Abstract
Objectives
We aimed at establishing the impact upon gadolinium administration on the conspicuity of active enhancing multiple sclerosis (MS) lesions using double inversion recovery (DIR) at 3T.
Methods
15 consecutive patients with MS (n=8) or a clinically isolated syndrome (n=7) underwent pre and post-contrast DIR in addition to T2-weighted, FLAIR, pre and post-contrast T1-weighted sequences. First, two neuroradiologists located and marked all the enhancing MS lesions visible in consensus. Second, two other neuroradiologists, blinded to other sequences than DIR, independently assessed the SI changes from pre to post-contrast DIR images for each enhancing lesion, according to a 4-point-scale: increased SI (grade 1), absence of change (grade 2), lesion being partially (grade 3) or completely masked on post-contrast DIR images (grade 4).
Results
246 MS lesions were detected including 26 enhancing on post-contrast T1-weighted images in 9 patients. The two blinded readers concluded to a decreased signal-intensity on post-contrast DIR images for all the 26 enhancing MS lesions (14 of grade 3 and 12 of grade 4). Inter-observer agreement was excellent, Kappa=0.85 (0.75 - 0.94). Using DIR post-contrast leads to altered signal-intensity of enhancing active MS lesions, ranging from partial to complete signal-loss.
Conclusion
Our study strongly suggests the use of DIR before gadolinium administration.
Key Points
• DIR has gained widespread use in MS.
• MRI protocols for MS patients usually contain several post-contrast sequences.
• Signal-intensity of enhancing MS lesions is altered using DIR post-contrast.
• Our study strongly suggests the use of DIR before gadolinium administration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The administration of gadolinium-based contrast agents is a common part of MR imaging examinations in patients with multiple sclerosis (MS). Demonstrating the presence of gadolinium-enhancing lesions is crucial for the diagnosis of MS [1], and most of the therapeutic strategies for MS also rely on contrast-enhanced MRI. Sensitivity of T1-weighted spin echo MR sequences for the detection of active MS lesions is progressively enhanced from 5 to 10 minutes after intra-venous injection of gadolinium [2]. Indeed, in the daily practice, the MRI protocols performed in MS patients usually contain several post-contrast MR sequences to further increase the delay-time between gadolinium administration and acquisition of T1-weighted images.
The double inversion recovery (DIR) MR sequence is based on a turbo spin echo (TSE) acquisition with variable refocusing flip angles (Brainview, Philips; Cube, General Electric; SPACE, Siemens) and includes two inversion recovery pulses designed for the suppression of cerebrospinal fluid (CSF) and normal white matter. DIR has gained widespread use in several clinical applications, including MS. Compared to conventional T2-weighted and FLAIR imaging, DIR improves the detection of cortical [3–7], infratentorial [8], spinal cord [9] and optic nerve [10] inflammatory lesions. Indeed, the diagnosis of these specific MS lesions may rely only on DIR images considering that T2-weighted and FLAIR images could be inconclusive. Even if DIR was initially described pre-contrast [5, 7], there is a temptation for radiologists to perform DIR, with its long acquisition time, after the gadolinium administration to further increase the delay-time before the acquisition of post-contrast T1-weighted images.
DIR is now widely used and a better knowledge of its potential pitfalls appears crucial. While the impact of gadolinium administration on T1-w MR sequences including a short tau inversion recovery (IR) pulse (TI of approximately 140 ms) is well known [11], the potential influence of gadolinium administration on the T2-weighted DIR sequence that includes a longer short tau IR pulse (ranging from 400 to 650 ms) is still not known. We hypothesized that the signal-intensity (SI) of active enhancing inflammatory lesions could be altered using post-contrast DIR considering the IR pulses used. The aim of this study was to establish the impact upon gadolinium administration on the conspicuity of active enhancing MS lesions using DIR at 3T. To test this hypothesis, we performed the DIR sequence pre- and post-contrast in a cohort of consecutive patients with MS and clinically isolated syndrome (CIS).
Materials and methods
Patients and MRI
From May 2014 to August 2014, 15 consecutive patients (seven with CIS and eight with relapsing remitting multiple sclerosis (RRMS), 11 women, four men; mean age 28 years, min: 20 years, max: 38 years) underwent 3T MRI (Achieva; Philips Medical Systems; Best, The Netherlands) using a 32-channel array head coil. The MRI protocol included pre- and post-contrast 3D DIR in addition to axial pre-contrast T1-weighted, T2-weighted, diffusion-weighted, 3D FLAIR and 3D TSE T1-weighted post-contrast MR sequences. Gadolinium (Dotarem; Guerbet, France) was administrated intravenously at a concentration of 0.1 mmol/kg following the acquisition of the pre-contrast DIR sequence. The delay time between intravenous gadolinium administration and image acquisition was 10 minutes and 15 minutes 30 seconds for DIR and post-contrast T1-weighted sequences, respectively. MR sequence parameters are detailed in Table 1.
