Abstract
Objectives
To investigate the relationship of dual-phase dual-energy CT (DE-CT) and tumour size in the evaluation of the response to anti-EGFR therapy in patients with advanced non-small cell lung cancer (NSCLC).
Methods
Dual-phase DE-CT was performed in 31 patients with NSCLC before the onset of anti-EGFR (erlotinib) therapy and as follow-up (mean 8 weeks). Iodine uptake (IU; mg/mL) was quantified using prototype software in arterial and venous phases; arterial enhancement fraction (AEF) was calculated. The change of IU before and after therapy onset was compared with anatomical evaluation in maximal transverse diameter and volume (responders vs. non-responders).
Results
A significant decrease of IU in venous phase was proved in responders according to all anatomical parameters (p=0.002–0.016). In groups of non-responders, a significant change of IU was not proved with variable trends of development. The most significant change was observed using the anatomical parameter of volume (cut-off 73 %). A significant difference of percentage change in AEF was proved between responding and non-responders (p=0.019–0.043).
Conclusion
Dual-phase DE-CT with iodine uptake quantification is a feasible method with potential benefit in advanced assessment of anti-EGFR therapy response. We demonstrated a decrease in vascularization in the responding primary tumours and non-significant variable development of vascularization in non-responding tumours.
Key Points
• Dual-phase DE-CT is feasible for vascularization assessment of NSCLC with anti-EGFR therapy.
• There was a significant decrease of iodine uptake in responding tumours.
• There was a non-significant and variable development in non-responding tumours.
• There was significant difference of AEF percentage change between responders and non-responders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Targeted therapy of non-small cell lung cancer (NSCLC) is currently widely used in advanced stages of this disease. Except for primarily anti-angiogenic substances, there are the tyrosine-kinase inhibitors, which act on the epidermal growth factor (EGFR), and this involves angiogenesis inhibitors whose benefit has been proven as maintenance therapy after first-line chemotherapy [1]. Their benefit has also been approved in second- and third-line monotherapy in the locally advanced NSCLCs in which chemotherapy failed [2–4].
Evaluating the effects of targeted therapy on tumour development is still a challenge for imaging methods. The standard method of evaluation using the RECIST criteria has significant limitations, which reflect only the development of the size of the primary tumour or metastatic lesions [5]. Compared to standard chemotherapy, however, different processes occur in the tumour during the targeted therapy. Necrosis development or haemorrhage are more common and may limit anatomical evaluation of the effect of therapy [6]. With regard to the basic principle of targeted therapy, a quantification of vascular supply using the parameter of microvascular density (MVD) is clearly most appropriate. However, the collection of a sample for an extensive histological examination is needed for this assessment.
A dynamic contrast (first-pass) examination using CT or MRI with the possibility of quantification of the perfusion parameters is the most similar method of evaluating vascularization with proven correlation with MVD [7]. In both methods, there are specific technical limitations. Moreover complex post-processing and evaluation that is still not standardized [8]. Dual-energy CT examination (DE-CT) has been proven to be a simple method with the possibility of precise quantification of the iodine content in different tissues [9]. Dual-energy CT examination was confirmed as an alternative to dynamic examination in several studies due to the possibility of exact quantification of the iodine content in the tumorous tissue [10]. In the case of dual-phase examination in the arterial and venous phases, there is an assumption of more accurate assessment of the vascularization state in the tumour tissue that is used more commonly, e.g. in liver metastases [11].
The aim of our study was to verify the relationship of the dual-phase DE-CT examination and the size of the primary tumour during evaluation of the response to anti-EGFR therapy in patients with advanced NSCLC in whom standard chemotherapy was not effective.
