Abstract
Objective
We aimed to evaluate the diagnostic potential of dual-time-point imaging with positron emission tomography (PET) using O-(2-[18F]fluoroethyl)-L-tyrosine (18F-FET) for non-invasive grading of cerebral gliomas compared with a dynamic approach.
Methods
Thirty-six patients with histologically confirmed cerebral gliomas (21 primary, 15 recurrent; 24 high-grade, 12 low-grade) underwent dynamic PET from 0 to 50 min post-injection (p.i.) of 18F-FET, and additionally from 70 to 90 min p.i. Mean tumour-to-brain ratios (TBRmean) of 18F-FET uptake were determined in early (20–40 min p.i.) and late (70–90 min p.i.) examinations. Time–activity curves (TAC) of the tumours from 0 to 50 min after injection were assigned to different patterns. The diagnostic accuracy of changes of 18F-FET uptake between early and late examinations for tumour grading was compared to that of curve pattern analysis from 0 to 50 min p.i. of 18F-FET.
Results
The diagnostic accuracy of changes of the TBRmean of 18F-FET PET uptake between early and late examinations for the identification of HGG was 81 % (sensitivity 83 %; specificity 75 %; cutoff - 8 %; p < 0.001), and 83 % for curve pattern analysis (sensitivity 88 %; specificity 75 %; p < 0.001).
Conclusion
Dual-time-point imaging of 18F-FET uptake in gliomas achieves diagnostic accuracy for tumour grading that is similar to the more time-consuming dynamic data acquisition protocol.
Key Points
• Dual-time-point imaging is equivalent to dynamic FET PET for grading of gliomas.
• Dual-time-point imaging is less time consuming than dynamic FET PET.
• Costs can be reduced due to higher patient throughput.
• Reduced imaging time increases patient comfort and sedation might be avoided.
• Quicker image interpretation is possible, as no curve evaluation is necessary.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Previous studies have shown that in addition to conventional magnetic resonance imaging (MRI), positron emission tomography (PET) using radiolabelled amino acids provides important diagnostic information in patients with cerebral glioma [1]. Most PET studies of cerebral gliomas have been performed with L-[methyl-11C]-methionine (11C-MET), but the short half-life of carbon-11 (20.4 min) limits the use of this tracer to PET centres with on-site radiotracer production. In recent years, 18F-labelled amino acids, such as O-(2-18F-fluoroethyl)-L-tyrosine (18F-FET) and 3,4-dihydroxy-6-[18F]fluoro-L-phenylalanine (18F-FDOPA), have been increasingly used for PET in brain tumour patients due to the logistical advantages of fluorine-18 (half-life, 109.8 min) [2–5]. 18F-FET is at present the best evaluated 18F-labelled amino acid for PET of brain tumours, and was approved by the Swiss Agency for Therapeutic Products as radiopharmaceutical for brain imaging with PET in 2014 [6]. The clinical impact of this tracer, especially to define the tumour volume for treatment planning, diagnosis of tumour recurrence and treatment monitoring, has been demonstrated [7–11].
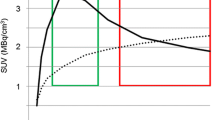
Several studies have shown that tracer uptake kinetics of 18F-FET in gliomas are variable depending on the grade of malignancy, a phenomenon that has not been observed with 11C-MET or 18F-FDOPA [12, 13]. Time–activity curves of 18F-FET uptake in patients with high-grade glioma (HGG) are characterised by an early peak of tracer uptake followed by a constant descent, whereas patients with low-grade glioma (LGG) typically show a steadily increasing curve pattern [14]. One intrinsic property of gliomas is a pronounced heterogeneity of the tumour tissue [15] and regionally different 18F-FET tracer uptake kinetics have been observed in heterogeneous gliomas during the first 50 min post-injection (p.i.), indicating different grades of malignancy [16]. Dynamic 18F-FET imaging, however, requires longer acquisition times, reducing the number of patients that can be investigated with one synthesis of 18F-FET. Thus far, the acquisition time using 18F-FET in brain tumours has been limited to 60 min and the uptake behaviour in a phase later than 60 min p.i. has not yet been investigated.
