Abstract
Objectives
To determine the feasibility of using a 70-kVp protocol compared with a 120-kVp protocol for cerebral CT angiography. An additional target was to investigate a possible reduction in the volume of contrast medium (CM) using the 70-kVp protocol.
Methods
Attenuation value and CNR for iodine were determined at various tube voltage settings using a phantom. Sixty-nine volunteers were randomly assigned to one of three protocols: group A (120-kVp and CM 64 mL), group B (70-kVp and CM 64 mL), or group C (70-kVp and CM 40 mL). The attenuation value, SNR, and CNR of cerebral arteries, subjective image quality, and radiation dose were compared among the groups.
Results
The vascular attenuation, SNR, and CNR of group B were significantly higher than those of group A. Group C had a significantly higher vascular attenuation than group A. Groups B and C were significantly better than group A with respect to subjective image quality. An effective dose of 70-kVp was 10 % lower than that of 120-kVp.
Conclusions
Using 70-kVp improved arterial enhancement, SNR, and CNR, and provided better subjective image quality, using a 10 % lower effective dose. Furthermore, the 70-kVp protocol may both reduce volume of CM by 37.5 % and improve arterial enhancement.
Key Points
• Cerebral CT angiography at 70-kVp substantially improved vascular enhancement
• Subjective image quality was better at 70-kVp, with lower radiation dose
• The volume of contrast media can be substantially reduced at 70-kVp
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the introduction of MDCT and the continuous technical advances, cerebral CT angiography (CTA) has been established as an effective minimally-invasive diagnostic method for the evaluation of patients with suspected cerebrovascular disease [1]. The diagnostic performance of cerebral CTA is largely dependent on adequate arterial enhancement (>250 HU), which is determined by the injection of a contrast medium (CM) as well as the scanning parameters [2, 3]. In clinical practice, a high concentration of iodine CM or a rapid injection rate of CM to increase intravascular iodine concentration are commonly used to improve arterial enhancement in CTA [1].
A low-tube-voltage protocol for CTA is a promising alternative that improves vascular enhancement without increasing the amount of intravascular iodine, since the lower X-ray energy of low tube voltage results in a greater photoelectric effect of iodine. The use of a low-tube-voltage (80–100 kVp) protocol for CTA has recently gained popularity, as this technique provides increased vascular enhancement while reducing the volume of CM and reducing radiation exposure for patients [3–12]. A recent study reported on the use of an x-ray tube that allowed for a 70-kVp tube voltage imaging protocol [13]. Although the main reason for the development of the 70-kVp protocol was to reduce radiation exposure in paediatric patients, it may also be used for CT scans in adults for parts of the body with a relatively low attenuation level, such as the neck [13].
The increased use of intravascular iodinated CM in radiographic imaging has resulted in a higher incidence of contrast-induced nephropathy (CIN) [14]. CIN is one of the common causes of acute renal dysfunction, significantly contributing to patient morbidity and mortality in high-risk patients [14–16]. A large volume of CM, in particular, is an important and independent risk factor for CIN [16–18].
The purpose of our study was to determinate the feasibility of using a 70-kVp protocol as compared with a conventional 120-kVp protocol for cerebral CTA with respect to vascular attenuation, image quality, and radiation dose. We also investigated the reduction in the volume of CM when the 70-kVp protocol was applied.
Materials and methods
Prior to conducting the clinical study, we performed a phantom study to establish the attenuation value and contrast-to-noise ratio (CNR) of iodine for CT scans with tube voltage protocols of 70, 80, 100, 120 and 140 kVp. This enabled us to quantify the increase in attenuation and CNR of the iodine at various tube voltage settings.
Phantom study
We used a 170-mm diameter cylindrical phantom filled with water to simulate the head of an adult [8]. A 25-mm diameter cylindrical tube filled with diluted CM was inserted into the centre of the phantom. We prepared six different dilutions (0.5 %, 1.0 %, 2.0 %, 3.0 %, 4.0 %, and 5.0 %) of CM (370 mg I/mL; Ultravist 370, Schering Korea, Seoul, Korea) in tap water.