Materials and methods
Patients selection
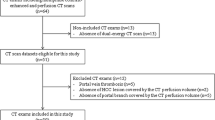
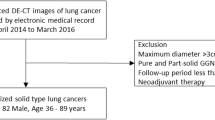
A prospectively conducted study of patients with NSCLC who were referred to third-line anti-EGFR therapy with erlotinib on the basis of failed standard chemotherapy treatment (progression according to RECIST 1.1 criteria) was performed within a routine diagnostic and therapeutic algorithm. Between March 2011 and August 2013, patients with advanced NSCLC treated with second-line chemotherapy underwent DE-CT examination in two post-contrast phases within a routine follow-up. The initial clinical stages of tumours were IIIB (10 patients) and IV (21 patients), median of maximal transversal diameter was 30.4 mm (12.5 – 98.6 mm). Majority of tumours were initially located peripherally (19; 61%) and in the left lung (17; 55%). Patients approved for anti-EGFR therapy according to routinely used obligatory rules were included in our study. Enrolled patients received erlotinib in standard doses (150 mg per day), continued until progression of disease (according RECIST 1.1) and underwent DE-CT follow-up. Patients with standard contraindications of contrast agent administration (renal insufficiency and history of severe allergic reaction) were excluded from the study. All performed examinations were part of standard diagnostic algorithm, however the specific CT protocol was used and the study was approved by the local ethic committee and patients signed informed consent.
Acquisition
All examinations were performed on a second generation dual-source CT (SOMATOM Definition Flash, Siemens Healthcare, Forchheim, Germany) in two circulatory phases (systemic arterial and late-venous) after intravenous injection of an iodine contrast medium (80 mL of iomeprol, 350 mg/mL, Bracco, Milan, Italy). The contrast medium was injected into an antecubital or cubital vein at a rate of 4 mL/s with a saline flush of 60 mL at the same rate. The scan of the arterial phase was started automatically 5 s after attenuation in the distal thoracic aorta increased to the default threshold (100 HU). For this purpose, we used the “BolusTracking” (Siemens Healthcare, Forchheim, Germany) monitoring system. The scan of the venous phase was started with a 20-s delay after the end of the arterial scan. The identical acquisition protocol was used for both scans – collimation 128 x 0.6 mm, gantry rotation 330 ms, pitch factor 0.9. Tube A was operated at a peak voltage of 80 KVp and a reference tube current of 160 mAs. Tube B was operated at 140Sn KVp and a reference tube current of 68 mAs. Data were reconstructed with a slice thickness of 0.75 mm (0.6 mm increment) for diameter measurement and 1 mm (0.75 mm increment) for analysis in the software prototype. Both series were acquired using iterative reconstruction techniques (SAFIRE, Siemens Healthcare, Forchheim, Germany) with average strength degree (3) and a convolution filter for soft tissue (I26f).
Postprocessing and measurements
All measurements were performed in consensus by two experienced radiologists (8 and 11 years) in oncologic imaging and qualified for using of the software prototype. The manual measurements of the tumour size were performed on multiplanar reformations (2 mm section width). The maximal orthogonal diameters were measured in a transverse plane, and volume measurement was part of the following post-contrast enhancement analysis. The tumour response was divided into two groups (responders and non-responders) according to maximal transverse diameter and volume difference. In the assessment of maximal transverse diameter, we used a 20 % cut-off (max_diam_20%) and in volume assessment, we used 73 % (volume_73%). Cut-off values of 0 % were used in both parameters (maximal diameter and volume).
Analysis of post-contrast enhancement was performed by using the Syngo.IPIPE prototype software application (Siemens Healthcare, Forchheim, Germany) with semi-automatic segmentation algorithms with the possibility for manual corrections. The value of absolute iodine uptake (IU) related to tumour size (mg/mL) was acquired in both scans in pre-treatment and follow-up DE-CT and were normalized to the reference value acquired using ROI (region of interest) placed in a near vessel. The value of the normalization ROI contains the iodine uptake at the given location and is interpreted as a 100 % iodine uptake reference value. The arterial enhancement fraction (AEF) value was calculated as a ratio of iodine uptake in the arterial and venous phase (iodine uptake in arterial phase/iodine uptake in venous phase x 100).