Image analysis
First, all the MRI examinations were assessed in consensus by two experienced neuroradiologists (X.L) and (J.P.P). For each subject, the readers were asked to locate and mark all the active enhancing inflammatory lesions visible using T2-weighted, pre-contrast DIR, 3D FLAIR, pre- and post-contrast T1-weighted MR sequences. Active inflammatory lesions were defined using the following criteria: size > 2 mm, enhancement on post contrast T1-weighted images associated with a corresponding parenchymal hyperintensity on 3D FLAIR, T2-weighted images and/or pre-contrast 3D DIR. Location (periventricular, deep white matter, intra-cortical, juxta-cortical or infra-tentorial) and size of each MS lesion (enhancing or not) were also collected.
Second, two neuroradiologists, with 10 (reader 1, S.B) and 8 (reader 2, P.L) years of experience, blinded to clinical data and to all the MR sequences other than DIR, independently analyzed pre- and post-contrast DIR images combined. For each of the previously marked enhancing MS lesion, they were asked to qualitatively assess the SI changes from pre- to post-contrast DIR images, according to a 4-point-scale: increased SI (grade 1), absence of change (grade 2), decreased SI: the lesion being partially (grade 3) or completely masked on post-contrast DIR images (grade 4). The readers were aware of gadolinium administration and assessed pre- and post-contrast DIR images synchronized and simultaneously using the available workstation (IntelliSpace PACS; Philips Healthcare; The Netherlands). Disagreements between the two blinded readers were resolved in consensus.
Quantitative analysis was performed by one senior neuroradiologist (S.B.) using the available workstation (iSite ViewForum, Philips Medical Systems, The Netherlands). Pre- and postcontrast DIR and post-contrast T1-weighted MR images were first automatically co-registered. For each patient, a region of interest (ROI) was manually drawn on post-contrast T1-weighted images encompassing each of the active enhancing MS lesions. The ROI was then automatically propagated on the co-registered pre- and post-contrast DIR images allowing for the measurement of signal-intensity changes.
Statistical analysis
Statistical analyses were performed using R Statistical Software (Version 3.1.2; http://www.r-project.org/). Inter-observer agreement was measured using the Cohen Kappa test. k values were interpreted as follows: k value of 0 indicates poor agreement; k value of 0.01–0.20, minor agreement; k value of 0.21–0.40, fair agreement; k value of 0.41–0.60, moderate agreement; k value of 0.61–0.80, good agreement; and k value of 0.81–1; excellent agreement. The signal-intensity values of all the enhancing MS lesions were compared between pre- and post-contrast DIR images using the Wilcoxon Signed-Rank non-parametric test.
Results
MS lesions
Based on the consensus reading, 246 inflammatory lesions were detected among the 15 patients (min number of lesions per patient: 3, max: 27) with a mean size of 6.5 mm (min: 2.9 mm, max: 23 mm). All these lesions were hyperintense on both FLAIR and pre-contrast DIR images. Most of the lesions were located in periventricular regions (n= 101, 41 %) while 13 lesions were infra-tentorial (5 %), 56 juxta-cortical (23 %), two intra-cortical (1 %) and 74 involved the deep white matter (30 %).
Of these 246 inflammatory lesions, 26 were enhanced on post-contrast T1-weighted images (two intra-cortical, seven juxta-cortical, three infra-tentorial, ten periventricular and four within the deep white matter) in nine patients (min number of lesions per patient: 1, max: 4) and were selected for blinded review.
In our cohort, all the lesions enhancing on post-contrast T1-weighted images were also visible on pre-contrast DIR images and FLAIR images.
Signal-intensity changes of enhancing lesions
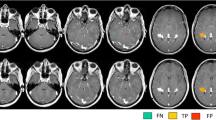
From the 26 enhancing active MS lesions, 12 were considered completely masked on post-contrast DIR images (grade 4, Fig. 1) by both the blinded readers.
Post-contrast T1-weighted (a), pre (b) and post-contrast (c) DIR coronal images in patient 10 with RRMS. The left frontal lesion appears hyperintense on pre-contrast DIR images (a, arrow) and is completely masked on post-contrast DIR images (b, arrow). This inflammatory lesion was rated “4” by the two blinded readers. The left frontal lesion, masked on post-contrast DIR images, enhances on post-contrast T1-weighted images (c, arrow)
According to reader 1, the 14 remaining enhancing lesions showed a partial decrease in SI (grade 3), 12 according to reader 2. Indeed, there were two disagreements between readers: two enhancing lesions (one periventricular and one within the deep white matter) that were considered without change in SI (grade 2) by reader 2 and with a partial decrease (grade 3) in SI according to reader 1.