We used tube voltage settings of 70, 80, 100, 120, and 140 kVp in a 128-slice multidetector CT (SOMATOM Definition AS+; Siemens, Forchheim, Germany), and chose the appropriate effective tube current–time product (mAseff) so as to make the phantom-volume CT dose index (CTDIvol) as close to 20.2 mGy as possible. This was achieved using a rotation time of 0.5 seconds, a pitch of 0.45, a field of view of 200 mm, and 94 mAseff with 140 kVp, 140 mAseff with 120 kVp, 231 mAseff with 100 kVp, and 464 mAseff with 80 kVp. However, at a gantry rotation time of 0.5 seconds and a pitch of 0.45, the maximum mAseff available was only 550 mAseff, and not 724 mAseff as would have theoretically been required, since the CT had a maximum tube current capacity of 500 mA. Therefore, the resultant CTDIvol for the 70-kVp protocol with 550 mAseff was 15.4 mGy, which was 23.8 % lower than that of the other protocols.
The attenuation value (Hounsfield units; HU) of each dilution of CM was measured using a 100-mm2 region of interest (ROI) in the centre of the tube. Image noise was defined as the mean standard deviation (SD) of the attenuation values measured at the centre of the tube and at the 3, 6, 9 and 12 o’clock positions at equidistant levels along the z-axis of the cylindrical water phantom. The contrast-to-noise ratio (CNR) of the tube filled with diluted CM was calculated using the following formula: CNR = (HU of the tube − mean HU of the water phantom)/image noise. The attenuation value (HU) and CNR of the tubes filled with diluted CM were plotted against the concentration of CM. Data were analyzed using Pearson’s correlation coefficients and linear regression analysis.
Normal volunteers
The study protocol was reviewed and approved by the institutional review board at our institution, and written informed consent was obtained from all participants. Sixty-nine healthy volunteers with normal renal function were enrolled in this study, and were randomly assigned to one of three CTA protocols: group A (120-kVp protocol and CM 64 mL at 4.0 mL/s), group B (70-kVp protocol and CM 64 mL at 4.0 mL/s), or group C (70-kVp protocol and CM 40 mL at 2.5 mL/s). The mean age of the patients was 45.1 ± 13.7 years (range, 25–67 years).
Imaging techniques
All CTs were performed using a 128-channel MDCT (SOMATOM Definition AS+; Siemens, Forchheim, Germany) with automatic tube current modulation software (CARE Dose 4D; Siemens). For group A, imaging parameters similar to those used in daily practice at our institution were applied, which included a collimation of 64 × 0.6 mm, slice acquisition of 128 × 0.6 mm using a z-flying focal spot technique, a gantry rotation time of 0.5 seconds, a pitch of 0.45, and an exposure setting of 120 kVp and 140 mAseff with automatic tube current modulation (CARE Dose 4D). The expected CTDIvol of the protocol used for group A was 20.2 mGy. The imaging volume ranged from the vertex of the skull to the posterior arch of the C1 vertebra. Axial scans were reconstructed at a thickness of 0.6 mm, and images were sent to online workstations (Aquarius iNtuition, TeraRecon Inc, San Mateo, CA, USA) for quantitative and qualitative image analysis.
For the 70-kVp protocol (groups B and C), the effective tube current–time product settings were intended to result in a CTDIvol that was as close as possible to that of the 120-kVp protocol. However, at a rotation time of 0.5 seconds and a pitch of 0.45, the maximum effective tube current–time product available on the scanner was only 550 mAseff, and not 724 mAseff, as would have theoretically been required. Therefore, the resultant CTDIvol for the 70-kVp protocol was 15.4 mGy. As a result, for the 70-kVp protocol, automatic tube current modulation software was not used in order to avoid further reducing the radiation dose. The remaining imaging parameters were the same as those used for the 120-kVp protocol.
CM with an iodine concentration of 370 mg I mL (Ultravist 370; Schering Korea, Seoul, Korea), warmed to 37 °C, was administered via an 18-gauge cannula placed in the right antecubital vein using a dual-head power injector (Dual Shot, Nemoto Kyorindo, Tokyo, Japan). For groups A and B, a total volume of 64 mL of CM was injected at a rate of 4.0 mL/s for 16 seconds, followed by 40 mL of saline injected at 4.0 mL/s. To evaluate the feasibility of volume reduction of CM at 70-kVp, the total volume of CM for group C was reduced by 37.5 %, to 40 mL. This percent reduction was chosen because the CNR of diluted CM in the 70-kVp protocol in the phantom study was a mean 39.7 % higher than that of the 120-kVp protocol. Therefore, a volume of 40 mL CM was injected at 2.5 mL/s for 16 seconds, followed by a 40-mL saline flush at a rate of 4 mL/s.
