Abstract
Objective
To evaluate optimal methodology for quantitative plaque volume analysis by coronary CT angiography (QCT).
Methods
Fifty-one coronary artery segments were evaluated and contour measurements based on two different methods [(1) no gap, or (2) fixed 0.3-mm gap between inner and outer boundary] were compared with intravascular ultrasound (IVUS). In addition, three different window width (WW) and level (WL) settings [fixed (740/220) Hounsfield unit (HU), adjusted (155 % and 65 % of mean luminal intensity of the segment, and aorta adjusted (155 % and 65 % of mean luminal intensity of central aorta)] were used for semiautomated plaque volume analysis.
Results
For boundary detection, the no gap method led to underestimation compared with IVUS (105.4 ± 82.3 vs. 136.1 ± 72.8 mm3, p < 0.001), while fixed 0.3-mm gap showed no difference between IVUS and QCT (136.1 ± 72.8 vs. 139.8 ± 93.9 mm3, p = 0.50). Comparison of the three different window settings demonstrated that the aorta adjusted setting underestimated (120.5 ± 74.3 vs. 136.1 ± 72.8 mm3, p = 0.003), while fixed setting showed the least mean difference compared with IVUS (3.8 ± 39.8 mm3, p = 0.50).
Conclusion
For plaque volumetric assessment, optimal results were obtained with fixed 0.3-mm gap with fixed HU setting (740/220).
Key Points
• Quantitative plaque volume analysis by coronary CT angiography has recently emerged.
• Different boundary detection methods and window width and level settings were evaluated.
• Fixed 0.3-mm gap with fixed HU setting (740/220) afforded optimal results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dynamic quantitative or qualitative changes of coronary atherosclerotic plaque have previously been demonstrated by numerous invasive studies [1–3] and have been shown to be closely related to overall prognosis [1]. Quantitative computed tomography (QCT) coronary atherosclerotic plaque analysis software has recently been introduced to provide feasible and accurate three-dimensional plaque assessment such as plaque volume, burden and characterization in a semi- or fully automated manner [4, 5]. Recently, atherosclerotic plaque volume or aggregated plaque volume percentage, a similar concept to aggregated three-dimensional plaque burden, demonstrated a strong correlation with lesion-specific ischaemia (fractional flow reserve, FFR) and demonstrated incremental performance improvement when it was combined with conventional two-dimensional cross-sectional parameters such as minimal luminal diameter (MLD) or minimal luminal area (MLA) [6]. Furthermore, when these features were combined with certain adverse plaque characteristics identified by CT such as positive remodelling (PR), low attenuated plaque (LAP) and spotty calcification (SC), further incremental discriminatory performance was demonstrated [7].
This innovative plaque analysis tool has been applied in a variety of settings including serial quantitative or qualitative follow-up assessment of coronary atherosclerotic plaque [8–10]. However, despite its utilization in variety of clinical settings, optimal and standardized methods for defining outer line plaque boundary and optimal window settings for semiautomated plaque assessment have not been well established. Therefore, we assessed different boundary detection methods and window settings in order to identify optimal semiautomated plaque analysis conditions, using intravascular ultrasound (IVUS) as the reference standard.
Materials and methods
Patient population and data acquisition
We retrospectively analysed 51 coronary segments, including 50 culprit lesions, from 50 consecutive patients with high pretest likelihood of coronary artery disease, who had undergone within a 60-day period both coronary computed tomography angiography (CCTA) and invasive coronary angiography (ICA) with iLAb intravascular ultrasound (IVUS-iLAb) from December 2010 to December 2012. Among 50 patients 62 % were male, mean age was 61.5 ± 12.0 years (age range 42–75 years), 58 % had type 2 diabetes mellitus, 78 % had hypertension, 80 % had dyslipidaemia and 66 % were current smokers, and we excluded patients with prior coronary artery bypass graft surgery, prior percutaneous coronary intervention, elevated serum creatinine level (≥1.5 mg/dL), allergy to iodinated contrast medium, pregnant state or haemodynamic instability.