Statistics
Standard descriptive statistics were used to assess the observations (median, range). Pre-treatment parameters of iodine uptake were compared with follow-up parameters between responding and non-responding tumours using the non-parametric Wilcoxon signed-rank test. The percentage change of iodine uptake was compared using the two-sample t-test. The medians of IU values change was set as cut-off for performance analysis (sensitivity, specificity, positive predictive value and negative predictive value). Increased values of IU and AEF were identified in cases of higher percentage change than the median in the complete cohort. The statistics were calculated using commercially available software (MedCalc software, Belgium). Box-plots and bar charts were used for visual comparison of the selected samples. All tests were performed at the 5 % level of significance.
Results
All together, 31 patients (20 male and 11 female) with NSCLC (17 adenocarcinomas, 10 squamous-cell carcinomas, and four others) were enrolled in the study. The average interval of both DE-CTs was 13 weeks (8–21). The quality of all CT examinations allow for analysis using the software prototype. During a follow-up interval (median of 43 weeks; range 12–112), 14 patients remain alive and 10 remain without disease progression. The complete baseline characteristic is summarized in Table 1. The overall mean value of DLP (dose length product) was 149.6 mGy x cm (117.6–211.3) and the mean effective dose was 2.4 mSv (1.9–3.4) for a single DE-CT scan.
Anatomical response assessment
Tumour response to approved anti-EGFR therapy was evaluated on the basis of selected parameters and tumours were divided into groups of responders and non-responders: 16 vs. 15 (max_diam_20%); 12 vs. 19 (max_diam_0%); 16 vs. 15 (volume_73%) and 12 vs. 19 (volume_0%). Median of maximal transversal diameter was 41.2 mm (22.8–136.4 mm) and volume was 19.5 mL (2.5–696.5). Complete results of the anatomical response evaluation of individual groups of patients are shown in Table 2.
Iodine content response assessment
A statistically significant decrease in IU was demonstrated in the responding tumours according to all the anatomical parameters. Strong significance of the IU decrease was demonstrated particularly in the venous phase (p=0.002–0.016), moderate significance was seen when assessing arterial phase (p=0.013–0.049). With regard to individual parameters for assessing anatomical response, the statistical significance was strongest using volume_73% (p=0.002; Fig. 1). The statistically significant change of IU was not proved in non-responding tumours in arterial phase (p=0.610–0.998) and also in venous phase (p=0.123–0.638).
In the ratio value of AEF, a weak statistically significant increase in non-responding tumours was demonstrated using the volume_73% parameter (p=0.042); while using other anatomical response parameters, the increase was not significant. In responding tumours, a minimal non-significant (p=0.252–0.597) decrease of median AEF was observed before and after anti-EGFR therapy.
A significant difference of AEF was proved in the evaluation of percentage change of IU between responders and non-responders (p=0.019–0.043). This significance was also stronger in comparison to IU change in single phases (Fig. 2). Complete results are presented in Table 3.
Bar charts showing mean percentage change in iodine uptake after anti-EGFR therapy onset according to maximal transversal diameter with 20 % cut-off (a) and volume with 73 % cut-off (b). Significant difference of AEF (arterial enhancement fraction) in percentage change between responders and non-responders
In comparison of IU values trend and anatomical response, the higher sensitivity, positive predictive value (PPV), and negative predictive value (NPV) of AEF over only venous phase IU was detected using max_diam_20 (sensitivity 66.7 % vs. 46.7 %; PPV 62.5 % vs. 58.3 %; NPV 66.7 % vs. 57.9 %) and also volume_73 (sensitivity 66.7 % vs. 40 %; PPV 62.5 % vs. 54.5 %; NPV 66.7 % vs. 55 %). Only specificity of AEF was lower in comparison to venous phase IU (62.5 % vs. 68.8 %) using both anatomical parameters.
