Abstract
The identification, quantification and characterization of coronary atherosclerotic plaque has a major influence on diagnosis and treatment of coronary artery disease (CAD). Recent studies have reported the ability of Computed Tomography Coronary Angiography (CTCA) to identify non-invasively coronary plaque features. In this study, we present a novel methodology for the identification of the plaque burden of the coronary artery and the volumetric quantification of calcified plaques (CP) and non-calcified plaques (NCP), utilizing CTCA images in comparison with virtual histology intravascular ultrasound (VH-IVUS). The proposed methodology includes seven steps: CTCA images pre-processing, blooming effect removal, vessel centerline extraction using Multistencil Fast Marching Method (MSFM), estimation of membership sigmoidal distribution functions, implementation of an extension of active contour models using prior shapes for the lumen, the outer wall and CP segmentation, detection and quantification of NCP and finally three-dimensional (3D) models construction. Bland Altman and correlation plot analyses were performed to assess the agreement between the presented methodology and VH-IVUS. Assessment of volume and length of lesion length in 18 lesions indicated good correlation with VH-IVUS. More specifically, the Pearson’s correlation (r) is (r = 0.93, p < 0.001) and (r = 0.92, p < 0.001) for CP and NCP volume, respectively, while the correlation for the length of lesion is (r = 0.84, p < 0.001) and (r = 0.95, p < 0.001) for CP and NCP, respectively.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Computed tomography angiography
- Coronary artery disease
- Coronary arteries
- Atherosclerotic plaque
- Calcified plaque
- Non-calcified plaque
- Level-set segmentation
1 Introduction
Atherosclerotic disease, the underlying cause of coronary artery disease (CAD), is one of the leading causes of mortality and morbidity in western societies. Atherosclerosis is characterized by molecular and cellular events, such as the deposition of low-density lipoprotein (LDL) molecules, smooth cell proliferation, calcification and fibrosis, causing compositional and geometric changes in coronary vessels. Several of these changes can be identified by Computed Tomography Coronary Angiography (CTCA), a non-invasive imaging modality, which accurately detects the inner and the outer wall of coronary arteries and also permits the characterization of atherosclerotic plaque composition [1].
In the literature, different studies were presented to examine the diagnostic accuracy of CTCA for the detection of coronary plaques. Dey et al. [2] examined the accuracy of CTCA to detect CP and NCP using an automated scan-specific threshold level-based approach. Similarly, in another study, proposed by Brodoefel et al. [3], an automated software was implemented to analyze the atherosclerotic plaque composition based on CT attenuation values. On the other hand, Graaf et al. [4] studied the correlation between the plaque volume derived by CTA automatic software (QAngio CT 1.1, Medis medical imaging systems) and the plaque volume provided by VH IVUS, which was defined as the gold standard. Jawaid et al. [5] implemented a SVM classifier to identify the abnormal coronary segments and further proposed a derivative-based method to localize the position and length of the NCP inside the segment.
The proposed study is dedicated to present a semi-automatic methodology, which permits the 3D models construction of the inner wall and the outer wall, as well as the detection and volumetric quantification of CP and NCP. The VH IVUS is used to validate the proposed study. Except of its semi-automated nature, the innovative aspect of this approach is that both the active contour models and the dynamic thresholding techniques are totally adapted to each CTCA image, and allow accurately 2D segmentation independently of the acquisition dose protocol.
2 Materials and Methods
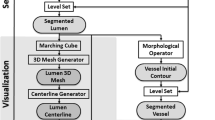
The proposed methodology consists of seven steps: the preprocessing, the blooming effect removal, the vessel centerline extraction, the estimation of membership functions, the inner wall, outer wall and CP segmentation, the NCP segmentation and finally 3D models construction.
2.1 Preprocessing
In this step, the Frangi Vesselness filter [6] is applied on the acquired axial DICOM CTCA images to detect the potential vessels regions.
2.2 Blooming Effect Removal
In this stage, we aim to improve the visualization of small high-density objects and to limit the blooming effect. The output CTCA image is considered as the convolution result of the input image with the system’s point spread function (PSF). Thus, we approximate the system’s PSF using a Gaussian kernel and we apply the Richardson Lucy algorithm on high intensity regions, to acquire the deblurred CTCA image [7].
2.3 Centerline Extraction
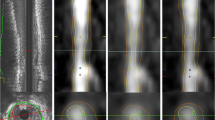
The implemented vessel centerline extraction approach, proposed by Metz et al. [8], is a minimum cost approach, based on the combination of the vesselness measure \( \left( {{\text{w}}_{\text{vessel}} } \right) \) and the lumen intensity \( \left( {{\text{w}}_{\text{lumen}} } \right) \) information. The considered cost function was defined as
where \( {\text{w}}_{\text{vessel}} \) is calculated in the pre-processing step, whereas the \( \left( {{\text{w}}_{\text{lumen}} } \right) \) is a generalized bell-shaped function.
2.4 Estimation of Memberships Functions
In this step, three membership functions were estimated to compensate three different intensity protocols for the discrimination of the lumen, the outer wall and the CP. More specifically, the selected intensity ranges depend both on the literature and the extracted mean luminal intensity.