After consensus reading, the readers concluded to a decrease in SI from pre- to post-contrast DIR images (14 of grade 3 and 12 of grade 4) for all the 26 inflammatory enhancing lesions studied (as initially stated by reader 1). There was no lesion with an increased or unchanged SI from pre- to post-contrast DIR images (grade 1 and 2, respectively).
Inter-observer agreement for the evaluation of SI changes between pre- and post-contrast DIR was excellent, Kappa of 0.85 (0.75 - 0.94).
The mean signal-intensity values of the active enhancing MS lesions were significantly different between pre- and post-gadolinium DIR images (mean: 56.3, min: 38.1, max: 74.5 and mean: 30.9, min: 14.7, max: 47.1 respectively; p<0.001) with a systematic decreased signal-intensity of enhancing inflammatory lesions on post-contrast DIR images compared to pre-contrast DIR images (mean: 46.2%, min: 6.1%, max: 79.2 %).
Discussion
MRI protocols for MS patients usually contain several post-contrast MR sequences to increase the delay-time before the acquisition of post-contrast T1-weighted images. For this purpose, performing DIR post-contrast could have been considered useful, considering its long acquisition time. However, in this technical development, we found that the use of DIR post-contrast systematically leads to a decreased signal-intensity of active enhancing MS lesions, ranging from partial to complete signal-loss.
Our results are of clinical relevance. Indeed, in the diagnostic work-up, it may be useful to correlate enhancing lesions on post-contrast T1-weighted images with a corresponding T2- hyperintensity (on T2-weighted, FLAIR or DIR images) to confirm inflammatory lesions and rule out a vascular enhancement. Considering that DIR could be superior to FLAIR and/or T2-weighted imaging for the detection of highly specific features of MS (such as cortical [3–7], infratentorial [8], spinal cord [9] and optic nerve [10] lesions), post-contrast DIR may lead to misdiagnoses (active lesions being hindered) with potential implications for patient care, particularly in patients with CIS or under treatment. A better understanding of the potential pitfalls using DIR also appears mandatory as the DIR sequence could potentially replace the conventional FLAIR sequence in some MRI protocols.
Post-contrast FLAIR was reported useful to detect subtle enhancement in the CSF space resulting from leptomeningeal brain blood barrier dysfunction when post-contrast T1W MRI does not detect these abnormalities [12]. In this prospective study, the FLAIR, post-contrast T1-weighted and DIR MR sequences were based on the same 3D TSE acquisition (“Brainview”) and performed with the same voxel size, providing an unbiased comparison. Indeed, FLAIR and DIR sequences differed solely by the use of an additional IR pulse with DIR, designed for the suppression of normal white matter (including a relatively short inversion time of 425 ms). Our results strongly suggest that the decrease in SI systematically observed for active enhancing lesions using post-contrast DIR is directly related to the use of this additional, short, IR pulse. The reason why the SI of some of the enhancing MS lesions was only partially decreased remains unclear. We may hypothesize that the drop in SI could be partly related to the degree of brain blood barrier disruption, resulting in various degrees of regional T1 shortening. Experimental studies may be required to assess the potential influence of gadolinium concentration on DIR images.
In the present study, we reported SI changes of active enhancing MS lesions, while Harris et al. [13] focused on patients with high-grade brain tumours. MS lesions are characterized by blood-brain barrier (BBB) breakdown, while patients with glioblastoma also exhibit significantly elevated blood volume. In their study, Harris et al., suggested that the decrease in SI observed on post-contrast DIR images could be related to the elevated blood tumoral volume considering the sensitivity of the DIR sequence to susceptibility differences between tissues [13]. We found that the signal loss observed on post-contrast DIR could also be observed in case of brain lesions with BBB breakdown and without neoangiogenesis.