Prediction of therapy effect
Values of IU and AEF were assessed as possible predictors of a favourable effect of anti-EGFR therapy (Table 3). Iodine uptake values were higher in responding tumours: moderate statistical significance was demonstrated in the venous phase (p=0.034). In the case of AEF, a statistically significant difference of pre-treatment values between responders and non-responders was observed in the volume_73% parameter, although the median value was generally higher in responding tumours.
Discussion
Limited anatomical evaluation of the effect of targeted therapy in NSCLC can have a major impact on patient survival. That is why there is an intensive effort, particularly in clinical research, to use functional imaging methods [12, 13]. These advanced methods are promising in detailed pre-treatment characterization of primary lung cancer or lymph nodes that may in the future allow choosing and optimizing therapy [14]. A relationship between the evaluation of perfusion and metabolic activity using FDG-PET/CT, which is increasingly being employed in the staging and follow-up of NSCLC, still remains to be fully clarified [15]. The results of studies comparing both parameters are unclear and did not conclusively prove a correlation between the perfusion parameters and SUV [7, 16]. Nevertheless, the evaluation of progression of metabolic activity or perfusion parameters in targeted therapy is more suitable than only anatomical assessment [6]. Indeed, both methods have their limitations, and this is a reason why other suitable methods of functional imaging are being sought [17, 18].
During the targeted therapy there is an early development of intra-tumour haemorrhage and necrosis, which may have a major impact not only on the assessment of the size, but also on routine density measurement that is a part of some new types of criteria [19]. The importance of CT examination using dual energy (DE-CT) has repeatedly been proved in various clinical indications including oncologic imaging [20]. A quantification of the iodine content that corresponds to the level of tissue perfusion at a concrete time-point, and hence to the degree of vascularization, is essential and could be used for advanced tumour evaluation [10]. This parameter is called iodine related attenuation (IRA) and, in addition to the simple determination of density using Hounsfield units (HU), the effect of various density levels of tumour tissue prior to administration of a contrast substance and the burden of intra-tumour haemorrhage are eliminated [21]. Up to now, only a small number of studies have been published focusing on the significance of DE-CT in the evaluation of the effect of anti-tumour therapy. Kim et al. demonstrated the benefit of determining IRA in NSCLC, especially with regard to the 14 % incidence of intra-tumour haemorrhage [19]. Furthermore, several studies have been published focusing on the use of DE-CT in monitoring the effect of therapy of other types of tumours or during experimental use. In both cases, the benefit of DE-CT was confirmed. [22, 23]. The IRA parameter has high reproducibility with the established calculation procedure, and recently the scanning using two different energies is no longer one-vendor dependent.. This fact is promising for the increase of clinical research in this field.
Iodine uptake and anatomical evaluation
In our study, we performed DE-CT in two post-contrast phases and evaluated IU in single phases, as well as the ratio value of AEF, assuming more accurate characteristics of the vascularization level in the tumour tissue. In the case of primary tumours, a statistically significant decrease of IU in the responding tumours was found using all anatomical parameters. The strongest significance of IU change was proven in the venous phase. These findings should correspond to the positive effect of anti-EGFR therapy and confirm the previous experience that the late post-contrast phase is more suitable for the assessment of IRA [24, 25]. In non-responding tumours, a decrease of the median of IU was also found, although this was not statistically significant.
The trend of IU development in the venous phase was clearly uniform in the responding tumours (e.g. only one case with IU increase in max_diameter_20%). In the non-responding tumours, the trend was variable (increase in eight cases and decrease in seven cases), which could possibly indicate a benefit of this method and future orientation of the research. In terms of comparison with already published studies, Lind et al. used perfusion CT and found a more significant decrease of tumour vascularization (blood flow) in cases of an anatomically favourable response to anti-EGFR therapy [26]. However, the unclear reproducibility of the whole-tumour quantification of perfusion was a limitation of this technique [27].