An automatic bolus-tracking program (CARE Bolus, Siemens, Forchheim, Germany) was used for initiating the CT after injection of CM. The ROI was located in the common carotid artery at the level of the hyoid bone, and a threshold of 160 HU was used for triggering the CTA. A delay of four seconds was added before every examination.
Quantitative image analysis
Vascular attenuation (in HU) in the internal carotid artery at the T-junction was measured [3, 8] by a board-certified radiologist with four years of experience in cerebral CTA evaluation. The ROI was carefully drawn to be as large as the lumen of the artery, omitting the outline of the lumen to avoid partial volume effects (range of ROI size: 1.44–6.60 mm2). The mean attenuation and the standard deviation (SD) of the brain parenchyma were measured at the centre of the left occipital lobe, avoiding vascular structures, using a 200 mm2 ROI [3, 8]. The SD of the brain parenchyma was defined as the image noise. To minimize bias from a single measurement, measurements were obtained three times for each artery or region of brain parenchyma. The mean of these values was used for subsequent calculations. The artery-to-brain parenchyma CNR was calculated using the following formula [3, 8, 19]: CNR = (mean arterial attenuation value – mean brain parenchyma attenuation value) / image noise.
Qualitative image analysis
Two radiologists with more than seven years of experience in the evaluation of neurovascular imaging independently reviewed the maximum intensity projections (axial, sagittal, and coronal) and volume-rendered images of the cerebral arteries. Two observers subjectively rated image quality on a five-point scale (1 = non-diagnostic; 2 = substandard; 3 = standard image quality; 4 = better than standard; 5 = excellent), in which a rating of 3 corresponded to the image quality expected with standard cerebral CTA. The two observers also independently scored the following image quality parameters: arterial enhancement, sharpness of the arterial boundary, visibility of small arterial details (based on the depiction of small arteries such as the superior cerebellar, anterior and posterior communicating, anterior choroid, and ophthalmic arteries), venous contamination, image noise, and overall image quality. As the use of a low-tube-voltage protocol for CT angiography may also increase venous enhancement, which may interrupt adequate evaluation of the arterial structure, we evaluated and graded the degree and influence of venous contamination on image quality. CT image datasets were randomized, and observers were blinded to the scanning parameters and volume of CM. A window level of 200 HU and width of 600 HU were fixed only during the qualitative assessment of arterial enhancement, venous contamination, and image noise in order to compare differences among the three groups.
Measurement of radiation exposure
The CTDIvol and dose–length product (DLP) of each scan were provided by the CT scanner, and an approximation of the effective dose was calculated for each subject by multiplying the DLP by a conversion coefficient (0.0023 mSv/mGy · cm) [20, 21].
Statistical analysis
All statistical analyses were performed using SPSS software (version 13.0 for Windows, SPSS Inc., Chicago, IL). Patient data (demographic and morphometric) and quantitative data were analyzed using student’s t tests. Qualitative data was analyzed using Mann–Whitney U tests. The Bonferroni correction was applied, and differences were considered to be significant when the P value was less than 0.05/2 (0.025) for two comparisons (between groups A and B and between groups A and C). The kappa statistic was used to assess interobserver agreement in scoring and was interpreted using the guidelines described by Landis and Koch [22].
Results
Phantom study
The attenuation value and CNR for a given dilution of iodine CM increased as the tube voltage decreased from 140 kVp to 70 kVp (Figs. 1 and 2). CNRs at the 70-, 80-, 100-, and 140-kVp settings were 59.6 %, 47.7 %, and 18.0 % higher, and 15.9 % lower, respectively, than those at the 120-kVp setting. The mean attenuation values at the 70-, 80-, 100-, and 140-kVp settings were 97.3 %, 64.8 %, and 23.7 % higher, and 15.2 % lower, respectively, than those obtained at 120 kVp. The mean image noise at 70, 80, 100, 120, and 140 kVp was 9.9, 8.8, 8.3, 8.0, and 8.1, respectively.
