Abstract
Objectives
To compare contrast effects of gadobutrol with gadoterate meglumine for brain MRI in multiple sclerosis (MS) in a multicentre, randomized, prospective, intraindividual study at 3 T.
Methods
Institutional review board approval was obtained. Patients with known or suspected active MS lesions were included. Two identical MRIs were performed using randomized contrast agent order. Four post-contrast T1 sequences were acquired (start time points 0, 3, 6 and 9 min). If no enhancing lesion was present in first MRI, second MRI was cancelled. Quantitative (number and signal intensity of enhancing lesions) and qualitative parameters (time points of first and all lesions enhancing; subjective preference regarding contrast enhancement and lesion delineation; global preference) were evaluated blinded.
Results
Seventy-four patients (male, 26; mean age, 35 years) were enrolled in three centres. In 45 patients enhancing lesions were found. Number of enhancing lesions increased over time for both contrast agents without significant difference (median 2 for both). Lesions signal intensity was significantly higher for gadobutrol (p < 0.05 at time points 3, 6 and 9 min). Subjective preference rating showed non-significant tendency in favour of gadobutrol.
Conclusion
Both gadobutrol and gadoterate meglumine can be used for imaging of acute inflammatory MS lesions. However, gadobutrol generates higher lesion SI.
Key Points
• Contrast-enhanced MRI plays a key role in the management of multiple sclerosis.
• Different gadolinium-based contrast agents are available.
• Number of visibly enhancing lesions increases over time after contrast injection.
• Gadobutrol and gadoterate meglumine do not differ in number of visible lesions.
• Gadobutrol generates higher signal intensity than gadoterate meglumine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance imaging (MRI) of the central nervous system plays a key role in the management of multiple sclerosis (MS). According to the 2010 McDonald criteria, the diagnosis of MS requires the proof of lesion dissemination in space and time, which both can be provided by MRI [1]. The simultaneous presence of gadolinium-enhancing and non-enhancing lesions on the baseline MRI suffices for the diagnosis of lesion dissemination in time [2]. During the course of disease, MRI has a markedly higher sensitivity for disease activity than the clinical assessment of relapses [3]. Signs of disease activity (new T2 and gadolinium-enhancing lesions) represent a good surrogate marker for treatment effects on relapses [4] and can predict the effects on relapses over longer follow-up periods [5].
Gadobutrol (Gadovist; Bayer HealthCare, Berlin, Germany) is the only 1.0 molar, macrocyclic, second-generation, gadolinium-based contrast agent (GBCA) approved for contrast-enhanced MRI of the central nervous system in the European Union. Several studies compared the contrast effects of gadobutrol to representatives of the most commonly used 0.5 molar GBCAs for imaging of brain tumours and brain metastases in patients and animals and found superior contrast enhancement characteristics of gadobutrol [6–8]. However, to our knowledge there is no published work comparing gadobutrol to a 0.5 molar GBCA in MS. Gadoterate meglumine (Dotarem; Guerbet, Villepinte, France) is a 0.5 molar GBCA with a comparable safety profile [9].
Therefore, the aim of this study was to compare the contrast effects of gadobutrol with gadoterate meglumine for morphologic brain MRI in acute inflammatory MS lesions in a multicentre, randomized, prospective and intraindividual design at 3 T.
Materials and methods
This prospective study was conducted in compliance with Good Clinical Practice (ICH-GCP) and any applicable regulatory requirement, and was registered at the European Union Drug Regulating Authorities Clinical Trials (EudraCT) register (number 2009-014857-34). Local institutional review board approval was obtained at each study centre involved before study initiation. All patients signed an approved informed consent form before study enrolment.
