Abstract
Objectives
To measure azygos, portal and aortic flow by two-dimensional cine phase-contrast magnetic resonance imaging (2D-cine PC MRI), and to compare the MRI values to hepatic venous pressure gradient (HVPG) measurements, in patients with cirrhosis.
Methods
Sixty-nine patients with cirrhosis were prospectively included. All patients underwent HVPG measurements, upper gastrointestinal endoscopy and 2D-cine PC MRI measurements of azygos, portal and aortic blood flow. Univariate and multivariate regression analyses were used to evaluate the correlation between the blood flow and HVPG. The performance of 2D-cine PC MRI to diagnose severe portal hypertension (HVPG ≥ 16 mmHg) was determined by receiver operating characteristic curve (ROC) analysis, and area under the curves (AUC) were compared.
Results
Azygos and aortic flow values were associated with HVPG in univariate linear regression model. Azygos flow (p < 10-3), aortic flow (p = 0.001), age (p = 0.001) and presence of varices (p < 10-3) were independently associated with HVPG. Azygos flow (AUC = 0.96 (95 % CI [0.91–1.00]) had significantly higher AUC than aortic (AUC = 0.64 (95 % CI [0.51–0.77]) or portal blood flow (AUC = 0.40 (95 % CI [0.25–0.54]).
Conclusions
2D-cine PC MRI is a promising technique to evaluate significant portal hypertension in patients with cirrhosis.
Key Points
• Noninvasive HVPG assessment can be performed with MRI azygos flow.
• Azygos MRI flow is an easy-to-measure marker to detect significant portal hypertension.
• MRI flow is more specific that varice grade to detect portal hypertension.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Portal hypertension is a major complication of cirrhosis. The direct measurement of portal pressure is highly invasive and is no longer performed in clinical practice. The current reference assessment method is measurement of the hepatic venous pressure gradient (HVPG) which, albeit indirect, has been shown to be the best predictor for the development of varices and to provide predictive prognostic values of the liver disease itself [1, 2]. In addition to its diagnostic and prognostic role of both compensated and decompensated cirrhosis, HVPG is an important tool for monitoring the pharmacological treatment of portal hypertension [3]. However, the HVPG technique has several limitations in clinical practice, two of which are the need for local expertise and its invasive nature. This underlines the need for noninvasive alternatives to measure portal pressure. Two-dimensional cine contrast-phase magnetic resonance imaging (2D-cine PC MRI) could be a promising safe, non-invasive, and reproducible technique for this purpose [4–6]. This technique can directly evaluate azygos and portal blood flow dynamics in patients with cirrhosis [6, 7]. The accuracy of the MRI-based azygos flow measurement for identifying high-risk hemorrhagic oesophageal varices in patients with chronic liver disease and portal hypertension has been recently reported [5]. However, the accuracy of this method for the noninvasive assessment of portal hypertension has not yet been evaluated in comparison with HVPG. The aims of the present study were to prospectively measure azygos, portal and aortic flow by 2D-cine PC MRI, and to compare the MRI values to HVPG measurements, the reference method, in patients with cirrhosis with or without oesophageal varices.
Materials and methods
Study population
This was a prospective single-centre study. All participating patients gave their written informed consent and the study was approved by the local Ethics Committee. Consecutive patients (n = 105) with suspected liver cirrhosis who were referred to our liver department were screened for inclusion between December 2012 and April 2014. Patients were eligible for the study if they had biopsy-proven liver cirrhosis (F4 according to the METAVIR classification) performed by the transjugular route with concomitant HVPG measurement [8]. Clinical (ascites, encephalopathy) and laboratory data (including liver function tests) were recorded. Child-Pugh and Model for End-Stage Liver Disease (MELD) scores classifying the severity of cirrhosis were also calculated.
Thirty-four patients had exclusion criteria including: encephalopathy (n = 2); concurrent β-blocker treatment (n = 8); a history of variceal haemorrhage (n = 14); contra-indications to MRI (n = 4), and no HVPG measurement (n = 2) or percutaneous liver-biopsy (n = 4). Of the 71 patients who were eligible for inclusion, two declined to participate. Consequently, 69 patients (45 men and 24 women; median age: 58 years) were included.