The aim of this study was to explore the diagnostic potential of dual-time-point 18F-FET PET imaging for tumour grading using a standard examination from 20 to 40 min p.i. and a delayed scan from 70 to 90 min p.i. The advantages of dual-time-point imaging are manifold, and include the following: a shorter imaging time leading to higher patient throughput that, of course, reduces costs (second patient injection after 30 instead 60 min). Moreover, the shorter imaging time leads to higher patient comfort / compliance (sedation may be avoided) and a concomitant reduction of motion artefacts. Finally, because of the fact that curve evaluation is not required, quicker image interpretation is facilitated. In this study, the dual-time-point imaging approach was compared to the results of dynamic 18F-FET PET during the first 50 min after tracer injection.
Materials and methods
Patient population
From April 2011 to April 2014, 36 adult patients (mean age, 49 ± 13 y, age range 27–75 y, 17 female and 19 male subjects) who were admitted for 18F-FET PET with suspicion of a cerebral glioma or a recurrence of a previously treated glioma were included in this prospective study. Twenty-one patients had primary tumours and 15 had recurrent tumours. Histopathological diagnosis was available in 33 patients from subsequent surgery and in three patients with low-grade gliomas and stable course from previous biopsies. Twelve patients had LGG of World Health Organisation (WHO) grade I and II, and 24 patients had HGG of WHO grade III and IV. Detailed information on the patient group is given in Table 1. The study was approved by the university ethics committee and the relevant federal authorities. All subjects gave prior written, informed consent for their participation in the study.
18F-FET PET imaging and data analysis
The amino acid 18F-FET was produced via nucleophilic 18F-fluorination with a radiochemical purity of greater than 98 %, a specific radioactivity greater than 200 GBq/μmol and a radiochemical yield of about 60 %, as described previously [17]. All patients fasted for at least 4 h before the PET studies, according to the German guidelines for brain tumour imaging using labelled amino acid analogues [18].
The dual imaging protocol in this prospective study was part of the clinical validation of a 3T MR-BrainPET hybrid system (Siemens Medical Systems, Inc.; 72 rings, axial field of view 19.2 cm). Together with the MR component, the BrainPET provides reliable quantitative data on tracer concentration, as reported previously [19]. During the validation period of this scanner, patients with cerebral gliomas first underwent a dynamic PET from 0 to 50 min p.i. of approximately 250 MBq of 18F-FET in the 3T MR-BrainPET hybrid system. Subsequently, patients were examined for another 20 minutes (70 to 90 min p.i.) in a conventional ECAT EXACT HR+ scanner (Siemens Medical Systems, Inc.) in 3D mode (32 rings, axial field of view 15.5 cm). Since the 3T MR-BrainPET does not provide a transmission source, attenuation correction was performed by a template-based approach using MRI [19]. The image data were corrected for random and scatter coincidences, and dead time prior to OP-OSEM reconstruction provided by the manufacturer (2 subsets, 32 iterations). The reconstructed dynamic data set consisted of 16 time frames (5 × 1 min; 5 × 3 min; 6 × 5 min).
For the ECAT EXACT HR+ scanner, attenuation correction of the PET data was based on a transmission scan measured with three rotating line sources (68Ge/68Ga). The data were corrected for random and scattered coincidences, and dead time prior to the iterative reconstruction using the OSEM algorithm (16 subsets, 6 iterations). The reconstructed data of the late scan (70 to 90 min p.i.) consisted of four time frames (4 × 5 min).