Estimation of signal intensity difference
We estimated a guiding value for the signal intensity (SI) difference between the two GBCAs. SI in spin echo (SE) sequences depends on the longitudinal (T1) or transverse (T2) relaxation times as described by the following equation [10]:
Echo time (TE) and repetition time (TR) are given by the used MRI sequence. T1 and T2 relaxation times after GBCA injection (T i ) can be estimated from relaxation times before GBCA injection (T i(0)), GBCA relaxivities (r i ) and GBCA concentrations (c(GBCA)):
For T1 and T2 relaxation times of brain white matter at 3 T, 1300 ms and 100 ms were used, respectively [11]. GBCA relaxivities in inflammatory brain lesions are unknown. We alternatively used the relaxivities in plasma at 37 °C [10]. Relative SI difference was calculated as
SI differences were computed for GBCA concentrations between 0.001 and 1 mmol/L.
Patients
Before commencement of the study, sample size calculations were performed with the result that a minimum of 43 evaluable patients were needed. Patients were enrolled at three participating university hospitals in Germany. Patients were eligible if they were between 18 and 85 years of age, had been diagnosed with MS, and presented with a known or suspected active MS lesion for a planned contrast-enhanced MR examination of the brain. Patients were ineligible if they had any contraindications for MRI or GBCA application (pregnancy or breast-feeding, impaired renal or hepatic function, allergies or history of anaphylactic reaction, inability to remain lying down for 30 min, metallic implants), were clinically unstable, had received any GBCA within 24 h before study enrolment or were scheduled for any therapy that may interfere with the comparability of the study procedures (e.g. corticosteroid application between the two examinations). Enrolled patients were prospectively randomized to one of two GBCA administration orders: gadobutrol first or gadoterate meglumine first.
Magnetic resonance imaging
MRI was performed on 3-T systems (Trio and Verio; Siemens Healthcare, Erlangen, Germany) using 32-channel head coils. The two MRI examinations in every individual patient were conducted on the same scanner. The imaging protocol consisted of transverse T1-weighted SE sequences before and after GBCA injection. Scan parameters were TR 550 ms, TE 9 ms, slice thickness 5 mm, FOV 22 × 22 cm, matrix 320 × 320. The acquisition of additional transverse T2-weighted turbo spin echo as well as transverse and sagittal T2-weighted fluid attenuated inversion recovery sequences was performed according to the Consortium of MS Centers consensus guidelines [12]. Parallel imaging was not used. To ensure identical slice positioning between the two MRI scans of each patient a vendor-provided automatic slice-positioning systems (AutoAlign; Siemens Healthcare, Erlangen, Germany) was used in all patients.
Intravenous GBCA administration was performed identically in both examinations. A dose of 0.1 mmol Gd/kg body weight (i.e. 0.1 ml/kg for gadobutrol and 0.2 ml/kg for gadoterate meglumine) was injected into an antecubital vein by manual or power injection, followed by a saline flush of 20 ml.
Post-contrast image acquisition began directly after the intravenous injection was finished. The transverse T1-weighted SE sequence was repeated consecutively four times with each scan lasting for 3 min and the entire post-contrast imaging lasting for 12 min. Post-contrast time points were labelled by sequence start time (i.e. 0, 3, 6 and 9 min post GBCA injection).
The first MRI in all patients was read by an on-site investigator. Only when a contrast-enhancing brain lesion was present was a second MRI scheduled. Otherwise, the patient was terminated from the study. The interval between the two MR imaging examinations was between 12 h (to prevent any effect of carry-over) and 4 days (to minimize the chance of lesion evolution).
Image evaluation
All study images (1 pre- and 4 post-contrast image sets in each examination) and case report forms were transferred to a central imaging core lab and a quality check (observance of study protocol, completeness of MRI examinations and correctness of MRI sequence parameters) was performed.
The examinations were evaluated by two experienced neuroradiologists with 13 years (reader 1) and 7 years (reader 2) of neuroradiology experience. Both readers were blinded to the GBCA used and the patients’ clinical and radiologic information. The read consisted of three sessions. In all sessions the reader could perform routine image-manipulation functions (e.g. window/level, zoom and pan) on all image sets.
In session 1 (quantitative step 1, both readers) the pre-contrast and one randomized corresponding post-contrast image set were displayed together. The technical adequacy of both image sets was evaluated [excellent, adequate (with artefacts, but tolerable for assessment), inadequate (not tolerable for further evaluation)] and the number of enhancing lesions on the post-contrast set was calculated. All post-contrast images of all examinations were displayed sequentially in a randomized order.