2.5 Segmentation of the Lumen, the Outer Wall and CP
In this step, an active contour model [9], which incorporates a prior shape [10] is implemented to segment the inner wall, the outer wall and the CP. The 2D segmentation approach includes four different stages: the update of lumen intensities, the approximation of an initial binary image, the calculation of the curve speed function and finally the sparse field algorithm implementation. More specifically, we modify the estimated lumen membership function. Based on the extracted vessel centerline, we consider only the pixels of the CTCA image, whose distance from the centerline is lower than the value obtained by dividing an estimation of lumen radius by the pixel spacing of the CTCA image. Consequently, an initial image-shape \( \varphi \) is estimated to implement the active contour models. This image \( \varphi \) is a binary image, whose pixels are 1’s, when the updated lumen membership function multiplied with a threshold value is larger than 500 HU. Except of the initial binary image \( \varphi \), a shape function \( \psi \) and a labelling function L are introduced [10, 11]. In this approach, the defined speed function to evolve the curve is defined by:
where \( {\text{E}}_{\text{cv}} \) is the Chan-Vese energy, \( {\text{E}}_{\text{shape}} \) is the shape comparison term and \( {\text{E}}_{\uppsi}\) is the labelling term. The sparse field algorithm algorithm implementation follows, aiming to extract the minimal representation of \( \varphi \). The sparse field algorithm is implemented twice, in order to achieve a smooth segmented shape for the inner and the outer wall.
A similar procedure is implemented for both the outer wall and the CP segmentation.
2.6 NCP Segmentation
The detection of NCP based on CTCA images remains a challenging problem, since their intensity range values depend on the luminal intensity and the acquisition dose protocol [12]. Furthermore, the segmentation of NCP could not be successfully achieved by implementing a level set based approach, since NCP intensity values are close to the ranges of the outer wall. Thus, in this study a dynamic threshold technique is applied in the region simultaneously outside the inner wall and inside the outer wall, which is considered as the region of interest (ROI). The main idea of the detection of NCP is the extraction of a critical intensity value, the mean lumen intensity (ml). This value corresponds to the mean intensity values of the pixels of the 50% of the image intensities. After the definition of ml value, the intensity value for the NCP segmentation ranges between 100 HU lower than ml and 100 higher than ml.
2.7 3D Models Construction
The 3D models for the lumen, the outer wall, the CP and the NCP are constructed based on the Marching Cubes approach [13], by applying a triangulation approach.
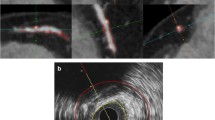
3 CTCA-IVUS Comparison
The presented methodology was compared using the corresponding frames of VH IVUS modality. The accurate registration was achieved based on anatomical landmarks, such as side-branches, ostia and CP. Furthermore, the VH IVUS images were analyzed based on a previously published study, introduced by Bourantas et al. [14], whereas the NCP volume is calculated based on the Simpson-rule method [15].
The accuracy of the presented methodology to detect plaque burden and to quantify the CP and NCP is evaluated using two different metrics for each plaque type, the plaque volume and the length of lesion. The plaque volume corresponds to the volume of plaque between the proximal and distal ends of the coronary lesion, whereas the length of lesion is the distance between the proximal and distal ends of the coronary lesion.
4 Results
In the validation procedure, we used totally 18 coronary arteries, 9 left anterior descending (LAD), 3 left circumflex (LCX) and 6 right coronary (RCA). The Bland Altman analysis and the correlation plots between the proposed CTCA based methodology and VH IVUS images analysis is illustrated in Fig. 1 and Fig. 2 for CP and NCP, respectively.
The evaluation procedure indicates that the CP and NCP extracted volumes and lengths of lesion derived from the proposed methodology correlate well with those derived by VH IVUS images analysis. More specifically, the Pearson’s correlation (r) is 0.93 and 0.92 for the CP and NCP volume, respectively, whereas the Pearson’s correlation (r) is 0.84, 0.95 for the CP and NCP length of lesion, respectively.
5 Discussion
In this work, a semi-automated methodology for the reconstruction of the lumen, the outer wall and the CP and NCP of coronary arteries is presented. The approach relies primarily on the active contour models, while the NCP detection is achieved by a dynamic threshold based approach. Although, level set based threshold techniques are promising approaches in the field of 2D segmentation, in case of NCP segmentation they are not applicable, due to the lower intensity values of NCP, which are close to the outer wall intensities.
Furthermore, the methodology of the inner and outer wall detection, proposed in this study has already been validated [16] using both manual annotations and IVUS modality. Thus, in this manner an accurate plaque burden region is assumed to be accurately identified and as a result the quantification of CP and NCP is successfully achieved.
Moreover, the basic innovative aspect of the presented methodology is its adaption to each CTCA image. It has been demonstrated that the mean luminal attenuation value differs using different acquisition protocols, as well as it varies between different patients. Contrary to others studies [2, 3], in our approach the selected threshold values are not fixed, but fully dynamic and adapted to different CTCA images.