Data analysis
After adjusting the BrainPET images to those of the ECAT EXACT HR+ scanner by applying a 2.5 mm 3D Gaussian filter (see supplementary data), images summed from 20 to 40 min p.i. (early scan), and 70 to 90 min p.i. (late scan) were compared. All images were motion-corrected and coregistered to morphological T1-weighted MRI using PMOD (Version 3.4, PMOD Technologies Ltd.). 18F-FET uptake in the tissue was expressed as standardised uptake value (SUV) by dividing the radioactivity (kBq/mL) in the tissue by the radioactivity injected per gram of body weight. Three-dimensional volumes-of-interest (VOI) were used for analysis. A spherical background VOI with a constant size was positioned in the hemisphere contralateral to the lesion in healthy brain tissue (volume of background VOI, 14.1 ml; 7220 voxels, 30 mm diameter). The tumour volume was determined by a 3D auto-contouring process using a tumour-to-brain ratio (TBR) of 1.6 or more in the early scan. This cutoff is based on a biopsy-controlled study of cerebral gliomas, in which a lesion-to-brain ratio of 1.6 best separated tumoral from peritumoral tissue [9]. Mean TBR (TBRmean) in both the early and late scan was calculated by dividing the mean SUV of the tumour VOI by the mean SUV of normal brain. Time–activity curves were generated by applying the tumour and brain VOIs to the entire dynamic data set. Time to peak (TTP; time in minutes from the beginning of the dynamic acquisition up to the maximum SUV of the lesion) was determined. Furthermore, the time–activity curves of the tumours from 0 to 50 min p.i. were assigned to one of the following curve patterns: (I) constantly increasing 18F-FET uptake, (II) 18F-FET uptake peaking between 20 and 40 min followed by a plateau, and (III) 18F-FET uptake peaking early (TTP < 20 min) followed by a constant descent [20]. The diagnostic accuracy of changes of 18F-FET uptake between early and late scans for tumour grading was compared to that based on curve patterns from 0 to 50 min p.i. Furthermore, the early and late PET images were visually evaluated for major changes in tumour volume, 18F-FET uptake and regional distribution of 18F-FET by three independent observers.
Statistical analysis
Descriptive statistics are provided as mean and SD or median and range. To compare SUV and TBRmean in the early and late scans, the Student’s t paired test was used. The diagnostic performance of TBRmean in the early scan, TTP and changes of tumoral TBRmean of 18F-FET uptake between the early and late scan to identify HGG was assessed by receiver-operating-characteristic (ROC) curve analyses using the histological confirmation as reference. Decision cutoff was considered optimal when the product of paired values for sensitivity and specificity reached its maximum. In addition, the area under the ROC curve (AUC), its standard error, and level of significance were determined as a measure of diagnostic quality. After the assignment of TACs of 18F-FET uptake to the three curve patterns, the Cohen’s κ-test was used to measure the degree of agreement among the raters (C.F., N.G., K.J.L); κ values between 0 and 0.20 were considered to indicate a positive but slight correlation; between 0.21 and 0.40, a fair correlation; between 0.41 and 0.60, a good correlation; between 0.61 and 0.80, a very good correlation; and greater than 0.80, an excellent correlation. The diagnostic performance of 18F-FET TAC patterns was evaluated by Fisher exact test for 2 × 2 contingency tables.
p values of 0.05 or less were considered significant. Statistical analyses were performed using SigmaPlot software (SigmaPlot Version 11.0, Systat Software Inc., San Jose, CA, USA) and PASW Statistics software (Release 22.0.0, SPSS Inc., Chicago, IL, USA).
Results
General comparison of 18F-FET uptake in the tumours in early and late scans
On average, SUVmean and TBRmean in the tumours in the early scans were significantly higher than in the late scans (2.53 ± 0.80 versus 2.29 ± 0.72, p = 0.001 and 2.20 ± 0.41 versus 1.97 ± 0.40; p = 0.001, respectively). Evaluation of the TBRmean value in the early scan did not reach the level of significance as a diagnostic test for the identification of HGG in ROC analysis in this patient group (sensitivity 83 %; specificity 58 %; accuracy 75 %; optimal cutoff, 2.0; AUC 0.65 ± 0.10; p = 0.15).
Grading of gliomas using dynamic 18F-FET PET (0-50 min p.i.)