Axial 0.6-mm-thick CT images of the phantom were obtained at each tube voltage with 4 % concentration CM diluted with tap water. A 4 % concentration of CM in the tube resulted in mean CT attenuation values most similar to those obtained for cerebral arteries in clinical studies (363.5 HU in 120 kVp, 695.2 HU in 70 kVp). The mean CT attenuation value and CNR were 339 HU and 41.8 at 140 kVp, 399 HU and 50.0 at 120 kVp, 494.0 HU and 56.5 at 100 kVp, 660.0 HU and 71.4 at 80 kVp, and 795.0 HU and 69.9 at 70 kVp, respectively.
Graphs shows the relationships between the contrast-to-noise ratio (CNR) and concentration of contrast medium (CM) dilution (A), and between the attenuation value and concentration of CM dilution (B). In A, the relationship between the CNR and concentration of CM dilution was as follows: for 70 kVp, y = 1,731.7x + 6.7 (R2 = 0.979); for 80 kVp, y = 1,593.5x + 7.2 (R2 = 0.979); for 100 kVp, y = 1,278.0x + 5.2 (R2 = 0.986); for 120 kVp, y = 1,123.4x + 3.4 (R2 = 0.990); and for 140 kVp, y = 942.8x + 2.8 (R2 = 0.986). In B, the relationship between CT attenuation value and concentration of CM dilution was as follows: for 70 kVp, y = 19,280x + 25.6 (R2 = 0.999); for 80 kVp, y = 15,966x + 24.2 (R2 = 0.999); for 100 kVp, y = 11,945x + 18.0 (R2 = 0.991); 120 kVp, y = 9,622.2x + 15.7 (R2 = 0.999); and 140 kVp, y = 8,159.7x + 13.49 (R2 = 0.999).
Clinical study
A total of 69 participants were enrolled in this study and were divided equally among each of the three groups. There were no significant differences in age, height, weight, or body mass index between groups A and B or between groups A and C (Table 1). Thus, further analyses and comparisons of attenuation measurements and radiation doses were considered to be valid.
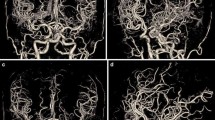
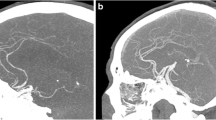
The mean vascular attenuation value at the internal carotid artery T-junction was 363.5 ± 74.9 HU (range, 241.0–501.0 HU) for group A (Fig. 3), 695.2 ± 92.6 HU (range, 542.5–942.0 HU) for group B (Fig. 4), and 601.4 ± 112.9 HU (range, 448.5–852.0 HU) for group C (Fig. 5). There were mean differences of 331.7 HU (p < 0.001) between groups A and B and of 237.9 HU (p < 0.001) between groups A and C (Table 2). In comparison to the 120-kVp protocol, the 70-kVp protocol had 91.2 % higher arterial attenuation, with the same amount of CM (64 mL), and 65.4 % higher vascular attenuation even though the volume of CM was reduced by 37.5 % (from 64 mL to 40 mL). The mean attenuation within the occipital lobe in groups B and C was 8.1 HU and 7.4 HU higher, respectively, than that of group A (p < 0.001). The mean image noise (SD of the occipital lobe) in groups B and C were 8.4 HU and 7.9 HU higher, respectively, than that of group A (p < 0.001), respectively. Although the noise level was significantly higher at the 70-kVp protocols, the mean SNR and CNR of the arteries for the 70-kVp protocol (group B) were 22.1 % and 26.9 % higher than those for the 120-kVp protocol (group A) (p = 0.006 and p = 0.002), respectively, when the same amount of CM was used (Table 2). The mean SNR and CNR in group C were 7.0 % and 10.1 % higher, respectively, than those of group A, but were not statistically significant (p = 0.341 and p = 0.204, respectively).
A 0.6-mm-thick axial reconstructed image (A), axial MIP image with a slab thickness of 8 mm (B), and volume-rendered image (C) of a 36-year-old woman who underwent cerebral CT angiography using 120 kVp and 64 mL contrast medium (group A protocol). Arterial attenuation was measured in the internal carotid artery at the T-junction (arrow). The mean attenuation value of the cerebral arteries (377.0 HU) in this patient was similar to the mean attenuation value (363.5 HU) of cerebral CT angiography in group A.