CCTA images were acquired using a 64-slice multidetector-row computed tomography scanner (Somatom Sensation 64, Siemens Medical Solutions, Forchheim, Germany) utilizing prospective or retrospective electrocardiographic gating. Patients with heart rates of 65 beats per minute or greater received metoprolol 50 mg orally. In addition, 0.3 mg of nitroglycerin was given sublingually to all patients prior to scanning unless contraindicated [11]. Then, 70 mL of contrast medium (iopamidol 370 mg/mL, Iopamiro 370, Bracco, Milan, Italy) at a flow rate of 5 mL/s followed by 50 mL of saline at a flow rate of 5 mL/s were administered into an antecubital vein via an 18-gauge catheter; the bolus tracking method was used by placing the region of interest (ROI) in the ascending aorta and image acquisition was started 5 s after reaching the predefined threshold of 140 Hounsfield units (HU). The scan parameters were as follows: 64 × 0.6 mm section collimation, 330 ms rotation time, 100 kVp or 120 kVp tube voltage (depending on body habitus), and 350–600 mAs tube current (depending on body habitus), and the images were reconstructed using a slice thickness of 0.75 mm, an increment interval of 0.5 mm and a medium-smooth convolution kernel of B36f. The estimated radiation dose for CTs ranged between 3 and 11 mSv. IVUS data were acquired by a commercially available IVUS system (iLab, Boston Scientific Scimed, Maple Grove, Minnesota). A motorized transducer pullback method was used throughout the length of the segment of interest after intracoronary administration of 200 μg of nitroglycerin, which permitted cross-sectional area (CSA) measurements at 0.5 mm/s and 30 frames per second.
IVUS and CCTA data analysis and co-registration
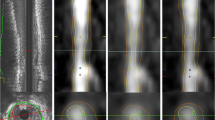
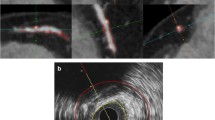
The IVUS images of lesion-containing target segments were independently analysed by an experienced IVUS reader in a blinded fashion using offline software (QCU-CMS v4.69, LKEB, Leiden University, the Netherlands; this is the research version of QIvus, Medis Medical Imaging Systems BV, Leiden, the Netherlands). CCTA images were analysed using QAngio CT Research Edition (v2.1.9.1, Medis Medical Imaging Systems BV, Leiden, the Netherlands) in a semiautomated manner; (1) starting with an automatic centreline extraction, (2) on the basis of these centrelines, straightened multi-planar reformatted (MPR) volumes were reconstructed for the segmentation and quantification, (3) longitudinal inner lumen and outer vessel wall contours were detected by automatic algorithm, (4) manual editing of both inner lumen and outer vessel wall delineations by transversal and longitudinal manner were performed.
The difference between lumen and vessel volume, which were respectively calculated by measuring the contour of the inner and outer boundary of the coronary artery, was defined as plaque volume. Subsequently, we measured plaque volume by CCTA utilizing different boundary detection methods (no gap vs. fixed 0.3-mm gap) and window settings (fixed vs. adjusted vs. aorta adjusted) to find the optimal setting for semiautomated plaque volume analysis. Each of these methods was then compared with IVUS-derived plaque volumes as the reference standard.
Initially, we compared the no gap method versus fixed 0.3-mm gap method utilizing the widely used fixed window setting 740/220 HU. The no gap method, which has been used most frequently in prior published studies for plaque analysis utilizing CCTA [6, 12] (Fig. 1), assumes that there is no gap between vessel wall and lumen. However, on the basis of histopathology studies, it is known that a space exists between the intima and media, which is where atherosclerotic changes occur [13, 14]. On the basis of these histopathology studies, we performed another analysis allowing a 0.3-mm gap between vessel wall and lumen in accordance with previous reports [15–17] (Fig. 1), which would then avoid the innate underestimation of plaque volume using CCTA.