All patients underwent independently upper gastrointestinal endoscopy (UGE) and 2D-cine PC MRI within the first week after inclusion. All operators were blinded to the results of the other examinations.
HVPG measurement
All patients underwent transjugular liver biopsy and HVPG measurement by the same physician with over 20 years of experience (PS), as previously described [1, 9]. (see Supplementary material 1).
Upper gastrointestinal endoscopy
All examinations were performed by one of our experienced experts. Oesophageal varices were categorized into three groups (Table 1) based on the following classification criteria: grade 0 = absence of varice; 1 = varices that collapse on inflation of the oesophagus with air; 2 = varices between grade 1 and 3; 3 = varices which are large enough to occlude the lumen.
Group 1 corresponds to grade 0 and 1, which do not require prophylaxis for the prevention of variceal haemorrhage; group 2 and group 3 correspond to grade 2 and 3, respectively.
2D-cine PC MRI blood flow examination
MR examinations were performed with 1.5-T imaging software (Avanto, VB 15; Siemens Medical Solutions, Erlangen, Germany) equipped with gradients with a peak strength of 45 mT/m and a maximal slope of 200 T/m. All of the patients were examined with electrocardiographic gating using a 32-channel coil system after they had fasted for at least 6 hours, as recommended in the literature [10].
True fast imaging with steady-state precession (FISP) MRI sequences was performed in the axial, coronal, and sagittal planes to determine the optimal plane for flow quantification. In the second step, velocity encoding (Venc) optimization was achieved by running test measurements with a discrete set of Venc values with a Venc-scout sequence.
Blood flow was then quantified using the cine phase-encoding sequence combined with parallel imaging in the generalized autocalibrating partially parallel acquisition (GRAPPA) algorithm for breath-hold acquisitions. The quantification was then performed along the orthogonal plane of the vessel. The aortic flow and portal venous flow encoding sequences were performed using a breath-hold technique, with the patient in full inspiration, whereas the azygos flow encoding sequence was performed during free breathing and respiratory gating.
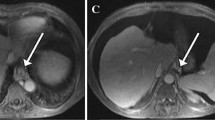
Aortic flow was imaged in the axial plane above the celiac trunk localized in the sagittal plane. Portal venous flow was measured in an orthogonal double-oblique plane to the sagittal plane and in the coronal oblique planes 20 mm proximal to the portal bifurcation. The azygos flow was measured in the axial plane at the most cephalic level of the parallel segment localized in the coronal and sagittal planes, below the azygos arch [5] (Fig. 1).
MR azygos and portal flow measurement in a 45-year-old man. True FISP MR sequences in a axial, b coronal and c sagittal planes were used to identify the most cephalic level of the parallel segment (arrow) located below the azygos arch (star). Azygos flow was measured in along a plane perpendicular to the course of the vessel localized with true FISP MR sequences (white line). True FISP MR sequences in d axial and e coronal planes were used to localize the main extra-hepatic portal vein (arrow). A plane was selected perpendicular (black line) to the main extra-hepatic portal vein to measure portal vein flow
Imaging sequence parameters are reported in Table 2.
Imaging interpretation
The flow data were processed at an independent workstation using flow analysis software (Syngo Argus Flow; Siemens Medical Solutions). Each data set was reconstructed to yield a magnitude image and a phase image (see Supplementary material 2).
Statistical analyses
Baseline characteristics of the study population were described by median and interquartile range (IQR) for continuous variables and by percentages for categorical variables. Values of the blood flows according to liver scores and varice grade were compared with the Kruskal Wallis test. Univariate and multivariate regression analysis were used to study the association between the HVPG and the different flows after common logarithm transformation to achieve a normal distribution. Multivariate analyses were adjusted for the variables that were determined to have a p value < 0.10 in univariate analysis. The MELD and Child-Pugh scores were entered in separate models, as these two scores are highly correlated.