In session 2 (qualitative, both readers) for each patient all image sets from both examinations were shown simultaneously in a matched-pairs fashion. All image sets were synchronized. The readers were asked at which time point the first enhancing lesion appeared and at which time point all contrast-enhancing lesions were visible for the first time for each GBCA. They were further asked for their subjective preference in a direct comparison of both contrast agents regarding contrast enhancement, lesion delineation from surrounding tissue/oedema as well as their global subjective preference (MR 1 better than MR 2, both MR studies equal, MR 2 better than MR 1).
In session 3 (quantitative step 2, only reader 1) the matched-pairs hanging protocol was used again. Circular regions of interest (ROIs) were placed around the enhancing parts of up to six representative MS lesions per examination, with an ROI size of at least 50 % of the total lesion size. The ROIs were copied automatically to the other time points and the corresponding second examination (same ROI size), but could be moved by the reader if necessary. For all ROIs the mean signal intensity, standard deviation (SD) and size (cm2) were saved.
Statistical analysis
Results are displayed as medians and range for the number of lesions, as means with standard deviation for continuous data, and absolute and relative frequencies for categorical data.
The preferences for one or the other GBCA were evaluated by logistic regression analysis across readers and by sign tests per reader’s assessment (secondary). For binary and ordinal scaled efficacy end points, logistic and multinomial regression analyses were performed on the basis of generalized estimation equations. For continuous data, a mixed linear model was used. Independence was used as a working correlation matrix taking into account the repeated measures design by two blinded readers and the intra-individual comparison of the GBCAs. The signal intensity was compared between the GBCAs across readers and by reader using mixed linear models as described above. The models were set up as Y = X β + Z u + ε with Y being the vector of observations, β being a vector of fixed effects [treatment group and baseline value (for changes from baseline)], u being a vector of independent and identical distributed random effects (reader and segment within a patient), X and Z being matrices of regressants and ε being the error term.
Statistical tests were performed two-sided, where p values below 0.05 were regarded as statistically significant.
The study was powered for hypothesis testing of the primary end point “overall assessment of contrast enhancement”. All secondary analyses are regarded as descriptive and hypothesis-generating.
Calculations were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA). Sample size calculations were performed with PASS 2005 (NCSS, Kaysville, UT, USA).
Results
Estimation of signal intensity difference
The estimated guiding value for the SI difference between the two GBCAs depends on the GBCA concentration and ranges between 0.02 % and 10.8 % (Fig. 1).
Estimated SI difference between the two GBCAs as a function of concentration. The calculation is based on certain assumptions stated in the manuscript. X-axis is scaled logarithmically to the base e. SI difference increases with GBCA concentration and peaks at approximately 0.25 mmol/L. Estimated intralesional GBCA concentration in MS lesions is below 0.14 mmol/L
Patients
Seventy-four consecutive patients were enrolled in a consecutive manner at the three study centres between January 2010 and October 2012. Table 1 summarizes the demography. Of the enrolled patients, 29 showed no contrast-enhancing lesion in the first MRI and hence were terminated from the study. Of these, 12 had received gadobutrol and 17 gadoterate meglumine.
Forty-five patients completed both MRI examinations and were included in the blinded read. No adverse events were reported during the study period for any patient. The median time interval between the two MRIs was 16 h (interquartile range, 14–21 h; total range, 12–94 h).
Quantitative analysis
For both GBCAs all image sets but one post-contrast set each were scored excellent or adequate by both readers.
No contrast-enhancing lesions were found on pre-contrast images. The number of enhancing lesions on post-contrast images did not show a significant difference between gadobutrol (median 2) and gadoterate meglumine (median 2) for all post-contrast time points (p > 0.05).
Lesion signal intensity was higher for gadobutrol for all post-contrast time points with the difference showing statistical significance for the time points 3, 6 and 9 min (Fig. 2; p = 0.04 at 3 min, 0.01 at 6 min and 0.02 at 9 min).