A 0.6-mm-thick axial reconstructed image (A), axial MIP image with a slab thickness of 8 mm (B), and volume-rendered image (C) of a 35-year-old man who underwent cerebral CT angiography using 70 kVp and 64 mL contrast medium (group B protocol). Arterial attenuation was measured in the internal carotid artery at the T-junction (arrow). The mean attenuation value of the cerebral arteries (698.0 HU) in this patient was most similar to the mean attenuation value (695.2 HU) for cerebral CT angiography obtained in group B.
A 0.6-mm-thick axial reconstructed image (A), axial MIP image with a slab thickness of 8 mm (B), and volume-rendered image (C) of a 65-year-old man who underwent cerebral CT angiography using 70 kVp and 40 mL contrast medium (Group C). Arterial attenuation was measured in the internal carotid artery at the T-junction (arrow). The mean attenuation value of the cerebral arteries (582.0 HU) of this patient was most similar to the mean attenuation value (601.4 HU) for cerebral CT angiography in group C.
The interobserver agreement for the subjective assessment of image quality between the two readers was substantial, with a kappa value of 0.744. In qualitative image analysis (Table 3), the images acquired using the 70-kVp protocols (groups B and C) were rated significantly higher than those obtained using the 120-kVp protocol (group A) with regard to arterial enhancement, sharpness of cerebral arterial boundaries, visibility of small arterial details, and overall image quality. Group A had statistically better subjective scores for venous contamination compared with group B, and better image noise compared with group C.
The mean CTDIvol and estimated effective dose of the 70-kVp protocols (groups B and C) were 10.5 % and 10.0 % lower, respectively, than those obtained using the 120-kVp protocol (group A), both of which were statistically significant (Table 2).
Discussion
Recently, some x-ray tubes within CT systems offer a 70-kVp setting, which is expected to be very helpful for angiographic examinations in children. When the mean photon energy of the polychromatic X-rays approaches the iodine K-edge energy of 33.2 keV, the attenuation of the iodine CM is increased as a result of a greater photoelectric effect [3, 8, 23]. In one study, the mean energy of the X-ray spectrum in CT using an X-ray tube with a 3–4 mm aluminum filter was reported to be 43.7 keV at 80 kVp, 51.6 keV at 100 kVp, 56.8 keV at 120 kVp, and 61.5 keV at 140 kVp [24]. The mean photon energy at the 70-kVp setting for CT has not yet been reported. However, since the 70-kVp setting had the highest iodine attenuation value in our phantom study, we presume that the mean photon energy of the 70-kVp setting would be closest to the iodine K-edge energy of 33.2 keV. In the clinical study, we also confirmed that vascular enhancement on CTA acquired with 70 kVp was significantly higher than that at 120 kVp when the same amount of CM was applied. Although the volume of CM administered was reduced by 37.5 %, the 70-kVp protocol had greater vascular enhancement compared with the 120-kVp protocol.
In previous cerebral CTA studies that used a 120-kVp setting, the mean attenuation of enhanced intracranial arteries varied from 190 HU to 351 HU according to the total amount of iodine injected and the injection rate of the CM [2, 3, 7, 8, 20, 25]. In the study that had the highest intracranial artery attenuation (351 HU), patients were given 100 mL of CM, with a 300 mg iodine/mL concentration [25]. The mean arterial attenuation in this study was 375 HU in the 120-kVp protocol when 64 mL CM with 370 mg iodine/mL was administered. In previous studies that have used a low-tube-voltage protocol (80–90 kVp), the mean attenuation of intracranial arteries ranged from 322 to 401 HU [3, 7, 8, 20]. The highest mean attenuation value (401 HU) for intracranial arteries was reported when patients were given 120 mL of CM and the 80-kVp protocol was applied [20]. When the 70-kVp protocol was used in our study, the mean arterial attenuation values were 708.6 HU and 584.6 HU with the administration of 64 mL and 40 mL of CM, respectively, at a concentration of 370 mg iodine/mL. To the best of our knowledge, 708.6 HU is the highest reported mean attenuation value of cerebral arteries for CTA.