The time–activity curves of the tumours from 0 to 50 min p.i. were assigned to the above-described pattern of curves by three independent observers (C.F., N.G., K.J.L.) blinded to the clinical diagnosis of the patients. The inter-observer agreement for the assignment of TACs to the three curve patterns showed a very good agreement, with a mean κ value of 0.74 ± 0.06. The evaluation of curve patterns yielded a significant result for the identification of HGG, i.e., the presence of curve patterns II and III achieved an accuracy of 83 % for the identification of HGG (sensitivity, 88 %; specificity, 75 %; p < 0.001). The evaluation of TTP yielded a lower accuracy of 69 % for the identification of HGG (sensitivity 58 %; specificity 92 %, cut-off, <35 min; AUC 0.76 ± 0.06; p = 0.01) (Table 2).
Grading of gliomas using dual point 18F-FET PET
TBRmean decreased by -10 ± 11 % between early and late scans. Patients with HGG showed a significantly stronger decrease of the TBRmean than patients with LGG (- 14 ± 10 % versus -2 ± 9 %; p < 0.01). The diagnostic accuracy of changes of the TBRmean of 18F-FET PET uptake for the identification of HGG in ROC analysis was 81 % (sensitivity 83 %, specificity 75 %, cutoff, - 8 %, AUC, 0.85 ± 0.06; p < 0.001). Thus, evaluation of 18F-FET PET uptake in the tumour between early and late scans using a dual imaging protocol achieved a similar accuracy as the evaluation of time–activity curves of the tumours from 0 to 50 min p.i. (Table 2).
General visual comparison of early and late scans
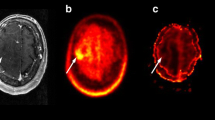
The visual comparison of early and late scans by three independent observers yielded a similar extent of tumour lesion in most patients. In 15 cases, tumour-to-brain contrast of 18F-FET uptake was rated as similar in early and late scans; in 13 cases, 18F-FET uptake was rated as decreasing in the late scan; and in seven cases, 18F-FET uptake was rated as increasing. The inter-observer agreement for rating of 18F-FET uptake showed a good agreement, with a mean κ value of 0.57 ± 0.02. An example of a patient with an increasing 18F-FET uptake in the tumour in the late scan indicating a LGG is shown in Fig. 1 (Pat. No. 12, recurrent oligoastrocytoma WHO grade II), and an example with decreasing 18F-FET uptake in the late scan indicating a HGG is shown in Fig. 2 (Patient No. 22, glioblastoma). In one patient with a recurrent oligoastrocytoma WHO grade II (Patient No. 3), a different intratumoral distribution pattern of 18F-FET in the early and late scan was noted. Analysis of the different parts of the tumour by smaller ROIs manually placed on the corresponding tumour areas demonstrated variable intratumoral tracer kinetics, as shown in Fig. 3.
Patient with a recurrent oligoastrocytoma of WHO grade II (Pat. No. 12). Contrast-enhanced T1-weighted MRI on the left, early 18F-FET PET (BrainPET, 20–40 min p.i.) in the middle, and late scan 18F-FET PET (ECAT HR+ PET 70–90 min p.i.) on the right. The tumour shows an increasing tumour-to-brain contrast in the late scan (+13 %)
Patient with a glioblastoma (Pat. No. 22). Contrast enhanced T1-weighted MRI on the left, early 18F-FET PET (BrainPET, 20–40 min p.i.) in the middle, and late scan 18F-FET PET (ECAT HR+ PET 70–90 min p.i.) on the right. The tumour shows a decreasing tumour-to-brain contrast indicating a high-grade tumour (-21 %)
Dynamic 18F-FET-PET of a patient with a recurrent, heterogeneous oligoastrocytoma of WHO grade II (Pat. No. 3). The first 50 min were acquired in the BrainPET; the last 20 min were acquired in the ECAT HR+ scanner. In this case, three different VOIs were defined in the tumour area in order to illustrate regional differences in the tracer uptake kinetics, which can be identified by dynamic imaging, but also by dual-point-imaging at 20–40 min and 70–90 min. Positions of the different tumour VOIs are indicated by arrows with corresponding colours in the sagittal brain slice on the right
Discussion
The aim of this study was to evaluate the diagnostic potential of dual-time-point imaging in 18F-FET PET of cerebral gliomas. To the best of our knowledge, the uptake behaviour of 18F-FET in human brain tumours has not yet been evaluated at later than 60 min after injection. Dual-time-point imaging has been successfully used; for example, with 18F-FDG to improve brain tumour identification and delineation of high-grade brain tumours [21, 22]. The median tumour volume depicted with late 18F-FDG PET, however, was only 55.4 % of that depicted in PET using 11C-MET. The discrepancies were especially large for anaplastic gliomas that partly consist of portions of low-grade glioma that generally exhibit low 18F-FDG uptake [22]. The influence of dual-time-point 18F-FDG PET on grading of brain tumours has not yet been investigated.