Qualitative analysis
Regarding the time point with the first enhancing lesion and the time point which shows all enhancing lesions, no statistically significant difference was found for both readers (Fig. 3; p > 0.05). For both GBCAs in some patients the first contrast-enhancing lesion became visible at the last time point (9 min).
In no parts of the subjective preference analysis was a statistically significant difference between the GBCAs found for both readers (p > 0.05). However, the combined analysis of both readers showed a tendency towards gadobutrol (Figs. 4 and 5).
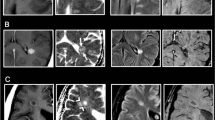
A 43-year-old woman with acute MS relapse. T1-weighted transverse SE images before (1a, 2a) and 0 min (1b, 2b), 3 min (1c, 2c), 6 min (1d, 2d) and 9 min (1e, 2e) after intravenous GBCA injection. Upper row shows MRI study with gadobutrol, lower row shows MRI study with gadoterate meglumine. The active MS lesion demonstrates a higher SI with gadobutrol
Discussion
This is the first published study comparing gadobutrol to gadoterate meglumine for brain MRI in acute inflammatory MS lesions. We found a significantly higher mean lesion SI for gadobutrol and a non-significant tendency in favour of a subjective preference of gadobutrol.
To facilitate the transfer of our results into clinical routine, the used imaging protocol was set up as close as possible to a routine diagnostic work-up, considering the published imaging guidelines [13, 14]. We therefore selected a robust conventional SE sequence and refrained from the quantification of the blood–brain barrier disruption (BBBD) using a dynamic contrast-enhanced MRI (DCE-MRI) approach. Moreover, we chose two commonly used GBCAs with a comparable safety profile.
Contrast enhancement in MS lesions changes over time naturally. Therefore, the time interval between the two MRI scans should be as short as possible. On the other hand, a certain time interval is necessary to allow for sufficient elimination of the first GBCA before the second scan. We defined 12 h as the minimum time interval. A longer period would have allowed for a more complete excretion of the first GBCA. However, all patients included in this study suffered from an acute MS relapse and in the majority of the patients therapy by corticosteroid injection could not be postponed longer than approximately 12 h from clinical views. As corticosteroids can rapidly reverse the BBBD [13], the second MRI scan had to be performed before therapy initiation. Based on a serum half-life of approximately 90 min for both used GBCAs (summaries of product characteristics), less than 1 % of the previously injected GBCA amount remains in the body after 12 h. Only patients with normal renal function were included in the study. The median time interval between the two MRI scans in all enrolled patients was 16 h. All pre-contrast images of this study were reviewed and no contrast-enhancing lesion was found. Finally, we randomized the GBCA injection order to distribute any potential residual carry-over effect between the two GBCAs.
Acute inflammatory MS lesions exhibit a BBBD, allowing extravasation of low molecular weight MRI GBCAs over time [15]. The accumulation of GBCAs in the extravascular extracellular space shortens the longitudinal relaxation time T1 and thus results in increased signal intensity in T1-weighted MRI sequences [10]. However, the time course and intensity of contrast enhancement in MS lesions can vary [16]. In a given lesion, the contrast depends on the magnetic field strength, local GBCA concentration and GBCA magnetic properties [10].
In today’s clinical practice the maximum broadly available magnetic field strength is 3 T. There are reports that imaging at 3 T can improve diagnostic sensitivity in MS [17, 18]. We therefore performed our study at this field strength.
The intralesional GBCA concentration at a given time point depends on the injected GBCA dose and the GBCA tracer kinetics and may differ between GBCAs. The GBCA standard dose is 0.1 mmol/kg body weight. Studies indicated that MS lesion detection may be improved with higher GBCA concentrations (0.2–0.3 mmol/kg body weight) [19, 20]. However, increasing the injected GBCA dose is expensive and is discouraged in view of nephrogenic systemic fibrosis [9].