Our study had some limitations. First, we included a small number of patients and all the lesions visible on pre-contrast DIR images were also detected using FLAIR in our cohort. Our main purpose was to show the influence of gadolinium on the SI of enhancing inflammatory lesions by the use of post-contrast DIR images. We did not include more patients since we demonstrated qualitatively and quantitatively a decreased signal-intensity of all the 26 enhancing inflammatory lesions in patients with CIS and RRMS on post-contrast DIR images compared to pre-contrast DIR images. We may hypothesize that the type of MS does not influence the occurrence of signal loss in enhancing MS lesions that are intrinsically characterized by a BBB breakdown. However, further studies are required to assess post-contrast DIR images in other types of MS and using DIR sequences from other MRI manufacturers. We did not formally demonstrate that the use of post-contrast DIR leads to misdiagnoses in the present study. Considering the literature comparing DIR with other MR sequences, we may assume that a larger cohort of patients could demonstrate the implication for patient care of this finding. Second, the described signal-loss of enhancing lesions on post-contrast DIR images may be directly related to the concentration in regional tissue of the gadolinium-based contrast agent. However, in our study, we used a single delay time between intravenous gadolinium administration and DIR acquisition for all the included patients. Indeed, the potential influence of shorter or longer delay times on post-contrast DIR images remains to be elucidated. Finally, we used Dotarem in our study while the different T1-relaxivities provided by the other commercially available contrast agents could also affect the described phenomenon.
Conclusion
Using DIR post-contrast leads to altered signal-intensity of enhancing active MS lesions, ranging from partial to complete signal-loss, with potential implications for patient care. Our study strongly suggests the use of DIR before gadolinium administration.
Abbreviations
- MS:
-
Multiple sclerosis
- RRMS:
-
Relapsing remitting multiple sclerosis
- CIS:
-
Clinically isolated syndrome
- DIS:
-
Dissemination of brain lesions in space
- DIT:
-
Dissemination of brain lesions in time
- SI:
-
Signal-intensity
- DIR:
-
Double inversion recovery
- FLAIR:
-
Fluid-attenuated inversion recovery
References
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302
Uysal E, Erturk SM, Yildirim H et al (2007) Sensitivity of immediate and delayed gadolinium-enhanced MRI after injection of 0.5 M and 1.0 M gadolinium chelates for detecting multiple sclerosis lesions. AJR. Am J Roentgenol 188(3):697–702
Calabrese M, De Stefano N, Atzori M et al (2007) Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch Neurol 64(10):1416–1422
Geurts JJ, Bo L, Pouwels PJ et al (2005) Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. AJNR Am J Neuroradiol 26(3):572–577
Geurts JJ, Pouwels PJ, Uitdehaag BM et al (2005) Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology 236(1):254–260
Seewann A, Kooi EJ, Roosendaal SD et al (2012) Postmortem verification of MS cortical lesion detection with 3D DIR. Neurology 78(5):302–308
Simon B, Schmidt S, Lukas C et al (2010) Improved in vivo detection of cortical lesions in multiple sclerosis using double inversion recovery MR imaging at 3 Tesla. Eur Radiol 20(7):1675–1683
Wattjes MP, Lutterbey GG, Gieseke J et al (2007) Double inversion recovery brain imaging at 3T: diagnostic value in the detection of multiple sclerosis lesions. AJNR Am J Neuroradiol 28(1):54–59
Riederer I, Karampinos DC, Settles M et al (2015) Double inversion recovery sequence of the cervical spinal cord in multiple sclerosis and related inflammatory diseases. AJNR Am J Neuroradiol 36(1):219–225
Hodel J, Outteryck O, Bocher AL et al (2014) Comparison of 3D double inversion recovery and 2D STIR FLAIR MR sequences for the imaging of optic neuritis: pilot study. Eur Radiol 24(12):3069–3075
Krinsky G, Rofsky NM, Weinreb JC (1996) Nonspecificity of short inversion time inversion recovery (STIR) as a technique of fat suppression: pitfalls in image interpretation. AJR Am J Roentgenol 166(3):523–526
Mathews VP, Caldemeyer KS, Lowe MJ et al (1999) Brain: gadolinium-enhanced fast fluid-attenuated inversion-recovery MR imaging. Radiology 211(1):257–263
Harris RJ, Cloughesy TF, Pope WB et al (2013) Pre- and post-contrast three-dimensional double inversion-recovery MRI in human glioblastoma. J Neuro-Oncol 112(2):257–266
Acknowledgments
The scientific guarantor of this publication is Prof Xavier Leclerc. David Chechin is an employee of Philips. All the other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise: Mr Mohamed Amine Benadjaoud kindly provided statistical advice for this manuscript. No complex statistical methods were necessary for this paper. Our institutional review board waived the requirement to obtain a signed informed consent from the subjects included since the present imaging protocol was similar to that used in clinical routine and did not require any additional gadolinium injection.
Our institutional review board waived the requirement to obtain a signed informed consent from the subjects included because the additional acquisition time related to the post-contrast DIR sequence remained limited. No study subjects or cohorts have been previously reported. Methodology: prospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hodel, J., Badr, S., Outteryck, O. et al. Altered signal intensity of active enhancing inflammatory lesions using post-contrast double inversion recovery MR sequence. Eur Radiol 27, 637–641 (2017). https://doi.org/10.1007/s00330-016-4416-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4416-1