Separate areas under the receiver operating characteristic curve (AUC-ROC) analysis were used to evaluate the diagnostic accuracy of the MR azygos, portal and aortic flow measurements for the diagnosis of a severe portal hypertension (HVPG ≥ 16 mmHg). AUCs and their 95 % confidence intervals (CI) were estimated for each flow and compared by a non-parametric method [11]. The best cutoff value of the azygos flow was chosen by maximizing the Youden index. The sensitivity, specificity, positive and negative predictive values, and likelihood ratios, with their corresponding exact 95 % CIs, are provided for azygos flow as well for varice grade ≥ 2 for the diagnosis of a severe portal hypertension (HVPG ≥ 16 mmHg) [12]. Univariate and multivariate logistic regressions were used to identify independent factors associated with severe portal hypertension (HVGP ≥ 16 mmHg). Multivariate models were adjusted for age, aortic and portal flows that had a p value < 0.10 in univariate analysis and additionally for the MELD (model 1) or Child-Pugh score (model 2) or varice grade (model 3).
The statistical analyses were performed using SAS 9.3 (SAS Institute Inc., North Carolina). The tests were two sided, and p values ≤ 0.05 were considered to be the threshold for statistical significance.
Results
Study population
The clinical and endoscopic features of the patients are summarized in Table 1. The two main causes of cirrhosis were hepatitis C virus (n = 28, 40.6 %) and alcohol (n = 20, 29 %). HVPG ranged from 3 to 25 mmHg, with a median of 16 mmHg (IQR [13–18]). A total of 32 patients (46 %) had grade 3 varices.
MRI mean blood flow values in all patients and in relation to the Child-Pugh score and varice grade are reported in Table 3.
HVPG and blood flow
The scatter plot of HVPG against log blood flows is presented in Fig. 2.
The azygos flow and HVPG were significantly correlated (R2 = 0.77; p < 10-3). On the contrary, no significant association was found between HVPG and the portal flow (R2 = 0.02, p = 0.07) or between HVPG and aortic flow (R2 = 0.14, p = 0.08).
In univariate analysis, HVPG was significantly associated with age, the severity of liver disease expressed by the MELD or the Child-Pugh score, varice group, and azygos and aortic blood flow. Conversely, no significant association was found between HVPG and the portal blood flow. When analyzed by multivariate linear regression, azygos and aortic blood flow, age and varice group remained independently associated with HVPG whatever the multivariate model. Neither the MELD nor the Child-Pugh score retained statistical significance (Table 4).
Distribution of each blood flow by varice grade and Child-Pugh score
Azygos blood flow measures were significantly different according to the varice group (p < 10-3) (Fig. 3a) and the Child-Pugh score (p < 10-3) (Fig. 3b).
Box plots of azygos flow (mL/s) according to each group of varices (Fig. 2a) and to Child-Pugh score (Fig. 2b). The boundary of boxes closest to zero indicates the 25th percentile, the line within boxes indicates the mean, and the boundary of boxes farthest from zero indicates the 75th percentile. The error bars indicate the smallest and largest values within 1.5 box lengths of the 25th and 75th percentiles. The outliers are represented as individual points. Varices groups were categorized as follows: Group 1: varices grade 0 or 1; Group 2: varices grade 2; Group 3: varices grade 3
In contrast, aortic blood flow measures were significantly different according to the Child-Pugh score (p = 0.01) but not to the varice group (p = 0.43). Portal blood flow measures were significantly different according to the varice group (p = 0.03) but not the Child-Pugh score.
Diagnostic accuracy of the flow measurements and varice grade ≥ 2 for HVPG ≥ 16 mmHg
ROC analysis (Fig. 4) for the diagnosis of severe portal hypertension (HVPG ≥ 16 mmHg), demonstrated that the azygos blood flow had a significantly higher area under the ROC curve (AUC = 0.96 (95 % CI [0.91–1.00]) than the aortic (AUC = 0.64 (95 % CI [0.51–0.77]) or portal blood flows (AUC = 0.40 (95 % CI [0.25–0.54]) (p < 10-3 for each comparison).