Our study demonstrates that two separate PET examinations performed at 20 to 40 min and 70 to 90 min p.i. of 18F-FET yield similar diagnostic information regarding tumour grade as a dynamic scan during the first 50 min p.i.. Thus, the decrease of the TBRmean of 18F-FET uptake between early and late scans showed a diagnostic accuracy for the identification of HGG that was similar to the evaluation of curve patterns of 18F-FET uptake. This result needs to be confirmed in a larger series of patients, but it appears that two separate scans with a longer interval lead to a robust signal concerning tumour grading.
In this study, the accuracy of dynamic 18F-FET PET in differentiating high-grade and low-grade glioma is in the range of 80 %, and thus, lower than that reported in earlier studies that observed an accuracy of more than 90 % in both primary and recurrent gliomas [20, 23, 24]. At present, it remains unclear whether these differences are caused by variations in data evaluation or patient selection. A recent study, however, showed that the specificity of dynamic 18F-FET PET for identification of high-grade gliomas was only 72 % [25], which is more consistent with the results in our study.
Dual-time-point imaging offers the possibility to identify HGG and LGG by simple visual comparison of early and late scans. Intensive tracer uptake in the early scan showing low uptake in the late scan may be indicative of high-grade glioma (Fig. 2). LGG that are characterised by an increasing 18F-FET uptake may be better visualized in the late scan, which could be helpful for planning of radiotherapy (Fig. 1). Owing to the fact that curve evaluation is not required, quicker scan interpretation is facilitated. Furthermore, it has been reported that in LGG, heterogeneous intratumoral 18F-FET kinetics may identify anaplastic foci [16, 25]. A hot spot in the early scan that disappears in the late scan may be indicative of an anaplastic focus (Fig. 3).
Furthermore, the implementation of two separate short scans may increase patient throughput and diminish the costs of the investigation. As shown in Fig. 4, two separate scans of 15 min duration allow interlaced scanning of two patients and reduce the interval between the two injections from 1 h to 30 min. This reduces the amount of radioactivity needed for investigation of two patients. Moreover, the second scan could be avoided if no pathological 18F-FET uptake can be identified in the first scan, which makes the presence of metabolically active tumour tissue unlikely. We assume that the actual time interval between the early and late scan is not critical, which is advantageous in routine clinical practice. Thus, 18F-FET uptake in the tumours in the late scans at 70 to 90 min was on average only 10 % lower than in the early scans, which also allowed appropriate imaging at later times in most cases. This may be of importance if a delay of imaging occurs due to unforeseen events. Moreover, the shorter acquisition times are more comfortable for patients and may reduce motion artefacts, and in younger children, the necessity of sedation or anaesthesia.
