and the FOM of CNR showed an 81 % increase. Wintersperger et al. noted that image quality in CTA depended more on CNR and signal-to-noise characteristics than absolute vessel opacification and image noise [21].
Qualitatively, the low-tube-voltage protocol showed greater image noise. However, the overall image quality scores were similar to those of the standard-tube-voltage protocol. This could be attributed to the higher mean aortic attenuations of the low-tube-voltage protocol, which help to compensate for higher image noise [26, 27]. These results support previous findings of good overall image quality, despite higher image noise in reduced tube voltage CTA [11, 12, 21, 27]. In this study, three endoleaks were found and the endoleak detection rate was the same for the two protocols.
This study has some limitations. First, different detector configurations caused some variability in the imaging parameters of standard tube voltage protocols. However, 71 % of the CT acquisitions in the standard tube voltage protocol were performed on Toshiba series CT units, with detector configurations of more than 64 rows. Second, we used a unified contrast medium dose that was not adjusted according to patient size. During arterial phase imaging, contrast medium accumulates in the vascular compartment, and the visceral organs can be effectively ignored [28]. Moreover, Awai and Hori noted that the central blood volume in litres is approximately 1.2 % of the patient weight in kilograms [29]. Third, the effectiveness of the saline flush was not considered in comparing the two protocols. We used a 40-ml saline flush to extend the duration of aortic enhancement in the low-tube-voltage protocol. Fourth, the average weight of the patients in this study was less than 70 kg. Therefore, the application of the current protocol in a heavier Western patient population requires further study. Finally, we focussed on the image quality and radiation dose, not diagnostic accuracy. Because of the limited sample size of patients with endoleaks, the accuracy of the low-tube-voltage protocol for endoleak detection could not be determined.
In summary, the application of the AIDR algorithm to an 80-kVp wide-volume CT aortography protocol can significantly reduce the radiation dose without affecting the diagnostic image quality. A reduction in tube voltage enables a reduction in the volume of contrast medium to 40 ml while maintaining uniform aortic enhancement.
Abbreviations
- AIDR:
-
adaptive iterative dose reduction
- CNR:
-
contrast-to-noise ratio
- CTA:
-
computed tomography angiography
- ED:
-
effective dose
- EVAR:
-
endovascular aortic repair
- FBP:
-
filtered-back projection
- FOM:
-
figure of merit
- IR:
-
iterative reconstruction
- ROI:
-
region of interest
- PACS:
-
picture-archiving and communication system
References
Nakayama Y, Awai K, Funama Y et al (2005) Abdominal CT with low tube voltage: preliminary observations about radiation dose, contrast enhancement, image quality, and noise. Radiology 237:945–951
Sigal-Cinqualbre AB, Hennequin R, Abada HT, Chen X, Paul JF (2004) Low-kilovoltage multi-detector row chest CT in adults: feasibility and effect on image quality and iodine dose. Radiology 231:169–174
Kalra MK, Maher MM, Toth TL et al (2004) Techniques and applications of automatic tube current modulation for CT. Radiology 233:649–657
Dewey M, Zimmermann E, Deissenrieder F et al (2009) Noninvasive coronary angiography by 320-row computed tomography with lower radiation exposure and maintained diagnostic accuracy: comparison of results with cardiac catheterization in a head-to-head pilot investigation. Circulation 120:867–875
Leipsic J, Nguyen G, Brown J, Sin D, Mayo JR (2010) A prospective evaluation of dose reduction and image quality in chest CT using adaptive statistical iterative reconstruction. AJR Am J Roentgenol 195:1095–1099
Huda W, Scalzetti EM, Levin G (2000) Technique factors and image quality as functions of patient weight at abdominal CT. Radiology 217:430–435
Kooiman J, Pasha SM, Zondag W et al (2012) Meta-analysis: serum creatinine changes following contrast enhanced CT imaging. Eur J Radiol 81:2554–2561
Marin D, Nelson RC, Schindera ST et al (2010) Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm—initial clinical experience. Radiology 254:145–153