Receiver operating characteristic (ROC) curves for mean azygos blood flow (mL/s), mean aortic blood flow (mL/s) and mean portal blood flow (mL/s) performances for the diagnosis of an HVPG ≥ 16. AUC (azygos flow) = 0.96 (95 % CI [0.91–1.00]); AUC (portal flow) = 0.40 (95 % CI [0.25–0.54]); AUC (aortic flow) = 0.64 (95 % CI [0.51–0.77])
The best cutoff value maximizing the Youden index for the diagnosis of an HVPG ≥ 16 mmHg was 4.4 mL/s. Table 5 summarizes the diagnostic performance of azygos blood flow with this cutoff value and of a varice grade ≥ 2 for the diagnosis of an HVPG ≥ 16 mmHg. Azygos blood flow measurement was as sensitive as varice grading but markedly more specific.
Results of the logistic regression analyzes indicated that azygos flow was associated with severe portal hypertension, whatever the model (Table 6).
Discussion
This study shows that 2D-cine PC MRI of azygos flow is a promising noninvasive method for assessing severe portal hypertension. We have established an easy-to-measure noninvasive marker to detect an HVPG ≥ 16 mmHg—azygos blood flow with an optimized cutoff value of 4.4 mL/s—which has a very high sensitivity and specificity. These results are clinically relevant as HVPG accurately estimates portal pressure in diseases in which the resistance to portal flow is located at the sinusoids, as is the case for the most common etiology of portal hypertension, namely liver cirrhosis [19]. Portal pressure measurement via HVPG stratifies cirrhosis into stages with defined outcomes, prognosis, and management strategies [13]. An HVPG of 10 mmHg defines the presence of clinically significant portal hypertension and is the best predictor of the development of varices, clinical decompensation and hepatocellular carcinoma [14, 15]. Additionally, in cases of decompensated cirrhosis, an HVPG ≥ 16 mmHg is an important predictor of poor outcome and identifies patients at risk of developing refractory ascites, bacterial infections, hepatorenal syndrome or recurrent variceal haemorrhage [16, 17]. Another key finding is that azygos flow measurement is as sensitive as, and markedly more specific than, varice grading for diagnosing significant portal hypertension (i.e., HVPG ≥ 16 mmHg). This is also clinically relevant, as it could result in primary pharmacological or endoscopic prophylaxis of variceal bleeding. We were not able to analyze the performance of MRI to stratify portal hypertension in patients based on an HVPG threshold of 10 mmHg because of the few patients in our population below this level. Moreover, our results highlight a significant association between azygos flow measured by 2D-cine PC MRI and HVPG with simple and multivariate linear regression analysis.
Interestingly, the analysis showed that azygos and aortic flow were independently associated with HVPG. Cirrhosis causes considerable changes in the haemodynamics of blood flow, including splanchnic inflow, hepatic resistance and increased portal venous pressure [18]. In portal hypertension, the higher variceal pressure increases tension in the variceal wall. This has been associated with increased azygos flow, a known indicator of oesophageal collateral vessel flow [20, 21]. Because gastro-oesophageal collaterals drain into the azygos system, the measurement of the flow therein indirectly reflects the degree of gastro-oesophageal collateralization [22]. Portal hypertension leads to the formation of portosystemic collaterals with numerous pathways. Gastrooesophageal varices are the most relevant portosystemic collaterals because their rupture leads to variceal haemorrhage, the most lethal complication of cirrhosis. Thus, it is important to develop a sensitive noninvasive test correlated with HVPG in patients with gastrooesophageal varices.
Our results show high sensitivity and high specificity for mean azygos flow to detect severe portal hypertension. This high specificity may reflect the fact that the patients in our study had large gastro-oesophageal varices.
Other methods, such as endoscopic ultrasound (EUS) and thermodilution, may also be used to study the azygos flow [23, 24]. However, these techniques are not only invasive, but also require specific expertise. Another problem with EUS is the inter-observer variability [25]. In contrast, optimal results for inter-observer and intra-observer variability, as well as reproducibility, with 2D-cine PC MRI flow measurement have previously been demonstrated [5]. The significant independent association between HVPG and aortic blood flow may be related to the development of a hyper-dynamic circulatory syndrome, which is coupled with the severity of portal hypertension [26]. Aortic flow is thus a well-known indirect marker of cardiac output.