Second, we assessed the three most widely used window width and level HU settings for CCTA in clinical practice, since semiautomated quantitative analysis is greatly dependent on them. These setting included fixed HU with window width (WW) and level (WL) of 740/220 [12], adjusted HU with 155 % and 65 % of mean luminal intensity of the disease-free proximal reference level for WW/WL [6, 18] and aorta adjusted HU with 155 % and 65 % of mean luminal intensity of the aortic root level for WW/WL [19].
The CCTA images were subsequently co-registered and compared with IVUS images as follows. During the IVUS analyses, the slices corresponding to bifurcation proximal and distal to each lesion were recorded. Next, using the extracted MPR stack from the CCTA data, the slice location of the corresponding bifurcations were also recorded. Next, using the slice numbers from IVUS and CCTA, the correspondence between lesion lengths was determined and length differences between the two modalities were corrected. Plaque volume defined as vessel volume minus lumen volume was evaluated (Fig. 1) [4, 5].
We also evaluated the overestimation effect of the calcium blooming artefact using the calcium score from the analysed lesion-containing segment. The difference of plaque volume between IVUS and CT was assessed, especially in mixed plaques.
Inter-observer variability between two expert readers was analysed in a blinded fashion for all segments using the four different combinations of boundary detection methods and window settings respectively. In addition, intra-observer variability for all segments was analysed regarding four different methods in a blinded fashion by one expert reader more than 30 days apart.
Statistical analysis
Mean±standard deviation when normally distributed or median [interquartile range (IQR)] if non-normally distributed were used for continuous variables. Absolute numbers or percentages were used for categorical variables. The paired t test and Pearson correlation coefficient using two-sided p values were used for comparing between IVUS and QCT, and Fisher’s Z transformation statistic was used for comparing two correlations. A p value of less than 0.05 was considered statistically significant. Bland–Altman plots with 95 % confidence intervals for correlation were calculated. The area under the receiver operating characteristic curve (AUC) models was employed to evaluate the discrimination of more than 50 % difference between IVUS and CT plaque volume with segment coronary artery calcium (CAC) score. Inter- or intra-observer variability was assessed by intraclass correlation coefficients (ICC) for absolute agreement of single measures between consistent raters. All statistical analysis was performed using commercially available statistics software (MedCalc, version 12.7.5, MedCalc software Inc., Mariakerke, Belgium).
Results
All 51 lesion-containing segments were assessed with CCTA and IVUS (Table 1). The lesion-containing segments evaluated were primarily located in the left anterior descending (n = 45, 88.2 %), followed by right coronary artery (n = 4, 7.8 %), left circumflex artery (n = 1, 2 %) and the first diagonal branch (n = 1, 2 %). The plaque composition profile of the lesions assessed were as follows: non-calcified plaques, 15 (29 %); calcified plaques, 6 (12 %); most of the lesions were composed of mixed plaque 30 (59 %) and 20 (39.2 %) plaques were located in the proximal segments of coronary arteries. The mean reference plaque volume derived from IVUS was 136.1 ± 72.8 mm3 and the mean segment length was 24.2 ± 7.2 mm.
Boundary gap setting
For boundary detection, the no gap/fixed window method led to significant underestimation compared with IVUS (105.4 ± 82.3 vs. 136.1 ± 72.8 mm3, p < 0.001), while the 0.3-mm gap/fixed window showed no significant difference between IVUS and QCT (136.1 ± 72.8 vs. 139.8 ± 93.9 mm3, p = 0.50) (Table 1; Figs. 2 and 3). Correlation between IVUS and QCT was slightly higher in the 0.3-mm gap/fixed window compared with no gap/fixed window, although these differences were not statistically significant (0.91 vs. 0.86, p = 0.15) (Table 1, Fig. 2). When compared with IVUS, the 0.3-mm gap/fixed window QCT measurements showed a smaller mean bias of −1.4 % with 95 % limits of agreement ranging from −61.8 to 59.1 %, while the no gap/fixed window QCT measurements showed a higher mean bias of 26.5 % with 95 % limits of agreement extending from −31.1 to 84.3 mm3 (Fig. 3).