GBCA magnetic properties, described by their T1 and T2 relaxivities, vary among different GBCA and depend in a complex manner on the physiological environments and the level of protein binding. The published relaxivities of gadobutrol in plasma at 37 °C (r1 = 5.0, r2 = 7.1) are higher than for gadoterate meglumine (r1 = 3.5, r2 = 4.9) [10]. However, SI in MRI is not directly proportional to the GBCA relaxivities and depends on different influencing factors. Nevertheless, we roughly estimated the theoretical SI difference between the two GBCAs. There are no published data on GBCA concentrations in inflammatory MS lesions. However, the results of an in vivo measurement of GBCA concentrations in a rat glioma model might serve as a reference [21]. In this study the GBCA concentration in the tumour (in mmol/L tumour volume) was between 0.9- and 1.4-fold the injected concentration (in mmol/kg body weight). Considering the variable SI in MS lesions, GBCA concentrations are likely to vary. Based on the GBCA standard dose for human examinations (0.1 mmol/kg body weight), the calculated SI difference was in the range of 2–10 %. Although our calculation is based on certain assumptions, the calculated and the empirically found SI difference (2–4 % for the different time points) are in good agreement.
We observed a continuous increase of SI and the reported number of MS lesions during the investigated time period of 12 min after GBCA injection for both GBCAs. In some patients the first enhancing lesion became visible at the last time point for both GBCAs. This finding is in keeping with the results of previously published studies on MS lesions [12, 19, 22] and tumours [23]. Bagheri et al. indicated that the SI and number of MS lesions might even rise during the first hour after contrast injection [24]. In contrast, there are also reports that the number of enhancing MS lesions can decrease at 30 min [25, 26]. Current imaging guidelines recommend a minimum scan delay after GBCA injection of 5 min [13] or performance of the GBCA injection before the first MRI sequence [14].
BBBD can occur in different brain pathologies from manifold pathophysiologic mechanisms [15]. BBBD in MS seems to result from heterogeneous pathogenetic mechanisms [27, 28]. There is evidence that the conventional binary approach, differentiating between enhancing and non-enhancing MS lesions, is a simplification of the pathophysiological processes in an inflammatory lesion. The BBBD seems to develop over a certain time and then gradually resolves again [17, 29]. Dichotomization in enhancing versus non-enhancing lesions results from the subjective assessment of the reporting radiologist; it may also result in the opposite judgment in the same patient depending on the detection threshold of the used MRI protocol. A solution to this dilemma could be the quantification of the BBBD by DCE-MRI [30–32]. However, this approach is complex and hardly used outside of specialized centres. The binary reporting approach is currently the widely accepted standard. Therefore, we performed the conventional qualitative image evaluation by two readers.
Our study has some limitations. First, the last post-contrast scan ended 12 min after contrast injection. A longer scan time might have revealed more slowly enhancing brain lesions. However, our focus was on the comparison of two different GBCAs. For all time points imaged, the SI of gadobutrol was higher than that of gadoterate meglumine. Considering the similar serum half-life and molecular weight of both GBCAs, it appears unlikely that this ratio would have reversed after a longer delay time. Second, we performed our study at 3 T only. The results at 1.5 T cannot be directly extrapolated from our results. Third, all MRI scanners used were manufactured by the same vendor, so that generalizations to other MRI scanners are to be done with care. However, we used a simple SE sequence, which is available on virtually every MRI scanner from all vendors. Fourth, in some of the enrolled patients no contrast-enhancing lesion was found in the first MRI. In these patients no second MRI was scheduled to limit patient stress and study complexity. This approach was validated by the study results. No patient with enhancing lesions in the first MRI lacked enhancing lesions in the second MRI. Finally, the GBCAs could not be blinded to the site investigators, as the gadolinium concentrations differed between them and different amounts had to be injected. However, the MRI parameter settings were fixed during the study for both GBCAs and the readers were blinded to the GBCA to ensure that bias towards one or the other GBCA is minimized. The study was powered for hypothesis testing of the primary end point “overall assessment of contrast enhancement”. All secondary analyses are regarded as descriptive and hypothesis-generating.