In contrast, there was no significant association between the portal flow and HVPG. There are conflicting reports about reduced or increased portal flow among patients with cirrhosis [27–29]. These discrepancies may be due to the stage of cirrhosis or to the physiologic features of portal hypertension and extrahepatic hemodynamic abnormalities [20, 30]. Portal hypertension results from both increased resistance to portal flow and increased portal blood inflow. Portal flow depends on both the portal venous inflow and the resistance of the portosystemic collaterals opposing blood flow. We hypothesize that the balance between intrahepatic resistance and altered splanchnic flow may be a key factor to explain our results. Reflecting the complexity of portal haemodynamics, Debatin et al. showed a lack of correlation between portal and azygos venous flow changes in patients with hypertension portal pre- and post- intrahepatic shunt placement [7].
Our results are in agreement with previous reports that have already highlighted the potential value of MRI in detecting high-risk oesophageal varices in patients with chronic liver disease and portal hypertension [4–6, 31]. Prior reports revealed an association between the risk of variceal haemorrhage and high azygos flow in patients with cirrhosis [5, 6]. In agreement with these data, we found a correlation between the azygos flow and the varice group.
In addition, our results showed a correlation between the MRI measured azygos flow and both the Child-Pugh and MELD scores, which is consistent with the results of previous studies [5]. As the Child-Pugh and MELD scores are valuable clinical tools in patients with cirrhosis, these results may have a major clinical impact. The Child-Pugh score is related to short-term prognosis in patients with acute variceal bleeding and is also a risk factor for oesophageal variceal haemorrhage. The Child-Pugh and MELD scores are currently used to define prognosis by modelling hepatic dysfunction. However, in contrast to MRI blood flow, these scores do not provide direct evidence of the stage or hemodynamic state of cirrhosis.
The robustness of the 2D-cine PC MRI blood flow sequence of this study has been validated in a previous study, both in vitro and in vivo in patients with or without portal hypertension [5]. Additionally, the sequence was combined with a retrospective ECG gating divided over 20 phases, resulting in high temporal resolution. We used the non breath-hold procedure for azygos flow evaluation. Breath-hold phase-contrast MRI flow has been reported to give different estimates of azygos flow in patients with portal hypertension compared with the non-breath-hold technique [32]. The breath-hold method is more susceptible to variations in respiratory phase and the consequent alterations in intrathoracic pressure. The measurement in different phases of respiration is thus more representative of the blood flow through the azygos vein than flow in either full inspiration or end expiration. Thus, breath-hold phase-contrast MRI flow was not a valid alternative to the non-breath-hold flow method.
Another noninvasive imaging technique recently developed to detect liver fibrosis and portal hypertension is MR elastography, which has proved to be promising for assessing severe portal hypertension and high-risk oesophageal varices [33].
Nevertheless, further prospective studies with more patients should be conducted to evaluate the role of noninvasive functional MR elastography in the clinical setting of cirrhosis and to compare liver/spleen MR and 2C MR flow in the same population.
Our study is limited by several technical and clinical points. First, 2D-cine PC MRI requires the use of double-oblique imaging planes, which are challenging and time consuming to position and coordinate with patient breath-holding or non-breath-holding. Second, the technique only allows visualization of two-dimensional anatomic collateral pathways of the portal venous system or azygos venous system. Such 2D anatomic information may be insufficient for a comprehensive flow evaluation, as the portosystemic collaterals associated with portal hypertension are highly variable and extend throughout the abdomen and the thorax. To overcome some of these limitations, 4D MRI flow mapping has recently emerged. 4D MR velocity mapping using temporally resolved 3D spatial encoding offers a combination of co-registered anatomic and hemodynamic information in the same examination. The feasibility of using radial 4D flow MRI to quantify blood flow in the hepatic and splanchnic vasculature of healthy volunteers and patients with portal hypertension has recently been reported [34]. Radial 4D flow MRI has large volumetric coverage including not only the portal circulation, hepatic arterial flow but also the azygos and portosystemic collaterals in a single breath hold with similar performance for flow parameter estimation when compared with standard Cartesian 4D flow and cine 2D phase-contrast measurements in vivo [35]. Nevertheless, the routine use of this technique is limited by the time-consuming reconstruction algorithm, which should be improved so as to provide images at the time of imaging.