Window setting
When comparing the three different window settings, the 0.3-mm gap/aorta adjusted window setting led to significant underestimation compared with IVUS (120.5 ± 74.3 vs. 136.1 ± 72.8 mm3, p = 0.003). The 0.3-mm gap/fixed window showed the least mean difference compared with IVUS with slight overestimation (mean difference 3.8 ± 39.8 mm3, p = 0.50), while the 0.3-mm gap/adjusted window led to slight underestimation (mean difference −7.6 ± 38.6 mm3, p = 0.16), although both these differences were not statistically significant when compared with IVUS (Table 1). The Bland–Altman analysis demonstrated that the 0.3-mm gap/fixed window (−1.4 %) setting had the least mean bias as compared to the 0.3-mm gap/adjusted window (6.2 %) or the 0.3-mm gap/aorta adjusted window (10.6 %) settings (Fig. 3).
CAC score and plaque volume analysis in mixed plaque
Among the 30 mixed plaques analysed, a linear correlation (r = 0.75; 95 % CI 0.54–0.88; p < 0.001) was found between CAC (Agatston) score in the lesion-containing segment and the difference in plaque volume between IVUS and CT when utilizing the 0.3-mm gap/fixed window setting (Fig. 4). The receiver operating area under the curve (AUC) was 0.84 (95 % CI 0.66–0.95, p < 0.001) for detection of more than 50 % plaque volume difference between IVUS and CT, with the cut-off value of CAC (Agatston) score being 78 (Fig. 4).
Inter and intra-observer variability
Both inter- and intra-observer variability was excellent as shown in Table 2.
Discussion
In this study, we aimed to evaluate the optimal setting of CCTA for plaque assessment. The fixed 0.3-mm gap method appeared to more accurately assess plaque volume than the no gap method when compared to the IVUS-derived measurements. Among the three different window settings, the aorta adjusted window setting significantly underestimated the plaque volume when compared to the fixed or adjusted setting; and the fixed window setting showed the least mean bias using the fixed 0.3-mm gap method. Furthermore, we found that the CAC score showed a linear association with incremental error in plaque volume measurement and that a CAC score of 78 or higher would result in more than 50 % difference in plaque volume measurement between QCT and IVUS.
Prior studies have noted that CCTA underestimates plaque volume as compared with IVUS [20, 21], especially in non-calcified [18, 21] and mixed plaque [18]. However, we found that if the intima and media thickness is taken into consideration using the fixed 0.3-mm gap method, there are significantly less plaque volume measurements as compared to the conventional no gap method; hence a more sensitive and accurate assessment of atherosclerotic plaque burden might be achieved with this method.
Multiple window width and level settings have previously been used for plaque assessment including fixed settings, such as 500/150 [22], 500/200 [23, 24], 700/200 [20, 25, 26], 740/220 [12] and 800/250 [27], and adjusted window settings including 155 % and 65 % of the mean luminal intensity or using the mean HU of the central aorta [19]; these variable window settings thus affect plaque quantification and characterization. A prior study by Achenbach et al. [20] assessed plaque volume in 83 segments using a fixed window setting of 700/200 HU and demonstrated underestimation of the plaque volume. Another study demonstrated similar findings while assessing non-calcified and mixed plaque using the window setting of 155 % (WW) and 65 % (WL) of the mean intensity of the lesion [18]. However, they demonstrated significant overestimation of plaque volume when assessing calcified plaques [18]. Another study by Moselewski et al. [22] using the fixed window setting of 500/150 HU showed different results; in their study the overall plaque volume was overestimated with moderate correlation with IVUS measurements (r = 0.55, p < 0.001), with the correlation being slightly better after excluding lesions with severe calcification (r = 0.60, p = 0.09). Marwan et al. [28] analysed the influence of various window settings on vessel wall and lumen area compared to IVUS and noted that all the window settings overestimated the vessel area, although the overall correlation was good. However, they noted that the 155 % (WW) and 65 % (WL) of mean luminal intensity showed the least overestimation. As for lumen area, all settings showed close correlation but slight underestimation when compared to IVUS-derived measurements, with the least underestimation seen when using the fixed setting of 700/200 HU, which is similar to our results. However, unlike our study most lesions in their study were non-calcified plaques and the proper window setting according to plaque composition was not considered. To our knowledge, our study is the first to evaluate the combination of the intima and media thickness (no gap vs. fixed 0.3-mm gap) and various window settings.