In conclusion, both gadobutrol and gadoterate meglumine can be used for imaging of acute inflammatory brain MS lesions. However, gadobutrol generates a significantly higher lesion SI. To improve lesion detection we recommend a scan delay after GBCA injection or performance of the GBCA injection before the first MRI sequence.
Abbreviations
- BBBD:
-
blood–brain barrier disruption
- DCE-MRI:
-
dynamic contrast-enhanced MRI
- GBCA:
-
gadolinium-based contrast agent
- MRI:
-
magnetic resonance imaging
- MS:
-
multiple sclerosis
- SE:
-
spin echo
- SI:
-
signal intensity
- TE:
-
echo time
- TR:
-
repetition time
References
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
Montalban X, Tintoré M, Swanton J et al (2010) MRI criteria for MS in patients with clinically isolated syndromes. Neurology 74:427–434
Filippi M, Preziosa P, Rocca MA (2014) Magnetic resonance outcome measures in multiple sclerosis trials: time to rethink? Curr Opin Neurol 27:290–299
Sormani MP, Bonzano L, Roccatagliata L, Cutter GR, Mancardi GL, Bruzzi P (2009) Magnetic resonance imaging as a potential surrogate for relapses in multiple sclerosis: a meta-analytic approach. Ann Neurol 65:268–275
Sormani MP, Bruzzi P (2013) MRI lesions as a surrogate for relapses in multiple sclerosis: a meta-analysis of randomised trials. Lancet Neurol 12:669–676
Koenig M, Schulte-Altedorneburg G, Piontek M et al (2013) Intra-individual, randomised comparison of the MRI contrast agents gadobutrol versus gadoteridol in patients with primary and secondary brain tumours, evaluated in a blinded read. Eur Radiol 23:3287–3295
Anzalone N, Scarabino T, Venturi C et al (2013) Cerebral neoplastic enhancing lesions: multicenter, randomized, crossover intraindividual comparison between gadobutrol (1.0M) and gadoterate meglumine (0.5M) at 0.1 mmol Gd/kg body weight in a clinical setting. Eur J Radiol 82:139–145
Anzalone N, Gerevini S, Scotti R, Vezzulli P, Picozzi P (2009) Detection of cerebral metastases on magnetic resonance imaging: intraindividual comparison of gadobutrol with gadopentetate dimeglumine. Acta Radiol 50:933–940
European Society of Urogenital Radiology (2014) ESUR guidelines on contrast media. European Society of Urogenital Radiology, Vienna. Available via http://www.esur.org/guidelines/. Accessed 15 Mar 2015
Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ (2005) Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Investig Radiol 40:715–724
Wansapura JP, Holland SK, Dunn RS, Ball WS Jr (1999) NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging 9:531–538
Filippi M, Yousry T, Rocca MA, Fesl G, Voltz R, Comi G (1997) Sensitivity of delayed gadolinium-enhanced MRI in multiple sclerosis. Acta Neurol Scand 95:331–334
Simon JH, Li D, Traboulsee A et al (2006) Standardized MR imaging protocol for multiple sclerosis: consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol 27:455–461
Lövblad KO, Anzalone N, Dörfler A et al (2010) MR imaging in multiple sclerosis: review and recommendations for current practice. AJNR Am J Neuroradiol 31:983–989
Heye AK, Culling RD, Valdés Hernández MD, Thrippleton MJ, Wardlaw JM (2014) Assessment of blood–brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. Neuroimage Clin 6:262–274
Jelescu IO, Leppert IR, Narayanan S, Araújo D, Arnold DL, Pike GB (2011) Dual-temporal resolution dynamic contrast-enhanced MRI protocol for blood–brain barrier permeability measurement in enhancing multiple sclerosis lesions. J Magn Reson Imaging 33:1291–1300
Leppert IR, Narayanan S, Araújo D et al (2014) Interpreting therapeutic effect in multiple sclerosis via MRI contrast enhancing lesions: now you see them, now you don't. J Neurol 261:809–816
Stankiewicz JM, Glanz BI, Healy BC et al (2011) Brain MRI lesion load at 1.5T and 3T versus clinical status in multiple sclerosis. J Neuroimaging 21:e50–e56