Third, the predictive values of MRI could be affected by the high proportion of patients in our population with major portal hypertension, as demonstrated by the high median HVPG. Nevertheless, this limitation is counterbalanced by the relatively large population size.
Fourth, as our study included patients with advanced portal hypertension (71 % of patients with varices that require preventive treatment for variceal rupture), we did not correlate MRI flow measurements with history of complications of portal hypertension, such as variceal haemorrhage.
In conclusion, our results demonstrate that 2D-cine PC MRI is a good technique for the indirect evaluation of significant portal hypertension in patients with cirrhosis.
Abbreviations
- MRI:
-
Magnetic resonance imaging
- HVPG:
-
Hepatic venous pressure gradient
- UGE:
-
Upper gastrointestinal endoscopy
- IQR:
-
Interquartile range
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
References
Groszmann RJ, Wongcharatrawee S (2004) The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology 39:280–282
Groszmann RJ, Garcia-Tsao G, Bosch J et al (2005) Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 353:2254–2261
Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodes J, Bosch J (2003) Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology 37:902–908
Burkart DJ, Johnson CD, Ehman RL, Weaver AL, Ilstrup DM (1993) Evaluation of portal venous hypertension with cine phase-contrast MR flow measurements: high association of hyperdynamic portal flow with variceal hemorrhage. Radiology 188:643–648
Gouya H, Vignaux O, Sogni P et al (2011) Chronic liver disease: systemic and splanchnic venous flow mapping with optimized cine phase-contrast MR imaging validated in a phantom model and prospectively evaluated in patients. Radiology 261:144–155
Wu MT, Pan HB, Chen C et al (1996) Azygos blood flow in cirrhosis: measurement with MR imaging and correlation with variceal hemorrhage. Radiology 198:457–462
Debatin JF, Zahner B, Meyenberger C et al (1996) Azygos blood flow: phase contrast quantitation in volunteers and patients with portal hypertension pre- and postintrahepatic shunt placement. Hepatology 24:1109–1115
Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24:289–293
Lebrec D, Sogni P, Vilgrain V (1997) Evaluation of patients with portal hypertension. Baillieres Clin Gastroenterol 11:221–241
Jajamovich GH, Dyvorne H, Donnerhack C, Taouli B (2014) Quantitative liver MRIcombining phase contrast imaging, elastography, and DWI: assessment ofreproducibility and postprandial effect at 3.0 T. PLoS One 9, e97355
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiving operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Albilllos A, Garcia-Tsao G (2011) Classification of cirrhosis: the clinical use of HVPG measurements. Dis Markers 31:121–128
Nagula S, Jain D, Groszmann RJ, Garcia-Tsao G (2006) Histological-hemodynamic correlation in cirrhosis-a histological classification of the severity of cirrhosis. J Hepatol 44:111–117
Ripoll C, Groszmann R, Garcia-Tsao G et al (2007) Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 133:481–488
Ripoll C, Groszmann RJ, Garcia-Tsao G et al (2009) Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol 50:923–928
Gluud C, Henriksen JH, Nielsen G (1988) Prognostic indicators in alcoholic cirrhotic men. Hepatology 8:222–227
Merkel C, Bolognesi M, Bellon S et al (1992) Prognostic usefulness of hepatic vein catheterization in patients with cirrhosis and esophageal varices. Gastroenterology 102:973–979
Bosch J, Mastai R, Kravetz D, Navasa M, Rodés J (1986) Hemodynamic evaluation of the patient with portal hypertension. Semin Liver Dis 6:309–317
Perello A, Escorsell A, Bru C et al (1999) Wedged hepatic venous pressure adequately reflects portal pressure in hepatitis C virus-related cirrhosis. Hepatology 30:1393–1397
Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W, Practice Guidelines Committee of the American Association for the Study of Liver Diseases, Practice Parameters Committee of the American College of Gastroenterology (2007) Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 46:922–938