In this study, we evaluated the effect of the calcified portion of the plaque on the overall plaque volume assessment, and we demonstrated a linear increase in plaque volume difference as compared with reference standard IVUS, as the overall calcium increased. Furthermore, we also proposed a CAC (Agatston) score 78 as a cut-off value for more than 50 % plaque volume difference between CT and IVUS. In the near future, we expect that the problems associated with calcium blooming artefact during plaque analysis will become less problematic, as the spatial and temporal resolution of CT scanners continue to improve, as well as the advent of new technology such as dual energy CT. These advances combined with techniques which further reduce the radiation dose during acquisition of cardiac CT [29] would allow QCT to be applied more frequently clinically. However, it should be noted that these advances would not only result in QCT being more universally used in clinical practice for CAD screening and prevention but also in the research field for the evaluation of certain drugs and their treatment response by directly measuring dynamic atherosclerotic plaque changes in a non-invasive, quantitative, timely and cost-effective manner [30]. Recent studies indicated its future possibilities, showing that statin use was strongly associated with a mortality benefit even in patients with non-obstructive CAD by CCTA [31], and another study proved that statin treatment slowed down the progression of coronary atherosclerosis by using CT plaque volume quantification [32].
Our study had limitations. We evaluated the lesion-containing segment based on difficulties with co-registration with IVUS. Therefore, the results may be variable depending on disease severity of each segment and may not be able to be extrapolated to per lesion analysis. However, IVUS is currently the gold standard method for plaque volume assessment; therefore, per segment analysis using IVUS as the reference standard would be the best possible option at the current time. Also, there are known limitations of CCTA plaque analysis such as the influence of calcium density (CAC score) on the overestimation of plaque volume, the attenuation of contrast-enhanced lumen, which contributes to the increasing overlap between mean density values [24, 33, 34], and lower spatial resolution compared to IVUS. However, despite these inherent limitations, we were able to identify optimal and standardized quantitative plaque analysis methods by comparing various pre-existing boundary detection and window settings, using currently validated software for automatic or semiautomatic measurements of plaque density and volume [5]. Lastly, all CCTA images were acquired with the limited contrast injection protocol and convolution kernel setting which may affect plaque imaging analysis [35, 36], and therefore our results may not be generalized to other scan settings. However, we have tried to use the most widely adopted scan protocol in clinical practice.
In conclusion, the fixed 0.3-mm gap method, which takes both media and intima into consideration, showed better agreement compared to the reference standard than the no gap method. In regards to the different window settings, all three settings showed good correlation with the reference method; however, the fixed window setting (740/220) demonstrated the smallest mean bias among the three. We therefore recommend the fixed 0.3-mm gap boundary detection method with fixed 740/220 window setting as the optimal methodology for semiautomated plaque volume assessment.