A limitation of this study is the heterogeneity of the patient group, which contains primary as well as recurrent tumours. Previous studies, however, have shown that the discriminatory power of dynamic 18F-FET PET is similar for the grading of primary and recurrent tumours [23, 24]. The aim of this study was to compare the diagnostic performance of dynamic 18F-FET PET and dual-time point imaging, and for this purpose, it is not essential to evaluate primary and recurrent tumours separately. Therefore, the results of this explorative study are not compromised by this shortcoming. Furthermore, the use of two different scanners may represent another shortcoming of the study design. The presented data set was obtained within a prospective study evaluating the performance of a novel 3T MR-BrainPET hybrid imaging system that required an additional late scan on a clinically approved PET. The resolution of the two different systems, however, was carefully adapted using a series of phantom studies (see supplementary material). Therefore, the data set should be adequate for the intended purpose.
In conclusion, dual-point imaging of 18F-FET by an early scan between 20 and 40 min and a late scan between 70 and 90 min p.i. may provide diagnostic information for tumour grading that is similar to a dynamic scan over 50 min. The results need to be confirmed in a larger series of patients, and may also be applied to other important applications of this method, such as the differentiation of recurrent metastases and radionecrosis [26].
Abbreviations
- 11C-MET:
-
L-[methyl-11C]-methionine
- 18F-FDOPA:
-
3,4-dihydroxy-6-[18F]fluoro-L-phenylalanine
- 18F-FET:
-
O-(2-18F-fluoroethyl)-L-tyrosine
- AUC:
-
Area under receiver-operating-characteristic curve
- HGG:
-
High-grade glioma
- LGG:
-
Low-grade glioma
- OP-OSEM:
-
Ordinary Poisson ordered subset expectation maximisation
- OSEM:
-
Ordered subset expectation maximisation
- ROC:
-
Receiver-operating-characteristic
- SUV:
-
Standardised uptake value
- SUVmean :
-
Mean standardised uptake value
- TAC:
-
Time-activity curve
- TBR:
-
Tumour-to-brain ratio
- TBRmean :
-
Mean tumour-to-brain ratio
- TTP:
-
Time to peak
- VOI:
-
Volume-of-interest
References
Herholz K, Langen KJ, Schiepers C, Mountz JM (2012) Brain tumors. Semin Nucl Med 42:356–370
Heiss WD (2014) Clinical impact of amino acid PET in gliomas. J Nucl Med 55:1831–1841
Langen KJ, Hamacher K, Weckesser M, Floeth F, Stoffels G, Bauer D et al (2006) O-(2-[18F]fluoroethyl)-L-tyrosine: uptake mechanisms and clinical applications. Nucl Med Biol 33:287–294
McConathy J, Yu W, Jarkas N, Seo W, Schuster DM, Goodman MM (2012) Radiohalogenated nonnatural amino acids as PET and SPECT tumor imaging agents. Med Res Rev 32:868–905
Wester HJ, Herz M, Weber W, Heiss P, Senekowitsch-Schmidtke R, Schwaiger M et al (1999) Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging. J Nucl Med 40:205–212
Swiss Agency for Therapeutic Products (2014) J Swissmedic 13:651
Dunet V, Rossier C, Buck A, Stupp R, Prior JO (2012) Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and Metaanalysis. J Nucl Med 53:207–214
Galldiks N, Langen K, Holy R, Pinkawa M, Stoffels G, Nolte K et al (2012) Assessment of treatment response in patients with glioblastoma using [18F]Fluoroethyl-L-Tyrosine PET in comparison to MRI. J Nucl Med 53:1048–1057
Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Muller HW et al (2005) O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 128:678–687
Pauleit D, Stoffels G, Bachofner A, Floeth FW, Sabel M, Herzog H et al (2009) Comparison of (18)F-FET and (18)F-FDG PET in brain tumors. Nucl Med Biol 36:779–787