Bosch J, Mastai R, Kravetz D, Bruix J, Rigau J, Rodés J (1985) Measurement of azygos venous blood flow in the evaluation of portal hypertension in patients with cirrhosis. Clinical and haemodynamic correlations in 100 patients. J Hepatol 1:125–139
Mahl TC, Groszmann RJ (1990) Pathophysiology of portal hypertension and variceal bleeding. Surg Clin North Am 70:251–266
Bosch J, Groszmann RJ (1984) Measurement of azygos venous blood flow by a continuous thermal dilution technique: an index of blood flow through gastroesophageal collaterals in cirrhosis. Hepatology 4:424–429
Sukigara M, Shimoji K, Ohata M et al (1988) Effects of propranolol and nitroglygerin on cephalad collateral venous flow in patients with cirrhosis: evaluation using transesophageal real-time two-dimensional Doppler echography. Am J Gastroenterol 83:1248–1254
Kok T, van der Jagt EJ, Haagsma EB, Bijleveld CM, Jansen PL, Boeve WJ (1999) The value of Doppler ultrasound in cirrhosis and portal hypertension. Scand J Gastroenterol Suppl 230:82–88
Garcia-Tsao G, Friedman S, Iredale J, Pinzani M (2010) Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology 51:1445–1449
Moreno AH, Burchell AR, Rousselot LM, Panke WF, Slafsky F, Burke JH (1967) Portal blood flow in cirrhosis of the liver. J Clin Invest 46:436–445
Okazaki K, Miyazaki M, Onishi S, Ito K (1986) Effects of food intake and various extrinsic hormones on portal blood flow in patients with liver cirrhosis demonstrated by pulsed Doppler with the Octoson. Scand J Gastroenterol 21:1029–1038
Ohnishi K, Sato S, Pugliese D, Tsunoda T, Saito M, Okuda K (1987) Changes of splanchnic circulation with progression of chronic liver disease studied by echo-Doppler flowmetry. Am J Gastroenterol 82:507–511
Ronot M, Asselah T, Paradis V et al (2010) Liver fibrosis in chronic hepatitis C virus infection: differentiating minimal from intermediate fibrosis with perfusion CT. Radiology 256:135–14221
Burkart DJ, Johnson CD, Ehman RL, Weaver AL, Ilstrup DM (1993) Evaluation of portal venous hypertension with cine phase-contrast MR flow measurements: high association of hyperdynamic portal flow with variceal hemorrhage. Radiology 188:643–648
Ng WH, Chan YL, Sung JY, Lee YT, Lee SF, Chung SS (2004) Comparison of breath-hold 2D phase-contrast with non breath-hold cine phase-contrast MRA in the assessment of azygos venous blood flow in portal hypertension. MAGMA 16:211–217
Ronot M, Lambert S, Elkrief L et al (2014) Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur Radiol 24:1394–1402
Roldán-Alzate A, Frydrychowicz A, Niespodzany E et al (2013) In vivo validation of 4D flow MRI for assessing the hemodynamics of portal hypertension. J Magn Reson Imaging 37:1100–1108
Dyvorne H, Knight-Greenfield A, Jajamovich G et al (2015) Abdominal 4D flow MR Imaging in a breath hold: combination of spiral sampling and dynamic compressed sensing for highly accelerated acquisition. Radiology 275:245–254
Acknowledgments
The scientific guarantor of this publication is Hervé Gouya. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Sophie Grabar, Biostatistics and Epidemiology Unit, Hotel-Dieu and Paris-Descartes University, Assistance Publique-Hôpitaux de Paris, Paris, France. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. This is not a study on animals. Study subjects have never been previously reported.
Methodology: prospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1
(DOCX 86 kb)
Supplementary material 2
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Gouya, H., Grabar, S., Vignaux, O. et al. Portal hypertension in patients with cirrhosis: indirect assessment of hepatic venous pressure gradient by measuring azygos flow with 2D-cine phase-contrast magnetic resonance imaging. Eur Radiol 26, 1981–1990 (2016). https://doi.org/10.1007/s00330-015-3991-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3991-x