Another basic innovative aspect of our approach is the incorporation of blooming effect removal. This deconvolution procedure implementation prevents the vessel stenosis overestimation and reduces the volumetric quantification of CP. Additionally, our algorithm allows the 3D reconstruction of the full arterial coronary tree and as result the CP and NCP can be visualized in critical regions, such as the bifurcations.
6 Conclusions
The presented methodology provides an accurate segmentation of the lumen, the outer wall, the CP and the NCP of 2D CTCA images, the plaque burden characterization, reliable coronary reconstruction and 3D representation of coronary anatomy and pathology. The validation procedure indicates that the proposed methodology correlates well with VH IVUS. Thus, its integration into a quantitative software may contribute to the diagnostic and prognostic value of CTCA and may provide a wide clinical and research applicability.
References
Voros, S., et al., Coronary Atherosclerosis Imaging by Coronary CT Angiography: Current Status, Correlation With Intravascular Interrogation and Meta-Analysis. JACC: Cardiovascular Imaging, 2011. 4(5): p. 537–548.
Dey, D., et al., Automated Three-dimensional Quantification of Noncalcified Coronary Plaque from Coronary CT Angiography: Comparison with Intravascular US. Radiology, 2010. 257(2): p. 516–522.
Brodoefel, H., et al., Accuracy of dual-source CT in the characterisation of non-calcified plaque: use of a colour-coded analysis compared with virtual histology intravascular ultrasound. The British Journal of Radiology, 2009. 82(982): p. 805–812.
de Graaf, M.A., et al., Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: cross-correlation with intravascular ultrasound virtual histology. The International Journal of Cardiovascular Imaging, 2013. 29(5): p. 1177–1190.
Jawaid, M., et al., Framework for detection and localization of coronary non-calcified plaques in cardiac CTA using mean radial profiles. Vol. 89. 2017.
Frangi, A.F., et al., Multiscale vessel enhancement filtering, in Medical Image Computing and Computer-Assisted Intervention — MICCAI’98: First International Conference Cambridge, MA, USA, October 11–13, 1998 Proceedings, W.M. Wells, A. Colchester, and S. Delp, Editors. 1998, Springer Berlin Heidelberg: Berlin, Heidelberg. p. 130–137.
Castillo-Amor, A.M., et al. Reduction of blooming artifacts in cardiac CT images by blind deconvolution and anisotropic diffusion filtering, in 11th International Symposium on Medical Information Processing and Analysis. 2015. International Society for Optics and Photonics.
Metz, C., et al., Coronary centerline extraction from CT coronary angiography images using a minimum cost path approach. Vol. 36. 2009. 5568–79.
Chan, T.F. and L.A. Vese, Active contours without edges. IEEE Transactions on Image Processing, 2001. 10(2): p. 266–277.
Chan, T. and Z. Wei. Level set based shape prior segmentation, in 2005 IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR’05). 2005.
Cremers, D., N. Sochen, and C. Schnörr, Towards Recognition-Based Variational Segmentation Using Shape Priors and Dynamic Labeling, in Scale Space Methods in Computer Vision: 4th International Conference, Scale Space 2003 Isle of Skye, UK, June 10–12, 2003 Proceedings, L.D. Griffin and M. Lillholm, Editors. 2003, Springer Berlin Heidelberg: Berlin, Heidelberg. p. 388–400.
Dalager, M.G., et al., Impact of luminal density on plaque classification by CT coronary angiography. The International Journal of Cardiovascular Imaging, 2011. 27(4): p. 593–600.
Lorensen, W.E. and H.E. Cline, Marching cubes: A high resolution 3D surface construction algorithm, in Proceedings of the 14th annual conference on Computer graphics and interactive techniques. 1987, ACM. p. 163–169.
V Bourantas, C., et al., A new methodology for accurate 3-dimensional coronary artery reconstruction using routine intravascular ultrasound and angiographic data: Implications for widespread assessment of endothelial shear stress in humans. Vol. 9. 2013.
Medina, R., et al., Three methods for accurate quantification of plaque volume in coronary arteries. The International Journal of Cardiovascular Imaging, 2003. 19(4): p. 301–311.
Kigka, V.I., et al., 3D reconstruction of coronary arteries and atherosclerotic plaques based on computed tomography angiography images. Biomedical Signal Processing and Control, 2018. 40(Supplement C): p. 286–294.
Acknowledgements
This work is funded by the European Commission: Project SMARTOOL, “Simulation Modeling of coronary ARTery disease: a tool for clinical decision support—SMARTool” GA number: 689068).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Kigka, V.I. et al. (2019). A Three-Dimensional Quantification of Calcified and Non-calcified Plaque Based on Computed Tomography Coronary Angiography Images: Comparison with Virtual Histology Intravascular Ultrasound. In: Lhotska, L., Sukupova, L., Lacković, I., Ibbott, G.S. (eds) World Congress on Medical Physics and Biomedical Engineering 2018. IFMBE Proceedings, vol 68/1. Springer, Singapore. https://doi.org/10.1007/978-981-10-9035-6_37
Download citation
DOI: https://doi.org/10.1007/978-981-10-9035-6_37
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-9034-9
Online ISBN: 978-981-10-9035-6
eBook Packages: EngineeringEngineering (R0)