Except evaluation of single phases that only present a “snapshot” of tumour vascularization, we have already used the ratio of AEF, as in our previous study, in which we found a possible benefit in the evaluation of the effect of standard chemotherapy, again with regard to the change of the size [24]. In the current study, a clear trend for the increase of this value in case of poor response to therapy was evident, but statistical significance was found only using volume_73% as a response parameter. On the other hand, we proved a statistically significant difference in the percentage change of AEF between responding and non-responding tumours. The real benefit of this value must be further evaluated. The assessment or imaging of this method was described in the case of liver lesion evaluation, especially in the detection of hepatocellular carcinoma [11, 28]. In general, this value has the potential for more specific quantitative assessment and correlates with the perfusion parameters [28]. Although most of the studies mainly used the determination of IRA in the late post-contrast phases, the importance of the IRA level in the systemic arterial phase, which would correspond to so-called first-pass of a contrast substance, i.e. especially the intravascular component of IRA, can be expected. In the late phase, IRA consists more of the presence of extravascular substances and an increase of the AEF ratio could indicate a progression of neoangiogenesis. Schmid-Bindert et al. proved a strong correlation between the maximal FDG uptake and the maximal IRA for primary lung tumours [25, 29].
We tried to assess the performance of AEF and single (venous phase) IU to confirm anatomical response and the higher sensitivity (66.7 %), but lower specificity (62.5 %) of AEF was proved. The importance of this fact is questionable, but could indicate possible added value of AEF as previously discussed and in accordance to our other results.
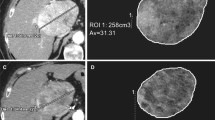
Except for the maximal diameter that is easily implemented in routine practice, there are also other possibilities of anatomical evaluation of lung cancer. In our study, we also used the volume assessment with the cut-off value of 73 %, which corresponds to the value of 20 % of the maximal diameter using in RECIST criteria [30]. With this parameter, a decrease in the IU values in the responding tumours was the most significant. Evaluation of the tumour volume can surely be considered a more accurate method compared to the maximal diameter. Nevertheless, its assessment is limited in clinical routine due to anatomical conditions (e.g. atelectasis or mediastinal invasion) and segmentation software is needed. The prototype we used was capable of very high-quality segmentation in all cases (Fig. 3). It should be noted that there was no tumour associated with atelectasis in our group. Using 0 % cut-off values did not provide any additional information over 20 % and 73 % cut-offs. This is consistent with clinical routine.
Response prediction
The possibility of predicting the effect of therapy would be very beneficial, and there are papers confirming a higher tendency to a good response in more vascularized tumour tissues, but the real possibility of pre-treatment prediction of effect is still unclear [11]. In our group, we found identical values of IU in the arterial phase, but in the venous phase, the values in subsequently responding tumours were higher, but we found moderate statistical significance in the case of one anatomical parameter (max_diameter_20%). In clinical routine, it is much more appropriate to make predictions on the basis of early assessment of the progression. The early effect of targeted therapy has been demonstrated in the experimental models very shortly after therapy onset [23]. Lind et al. statistically demonstrated a significantly better clinical outcome in tumours with a higher decrease compared to the median in their group [26]. With regard to the limited group size and discontinuation of therapy in cases of progression of the disease during follow-up, we could not evaluate the outcome. Assessment of other parameters or characteristics for therapy response prediction was not possible particularly due to limited and heterogeneous cohort.
Limitations
The performed study has several limitations that should be mentioned. Except for a low number of subjects and the heterogeneity of the histological types, the influence of differences in previous chemotherapy is limiting. This therapy has an indirect effect on the perfusion parameters in the primary tumour and could affect the possibility of mutual comparison of the single tumours. A much greater benefit would result if this method was verified using anti-EGFR as a first-line therapy in people with an appropriate gene mutation. In our study, we observed only primary tumours separately without assessing the lymph nodes or remote metastases. A clear benefit was the possibility to compare the determination of the IU values with the perfusion parameters using the dynamic CT examination; however, this would be a further burden for patients. The range of intervals between anti-EGFR therapy onset and follow-up CT (8–21 weeks) could affect the significance of presented results.