Uysal E, Erturk SM, Yildirim H, Seleker F, Basak M (2007) Sensitivity of immediate and delayed gadolinium-enhanced MRI after injection of 0.5 M and 1.0 M gadolinium chelates for detecting multiple sclerosis lesions. AJR Am J Roentgenol 188:697–702
Filippi M, Rovaris M, Capra R et al (1998) A multi-centre longitudinal study comparing the sensitivity of monthly MRI after standard and triple dose gadolinium-DTPA for monitoring disease activity in multiple sclerosis. Implications for phase II clinical trials. Brain 121:2011–2020
Le Duc G, Corde S, Charvet AM et al (2004) In vivo measurement of gadolinium concentration in a rat glioma model by monochromatic quantitative computed tomography: comparison between gadopentetate dimeglumine and gadobutrol. Investig Radiol 39:385–393
Silver NC, Good CD, Barker GJ et al (1997) Sensitivity of contrast enhanced MRI in multiple sclerosis. Effects of gadolinium dose, magnetization transfer contrast and delayed imaging. Brain 120:1149–1161
Engelhorn T, Schwarz MA, Eyupoglu IY, Kloska SP, Struffert T, Doerfler A (2010) Dynamic contrast enhancement of experimental glioma an intra-individual comparative study to assess the optimal time delay. Acad Radiol 17:188–193
Bagheri MH, Meshksar A, Nabavizadeh SA, Borhani-Haghighi A, Ashjazadeh N, Nikseresht AR (2008) Diagnostic value of contrast-enhanced fluid-attenuated inversion-recovery and delayed contrast-enhanced brain MRI in multiple sclerosis. Acad Radiol 15:15–23
Hashemi H, Behzadi S, Ghanaati H et al (2014) Evaluation of plaque detection and optimum time of enhancement in acute attack multiple sclerosis after contrast injection. Acta Radiol 55:218–224
Filippi M, Capra R, Campi A et al (1996) Triple dose of gadolinium-DTPA and delayed MRI in patients with benign multiple sclerosis. J Neurol Neurosurg Psychiatry 60:526–530
Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47:707–717
Rosenberg GA (2012) Neurological diseases in relation to the blood–brain barrier. J Cereb Blood Flow Metab 32:1139–1151
Soon D, Tozer D, Altmann D, Tofts P, Miller D (2007) Quantification of subtle blood–brain barrier disruption in non-enhancing lesions in multiple sclerosis: a study of disease and lesion subtypes. Mult Scler 13:884–894
Kermode AG, Thompson AJ, Tofts P et al (1990) Breakdown of the blood–brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Pathogenetic and clinical implications. Brain 113:1477–1489
Tofts PS, Kermode AG (1991) Measurement of the blood–brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med 17:357–367
Ingrisch M, Sourbron S, Morhard D et al (2012) Quantification of perfusion and permeability in multiple sclerosis: dynamic contrast-enhanced MRI in 3D at 3T. Investig Radiol 47:252–258
Acknowledgments
The scientific guarantor of this publication is Prof. Dr. A. Doerfler. The authors of this manuscript declare relationships with the following companies: Carsten Schwenke works as a freelance statistician for Bayer on an honorary basis. Norbert Hosten is a stockholder of Siemens and is an institutional grant recipient of Siemens and Bayer. This study has received funding by Bayer. One of the authors has significant statistical expertise. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. No study subjects or cohorts have been previously reported. Methodology: prospective, randomised controlled trial, multicentre study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saake, M., Langner, S., Schwenke, C. et al. MRI in multiple sclerosis: an intra-individual, randomized and multicentric comparison of gadobutrol with gadoterate meglumine at 3 T. Eur Radiol 26, 820–828 (2016). https://doi.org/10.1007/s00330-015-3889-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3889-7