The use of a low tube voltage for CT increases the attenuation of iodine and substantially reduces the radiation dose. In our study, the 70-kVp protocol (groups B and C) had a 10.5 % lower CTDIvol and a 10.0 % lower effective dose than the 120-kVp protocol (group A). Images obtained using low-dose CT are inevitably noisier than normal-dose CT images. Although we increased the effective tube current–time product in the 70-kVp protocol to compensate for this expected increase in image noise, the 70-kVp protocol (groups B and C) had a higher image noise, at 8.1 HU, and the subjective score for image noise was lower than that for the 120-kVp protocol. Despite this higher image noise, however, the 70-kVp protocol (group B) had significantly higher SNR and CNR than the 120-kVp protocol (group A), and a better subjective score for overall image quality.
The mechanism underlying CIN has yet to be fully elucidated [26]. However, the volume of injected CM is the primary risk factor and strongest predictor of the development of CIN [16–18], and therefore, attempts should be made to minimize the amount of CM used. When a 37.5 % lower CM volume (40 mL instead of 64 mL) was used in the 70-kVp protocol (group C), while the mean attenuation value of the cerebral arteries was significantly higher, the SNR and CNR were not different from those in the 120-kVp protocol (group A). The subjective scores for arterial enhancement, sharpness of the cerebral arterial boundary, visibility of small arterial details, and overall image quality were better for the 70-kVp protocol (group C). These data demonstrate that the use of low-kVp CTA protocols for cerebral CTA not only reduces radiation exposure, but also decreases the amount of CM required, while improving arterial enhancement and image quality. In addition, the risk of volume-dependent CIN is reduced and the safety of the examination is increased, especially in elderly patients with borderline renal function [9].
The subjective score for venous contamination in group B was statistically worse than that of group A. A low-tube-voltage protocol for CT angiography also increases venous enhancement, which may interfere with adequate evaluation of the cerebral arteries. Group C, which used a 37.5 % lower CM volume, had a subjective score for venous contamination that was comparable to that in group A. As the reduction in CM volume for cerebral CTA decreases venous enhancement as well as arterial enhancement, the venous contamination score for group C might have been improved compared to group B.
Our study had several limitations. First, because of ethical concerns, it was not possible to perform intra-individual comparisons among the three protocols. However, there were no significant differences in patient characteristics among the three groups, and thus quantitative and qualitative analyses and comparisons were possible across groups. Second, the sample size in each group was relatively small. However both phantom and clinical studies showed good correlation between the attenuation value and CT tube voltage setting. Additionally, the 70-kVp protocol had a significantly higher SD in attenuation in the occipital lobe, which was a quantitative marker of image noise. Promising techniques such as iterative reconstruction have recently been applied to reduce image noise [27]. Iterative reconstruction was not used in this study, however, and further studies are needed to explore the value of this technique.
In conclusion, the use of the 70-kVp protocol in cerebral CTA substantially improved arterial enhancement, SNR, and CNR, and provided better subjective image quality compared with the 120-kVp protocol, with a 10 % lower effective dose of radiation. Even with a 37.5 % lower volume of CM, the 70-kVp protocol had higher arterial enhancement and better image quality, while at the same time maintaining the SNR and CNR. Considering the safety of the examination, the 70-kVp protocol may not only reduce radiation exposure, but also decrease the volume of CM and in turn reduce the risk of CIN in patients with impaired renal function.