Goshima S, Kanematsu M, Nishibori H et al (2011) CT of the pancreas: comparison of anatomic structure depiction, image quality, and radiation exposure between 320-detector volumetric images and 64-detector helical images. Radiology 260:139–147
Gervaise A, Osemont B, Lecocq S et al (2012) CT image quality improvement using Adaptive Iterative Dose Reduction with wide-volume acquisition on 320-detector CT. Eur Radiol 22:295–301
Nakayama Y, Awai K, Funama Y et al (2006) Lower tube voltage reduces contrast material and radiation doses on 16-MDCT aortography. AJR Am J Roentgenol 187:W490–W497
Schindera ST, Graca P, Patak MA et al (2009) Thoracoabdominal-aortoiliac multidetector-row CT angiography at 80 and 100 kVp: assessment of image quality and radiation dose. Invest Radiol 44:650–655
Szucs-Farkas Z, Kurmann L, Strautz T, Patak MA, Vock P, Schindera ST (2008) Patient exposure and image quality of low-dose pulmonary computed tomography angiography: comparison of 100- and 80-kVp protocols. Invest Radiol 43:871–876
Paul NS, Blobel J, Prezelj E et al (2010) The reduction of image noise and streak artifact in the thoracic inlet during low dose and ultra-low dose thoracic CT. Phys Med Biol 55:1363–1380
Huda W, Magill D, He W (2011) CT effective dose per dose length product using ICRP 103 weighting factors. Med Phys 38:1261–1265
ICRP (2007) The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 37:1–332
Schoellnast H, Tillich M, Deutschmann MJ, Deutschmann HA, Schaffler GJ, Portugaller HR (2004) Aortoiliac enhancement during computed tomography angiography with reduced contrast material dose and saline solution flush: influence on magnitude and uniformity of the contrast column. Invest Radiol 39:20–26
Brooks RA, Di Chiro G (1976) Statistical limitations in x-ray reconstructive tomography. Med Phys 3:237–240
Bae KT (2010) Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 256:32–61
Kim DJ, Kim TH, Kim SJ et al (2008) Saline flush effect for enhancement of aorta and coronary arteries at multidetector CT coronary angiography. Radiology 246:110–115
Wintersperger B, Jakobs T, Herzog P et al (2005) Aorto-iliac multidetector-row CT angiography with low kV settings: improved vessel enhancement and simultaneous reduction of radiation dose. Eur Radiol 15:334–341
Prakash P, Kalra MK, Kambadakone AK et al (2010) Reducing abdominal CT radiation dose with adaptive statistical iterative reconstruction technique. Invest Radiol 45:202–210
Sagara Y, Hara AK, Pavlicek W, Silva AC, Paden RG, Wu Q (2010) Abdominal CT: comparison of low-dose CT with adaptive statistical iterative reconstruction and routine-dose CT with filtered back projection in 53 patients. AJR Am J Roentgenol 195:713–719
Singh S, Kalra MK, Hsieh J et al (2010) Abdominal CT: comparison of adaptive statistical iterative and filtered back projection reconstruction techniques. Radiology 257:373–383
Tatsugami F, Matsuki M, Nakai G et al (2012) The effect of adaptive iterative dose reduction on image quality in 320-detector row CT coronary angiography. Br J Radiol 85:e378–e382
Watanabe H, Kanematsu M, Miyoshi T et al (2010) Improvement of image quality of low radiation dose abdominal CT by increasing contrast enhancement. AJR Am J Roentgenol 195:986–992
Iezzi R, Cotroneo AR, Giammarino A, Spigonardo F, Storto ML (2011) Low-dose multidetector-row CT-angiography of abdominal aortic aneurysm after endovascular repair. Eur J Radiol 79:21–28
Bae KT, Heiken JP, Brink JA (1998) Aortic and hepatic peak enhancement at CT: effect of contrast medium injection rate—pharmacokinetic analysis and experimental porcine model. Radiology 206:455–464
Awai K, Hori S (2003) Effect of contrast injection protocol with dose tailored to patient weight and fixed injection duration on aortic and hepatic enhancement at multidetector-row helical CT. Eur Radiol 13:2155–2160
Acknowledgments
This study was supported by a grant from the Chang Gung Medical Research Program (CMRPG1B0061). We acknowledge the assistance of the dedicated radiology staff at Chang Gung Memorial Hospital Linkou.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, CM., Chu, SY., Hsu, MY. et al. Low-tube-voltage (80 kVp) CT aortography using 320-row volume CT with adaptive iterative reconstruction: lower contrast medium and radiation dose. Eur Radiol 24, 460–468 (2014). https://doi.org/10.1007/s00330-013-3027-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-3027-3