Abbreviations
- AUC:
-
receiver operating area under the curve
- CACS:
-
coronary artery calcium score
- CAD:
-
coronary artery disease
- CCTA:
-
coronary computed tomography angiography
- CI:
-
confidence interval
- HU:
-
Hounsfield unit
- IVUS:
-
intravascular ultrasound
- QCT:
-
quantitative computed tomography
- WL:
-
window level
- WW:
-
window width
References
Stone GW, Maehara A, Lansky AJ et al (2011) A prospective natural-history study of coronary atherosclerosis. N Engl J Med 364:226–235
Kubo T, Maehara A, Mintz GS et al (2010) The dynamic nature of coronary artery lesion morphology assessed by serial virtual histology intravascular ultrasound tissue characterization. J Am Coll Cardiol 55:1590–1597
Tian J, Dauerman H, Toma C et al (2014) Prevalence and characteristics of TCFA and degree of coronary artery stenosis: an OCT, IVUS, and angiographic study. J Am Coll Cardiol 64:672–680
Boogers MJ, Broersen A, van Velzen JE et al (2012) Automated quantification of coronary plaque with computed tomography: comparison with intravascular ultrasound using a dedicated registration algorithm for fusion-based quantification. Eur Heart J 33:1007–1016
de Graaf MA, Broersen A, Kitslaar PH et al (2013) Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: cross-correlation with intravascular ultrasound virtual histology. Int J Cardiovasc Imaging 29:1177–1190
Nakazato R, Shalev A, Doh JH et al (2013) Aggregate plaque volume by coronary computed tomography angiography is superior and incremental to luminal narrowing for diagnosis of ischemic lesions of intermediate stenosis severity. J Am Coll Cardiol 62:460–467
Park HB, Heo R, ó Hartaigh B et al (2015) Atherosclerotic plaque characteristics by CT Angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging 8:1–10
Inoue K, Motoyama S, Sarai M et al (2010) Serial coronary CT angiography-verified changes in plaque characteristics as an end point: evaluation of effect of statin intervention. JACC Cardiovasc Imaging 3:691–698
Shimojima M, Kawashiri MA, Nitta Y et al (2012) Rapid changes in plaque composition and morphology after intensive lipid lowering therapy: study with serial coronary CT angiography. Am J Cardiovasc Dis 2:84–88
Zeb I, Li D, Nasir K et al (2013) Effect of statin treatment on coronary plaque progression – a serial coronary CT angiography study. Atherosclerosis 231:198–204
Takx RA, Sucha D, Park J, Leiner T, Hoffmann U (2015) Sublingual nitroglycerin administration in coronary computed tomography angiography: a systematic review. Eur Radiol 25:3536–3542
Papadopoulou SL, Neefjes LA, Garcia-Garcia HM et al (2012) Natural history of coronary atherosclerosis by multislice computed tomography. JACC Cardiovasc Imaging 5:S28–S37
Libby P, Ridker PM, Hansson GK (2011) Progress and challenges in translating the biology of atherosclerosis. Nature 473:317–325
Libby P (2013) Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 368:2004–2013
Waller BF, Orr CM, Slack JD, Pinkerton CA, Van Tassel J, Peters T (1992) Anatomy, histology, and pathology of coronary arteries: a review relevant to new interventional and imaging techniques–Part I. Clin Cardiol 15:451–457
Miyao Y, Kugiyama K, Kawano H et al (2000) Diffuse intimal thickening of coronary arteries in patients with coronary spastic angina. J Am Coll Cardiol 36:432–437
Nissen SE, Gurley JC, Grines CL et al (1991) Intravascular ultrasound assessment of lumen size and wall morphology in normal subjects and patients with coronary artery disease. Circulation 84:1087–1099
Leber AW, Becker A, Knez A et al (2006) Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol 47:672–677
Bischoff B, Kantert C, Meyer T et al (2012) Cardiovascular risk assessment based on the quantification of coronary calcium in contrast-enhanced coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging 13:468–475
Achenbach S, Moselewski F, Ropers D et al (2004) Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation 109:14–17
Otsuka M, Bruining N, Van Pelt NC et al (2008) Quantification of coronary plaque by 64-slice computed tomography: a comparison with quantitative intracoronary ultrasound. Investig Radiol 43:314–321
Moselewski F, Ropers D, Pohle K et al (2004) Comparison of measurement of cross-sectional coronary atherosclerotic plaque and vessel areas by 16-slice multidetector computed tomography versus intravascular ultrasound. Am J Cardiol 94:1294–1297