It can be concluded that dual phase DE-CT is a relatively simple method that could provide benefit in the objective assessment of the effect of targeted (anti-EGFR) therapy in persons with NSCLC. By using this method, we were able to demonstrate a decrease in vascularization in the primary tumours with favourable response to anti-EGFR therapy following the failure of standard chemotherapy. On the contrary, the insignificant changes were seen in the tumours with unfavourable response. Assessment of AEF demonstrated also additional value and should be considered as a possible parameter in advanced response assessment.
References
Cappuzzo F, Ciuleanu T, Stelmakh L et al (2010) Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 11:521–529
Al-Farsi A, Ellis PM (2014) Treatment paradigms for patients with metastatic non-small cell lung cancer, squamous lung cancer: first, second, and third-line. Front Oncol 27:157
Shepherd FA, Rodrigues Pereira J, Ciuleanu T et al (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123–132
Genestreti G, Grossi F, Genova C et al (2014) Third- and further-line therapy in advanced non-small-cell lung cancer patients: an overview. Future Oncol 10:2081–2096
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Nishino M, Hatabu H, Johnson BE, McLoud TC (2014) State of the art: Response assessment in lung cancer in the era of genomic medicine. Radiology 271:6–27
Miles KA, Lee TY, Goh V et al (2012) Current status and guidelines for the assessment of tumour vascular support with dynamic contrast-enhanced computed tomography. Eur Radiol 22:1430–1441
Brix G, Griebel J, Kiessling F, Wenz F (2010) Tracer kinetic modelling of tumour angiogenesis based on dynamic contrast-enhanced CT and MRI measurements. Eur J Nucl Med Mol Imaging 37:S30–S51
Johnson TR, Krauss B, Sedlmair M et al (2007) Material differentiation by dual energy CT: initial experience. Eur Radiol 17:1510–1517
Zhang LJ, Zang GF, Wu SY, Xu J, Lu GM, Schoepf UJ (2013) Dual-energy CT imaging of thoracic malignancies. Cancer Imaging 13:81–91
Joo I, Lee JM, Kim KW, Klotz E, Han JK, Choi BI (2011) Liver metastases on quantitative color mapping of the arterial enhancement fraction from multiphasic CT scans: evaluation of the hemodynamic features and correlation with the chemotherapy response. Eur J Radiol 80:e278–e283
Tiseo M, Ippolito M, Scarlattei M et al (2014) Predictive and prognostic value of early response assessment using 18FDG-PET in advanced non-small cell lung cancer patients treated with erlotinib. Cancer Chemother Pharmacol 73:299–307
Tacelli N, Santangelo T, Scherpereel A et al (2013) Perfusion CT allows prediction of therapy response in non-small cell lung cancer treated with conventional and anti-angiogenic chemotherapy. Eur Radiol 23:2127–2136
van Elmpt W, Zegers CM, Das M, De Ruysscher D (2014) Imaging techniques for tumour delineation and heterogeneity quantification of lung cancer: overview of current possibilities. J Thorac Dis 6:319–327
Sauter AW, Winterstein S, Spira D et al (2012) Multifunctional profiling of non-small cell lung cancer using 18F-FDG PET/CT and volume perfusion CT. J Nucl Med 53:521–529
van Elmpt W, Das M, Hüllner M et al (2013) Characterization of tumor heterogeneity using dynamic contrast enhanced CT and FDG-PET in non-small cell lung cancer. Radiother Oncol 109:65–70
Goh V, Ng QS, Miles K (2012) Computed tomography perfusion imaging for therapeutic assessment: has it come of age as a biomarker in oncology? Invest Radiol 47:2–4
Shim SS, Lee KS, Kim BT et al (2005) Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology 236:1011–1019