Pöpperl G, Gotz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K (2004) Value of O-(2-[18F]fluoroethyl)- L-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging 31:1464–1470
Kratochwil C, Combs SE, Leotta K, Afshar-Oromieh A, Rieken S, Debus J et al (2014) Intra-individual comparison of (1)(8)F-FET and (1)(8)F-DOPA in PET imaging of recurrent brain tumors. Neuro Oncol 16:434–440
Moulin-Romsee G, D'Hondt E, de Groot T, Goffin J, Sciot R, Mortelmans L et al (2007) Non-invasive grading of brain tumours using dynamic amino acid PET imaging: does it work for 11C-methionine? Eur J Nucl Med Mol Imaging 34:2082–2087
Weckesser M, Langen KJ, Rickert CH, Kloska S, Straeter R, Hamacher K et al (2005) O-(2-[18F]fluorethyl)-L-tyrosine PET in the clinical evaluation of primary brain tumours. Eur J Nucl Med Mol Imaging 32:422–429
Paulus W, Peiffer J (1989) Intratumoral histologic heterogeneity of gliomas. A quantitative study. Cancer 64:442–447
Kunz M, Thon N, Eigenbrod S, Hartmann C, Egensperger R, Herms J et al (2011) Hot spots in dynamic (18)FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol 13:307–316
Hamacher K, Coenen HH (2002) Efficient routine production of the 18F-labelled amino acid O-2-18F fluoroethyl-L-tyrosine. Appl Radiat Isot 57:853–856
Langen KJ, Bartenstein P, Boecker H, Brust P, Coenen HH, Drzezga A et al (2011) [German guidelines for brain tumour imaging by PET and SPECT using labelled amino acids]. Nuklearmedizin 50:167–173
Herzog H, Langen KJ, Weirich C, Rota Kops E, Kaffanke J, Tellmann L et al (2011) High resolution BrainPET combined with simultaneous MRI. Nuklearmedizin 50:74–82
Calcagni ML, Galli G, Giordano A, Taralli S, Anile C, Niesen A et al (2011) Dynamic O-(2-[18F]fluoroethyl)-L-tyrosine (F-18 FET) PET for glioma grading: assessment of individual probability of malignancy. Clin Nucl Med 36:841–847
Kim DW, Jung SA, Kim CG, Park SA (2010) The efficacy of dual time point F-18 FDG PET imaging for grading of brain tumors. Clin Nucl Med 35:400–403
Prieto E, Marti-Climent JM, Dominguez-Prado I, Garrastachu P, Diez-Valle R, Tejada S et al (2011) Voxel-based analysis of dual-time-point 18F-FDG PET images for brain tumor identification and delineation. J Nucl Med 52:865–872
Pöpperl G, Kreth FW, Herms J, Koch W, Mehrkens JH, Gildehaus FJ et al (2006) Analysis of 18F-FET PET for grading of recurrent gliomas: is evaluation of uptake kinetics superior to standard methods? J Nucl Med 47:393–403
Pöpperl G, Kreth FW, Mehrkens JH, Herms J, Seelos K, Koch W et al (2007) FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur J Nucl Med Mol Imaging 34:1933–1942
Jansen NL, Graute V, Armbruster L, Suchorska B, Lutz J, Eigenbrod S et al (2012) MRI-suspected low-grade glioma: is there a need to perform dynamic FET PET? Eur J Nucl Med Mol Imaging 39:1021–1029
Galldiks N, Stoffels G, Filss CP, Piroth MD, Sabel M, Ruge MI et al (2012) Role of O-(2-18F-Fluoroethyl)-L-Tyrosine PET for Differentiation of Local Recurrent Brain Metastasis from Radiation Necrosis. J Nucl Med 53:1367–1374
Acknowledgments
The authors thank Suzanne Schaden, Elisabeth Theelen and Kornelia Frey for assistance in the patient studies; and Johannes Ermert, Silke Grafmüller, Erika Wabbals and Sascha Rehbein for radiosynthesis of 18F-FET. The scientific guarantor of this publication is Prof. Dr. Karl-Josef Langen. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: prospective, diagnostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 52.5 kb)
Rights and permissions
About this article
Cite this article
Lohmann, P., Herzog, H., Rota Kops, E. et al. Dual-time-point O-(2-[18F]fluoroethyl)-L-tyrosine PET for grading of cerebral gliomas. Eur Radiol 25, 3017–3024 (2015). https://doi.org/10.1007/s00330-015-3691-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3691-6