van der Giessen AG, Toepker MH, Donelly PM et al (2010) Reproducibility, accuracy, and predictors of accuracy for the detection of coronary atherosclerotic plaque composition by computed tomography: an ex vivo comparison to intravascular ultrasound. Investig Radiol 45:693–701
Ferencik M, Chan RC, Achenbach S et al (2006) Arterial wall imaging: evaluation with 16-section multidetector CT in blood vessel phantoms and ex vivo coronary arteries. Radiology 240:708–716
Hoffmann U, Moselewski F, Nieman K et al (2006) Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol 47:1655–1662
Schepis T, Marwan M, Pflederer T et al (2010) Quantification of non-calcified coronary atherosclerotic plaques with dual-source computed tomography: comparison with intravascular ultrasound. Heart 96:610–615
Hausleiter J, Meyer T, Hadamitzky M, Kastrati A, Martinoff S, Schomig A (2006) Prevalence of noncalcified coronary plaques by 64-slice computed tomography in patients with an intermediate risk for significant coronary artery disease. J Am Coll Cardiol 48:312–318
Marwan M, Pflederer T, Schepis T et al (2011) Coronary vessel and luminal area measurement using dual-source computed tomography in comparison with intravascular ultrasound: effect of window settings on measurement accuracy. J Comput Assist Tomogr 35:113–118
Zhang LJ, Wang Y, Schoepf UJ et al (2015) Image quality, radiation dose, and diagnostic accuracy of prospectively ECG-triggered high-pitch coronary CT angiography at 70 kVp in a clinical setting: comparison with invasive coronary angiography. Eur Radiol. doi:10.1007/s00330-015-3868-z
Park HB, Lee BK, Shin S et al (2015) Clinical feasibility of 3D automated coronary atherosclerotic plaque quantification algorithm on coronary computed tomography angiography: comparison with intravascular ultrasound. Eur Radiol 25:3073–3083
Chow BJ, Small G, Yam Y et al (2015) Prognostic and therapeutic implications of statin and aspirin therapy in individuals with nonobstructive coronary artery disease: results from the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter registry) registry. Arterioscler Thromb Vasc Biol 35:981–989
Hoffmann H, Frieler K, Schlattmann P, Hamm B, Dewey M (2010) Influence of statin treatment on coronary atherosclerosis visualised using multidetector computed tomography. Eur Radiol 20:2824–2833
Cademartiri F, Mollet NR, Runza G et al (2005) Influence of intracoronary attenuation on coronary plaque measurements using multislice computed tomography: observations in an ex vivo model of coronary computed tomography angiography. Eur Radiol 15:1426–1431
Halliburton SS, Schoenhagen P, Nair A et al (2006) Contrast enhancement of coronary atherosclerotic plaque: a high-resolution, multidetector-row computed tomography study of pressure-perfused, human ex-vivo coronary arteries. Coron Artery Dis 17:553–560
Seifarth H, Raupach R, Schaller S et al (2005) Assessment of coronary artery stents using 16-slice MDCT angiography: evaluation of a dedicated reconstruction kernel and a noise reduction filter. Eur Radiol 15:721–726
La Grutta L, Galia M, Gentile G et al (2013) Comparison of iodinated contrast media for the assessment of atherosclerotic plaque attenuation values by CT coronary angiography: observations in an ex vivo model. Br J Radiol 86:20120238
Acknowledgments
The scientific guarantor of this publication is Hyuk-Jae Chang, MD, PhD. All authors disclose no conflict of interest. This study has received funding from the Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (MSIP) (No. 2012027176). No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was waived by the institutional review board.
Methodology: retrospective, cross sectional study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
All authors disclose no conflict of interest.
Additional information
Ran Heo and Hyung-Bok Park contributed equally to this work.
Rights and permissions
About this article
Cite this article
Heo, R., Park, HB., Lee, B.K. et al. Optimal boundary detection method and window settings for coronary atherosclerotic plaque volume analysis in coronary computed tomography angiography: comparison with intravascular ultrasound. Eur Radiol 26, 3190–3198 (2016). https://doi.org/10.1007/s00330-015-4121-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4121-5