Kim YN, Lee HY, Lee KS et al (2012) Dual-energy CT in patients treated with anti-angiogenic agents for non-small cell lung cancer: new method of monitoring tumor response? Korean J Radiol 13:702–710
Simons D, Kachelriess M, Schlemmer HP (2014) Recent developments of dual-energy CT in oncology. Eur Radiol 24:930–939
Toepker M, Moritz T, Krauss B et al (2012) Virtual non-contrast in second-generation, dual-energy computed tomography: reliability of attenuation values. Eur J Radiol 81:398–405
Uhrig M, Simons D, Ganten MK, Hassel JC, Schlemmer HP (2015) Histogram analysis of iodine maps from dual energy computed tomography for monitoring targeted therapy of melanoma patients. Future Oncol 11:591–606
Knobloch G, Jost G, Huppertz A, Hamm B, Pietsch H (2014) Dual-energy computed tomography for the assessment of early treatment effects of regorafenib in a preclinical tumor model: comparison with dynamic contrast-enhanced CT and conventional contrast-enhanced single-energy CT. Eur Radiol 24:1896–1905
Baxa J, Vondráková A, Matoušková T et al (2014) Dual-phase dual-energy CT in patients with lung cancer: assessment of the additional value of iodine quantification in lymph node therapy response. Eur Radiol 24:1981–1988
Tawfik AM, Razek AA, Kerl JM, Nour-Eldin NE, Bauer R, Vogl TJ (2014) Comparison of dual-energy CT-derived iodine content and iodine overlay of normal, inflammatory and metastatic squamous cell carcinoma cervical lymph nodes. Eur Radiol 24:574–580
Lind JS, Meijerink MR, Dingemans AM et al (2010) Dynamic contrast-enhanced CT in patients treated with sorafenib and erlotinib for non-small cell lung cancer: a new method of monitoring treatment? Eur Radiol 20:2890–2898
Ng QS, Goh V, Fichte H et al (2006) Lung Cancer perfusion at multi-detector row CT:reproducibility of whole tumor quantitative measurements. Radiology 239:547–553
Kim KW, Lee JM, Klotz E et al (2009) Quantitative CT colour mapping of the arterial enhancement fraction of the liver to detect hepatocellular carcinoma. Radiology 250:425–434
Schmid-Bindert G, Henzler T, Chu TQ et al (2012) Functional imaging of lung cancer using dual energy CT: how does iodine related attenuation correlate with standardized uptake value of 18FDG-PET-CT? Eur Radiol 22:93–103
Werner-Wasik M, Xiao Y, Pequinot E, Curran WJ, Hauck W (2001) Assessment of lung cancer response after nonoperative therapy: tumor diameter, bidimensional product, and volume. A serial CT scan-based study. Int J Radiat Oncol Biol Phys 51:56–61
Acknowledgments
The scientific guarantor of this publication is univ. prof. Jiří Ferda, Ph.D. The authors of this manuscript declare relationships with the following companies: T. Flohr, B. Schmidt and M.Sedlmair are employees of Siemens HealthCare, Germany. This research was supported by the Charles University Research Fund (project number P36) and by the Ministry of Health, Czech Republic – the project of conceptual development of research organization (Faculty Hospital in Pilsen – FNPl, 00669806). One of the authors has significant statistical expertise. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. No subjects or cohorts have been previously reported. Methodology: prospective, diagnostic and prognostic, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baxa, J., Matouskova, T., Krakorova, G. et al. Dual-Phase Dual-Energy CT in Patients Treated with Erlotinib for Advanced Non-Small Cell Lung Cancer: Possible Benefits of Iodine Quantification in Response Assessment. Eur Radiol 26, 2828–2836 (2016). https://doi.org/10.1007/s00330-015-4092-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4092-6