References
Lell MM, Anders K, Uder M et al (2006) New techniques in CT angiography. Radiographics 26:S45–62
Tamura Y, Utsunomiya D, Sakamoto T et al (2010) Reduction of contrast material volume in 3D angiography of the brain using MDCT. AJR Am J Roentgenol 195:455–458
Waaijer A, Prokop M, Velthuis BK, Bakker CJ, de Kort GA, van Leeuwen MS (2007) Circle of Willis at CT angiography: dose reduction and image quality—reducing tube voltage and increasing tube current settings. Radiology 242:832–839
Schindera ST, Graca P, Patak MA et al (2009) Thoracoabdominal-aortoiliac multidetector-row CT angiography at 80 and 100 kVp: assessment of image quality and radiation dose. Invest Radiol 44:650–655
Heyer CM, Mohr PS, Lemburg SP, Peters SA, Nicolas V (2007) Image quality and radiation exposure at pulmonary CT angiography with 100- or 120-kVp protocol: prospective randomized study. Radiology 245:577–583
Schueller-Weidekamm C, Schaefer-Prokop CM, Weber M, Herold CJ, Prokop M (2006) CT angiography of pulmonary arteries to detect pulmonary embolism: improvement of vascular enhancement with low kilovoltage settings. Radiology 241:899–907
Bahner ML, Bengel A, Brix G, Zuna I, Kauczor HU, Delorme S (2005) Improved vascular opacification in cerebral computed tomography angiography with 80 kVp. Invest Radiol 40:229–234
Cho ES, Chung TS, Oh DK et al (2012) Cerebral computed tomography angiography using a low tube voltage (80 kVp) and a moderate concentration of iodine contrast material: a quantitative and qualitative comparison with conventional computed tomography angiography. Invest Radiol 47:142–147
Szucs-Farkas Z, Schaller C, Bensler S, Patak MA, Vock P, Schindera ST (2009) Detection of pulmonary emboli with CT angiography at reduced radiation exposure and contrast material volume: comparison of 80 kVp and 120 kVp protocols in a matched cohort. Invest Radiol 44:793–799
Szucs-Farkas Z, Schibler F, Cullmann J et al (2011) Diagnostic accuracy of pulmonary CT angiography at low tube voltage: intraindividual comparison of a normal-dose protocol at 120 kVp and a low-dose protocol at 80 kVp using reduced amount of contrast medium in a simulation study. AJR Am J Roentgenol 197:W852–859
Sahani DV, Kalva SP, Hahn PF, Saini S (2007) 16-MDCT angiography in living kidney donors at various tube potentials: impact on image quality and radiation dose. AJR Am J Roentgenol 188:115–120
Hunsaker AR, Oliva IB, Cai T et al (2010) Contrast opacification using a reduced volume of iodinated contrast material and low peak kilovoltage in pulmonary CT angiography: Objective and subjective evaluation. AJR Am J Roentgenol 195:W118–124
Gnannt R, Winklehner A, Goetti R, Schmidt B, Kollias S, Alkadhi H (2012) Low kilovoltage CT of the neck with 70 kVp: comparison with a standard protocol. AJNR Am J Neuroradiol 33:1014–1019
Mehran R, Aymong ED, Nikolsky E et al (2004) A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 44:1393–1399
Gleeson TG, Bulugahapitiya S (2004) Contrast-induced nephropathy. AJR Am J Roentgenol 183:1673–1689
Tepel M, Aspelin P, Lameire N (2006) Contrast-induced nephropathy: a clinical and evidence-based approach. Circulation 113:1799–1806
Mehran R, Nikolsky E (2006) Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl:S11–15
Barrett BJ, Parfrey PS (2006) Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med 354:379–386
Furtado AD, Adraktas DD, Brasic N et al (2010) The triple rule-out for acute ischemic stroke: imaging the brain, carotid arteries, aorta, and heart. AJNR Am J Neuroradiol 31:1290–1296
Ertl-Wagner BB, Hoffmann RT, Bruning R et al (2004) Multi-detector row CT angiography of the brain at various kilovoltage settings. Radiology 231:528–535
Commission of the European Community (1999) European guidelines on quality criteria for computed tomography. Report EUR 16262 EN. Available via http://www.drs.dk/guidelines/ct/quality/htmlindex.htm. Accessed 31 Jan 2013
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Curry TSDJ, Murry RC (1990) Basic interactions between X-rays and matter. In: Curry TS, Dowdey JE, Murry RC (eds) Christensen’s physics of diagnostic radiology, 4th edn. Lippincott Williams & Wilkins, Philadelphia, pp 61–69
Huda W, Scalzetti EM, Levin G (2000) Technique factors and image quality as functions of patient weight at abdominal CT. Radiology 217:430–435
Yoon DY, Lim KJ, Choi CS et al (2007) Sixteen-detector row CT angiography of the brain: comparison of 3 different volumes of the contrast material. J Comput Assist Tomogr 31:671–676
Tumlin J, Stacul F, Adam A et al (2006) Pathophysiology of contrast-induced nephropathy. Am J Cardiol 98:14K–20K
Machida H, Takeuchi H, Tanaka I et al (2013) Improved delineation of arteries in the posterior fossa of the brain by model-based iterative reconstruction in volume-rendered 3D CT angiography. AJNR Am J Neuroradiol 34:971–975
Acknowledgements
The scientific guarantor of this publication is Tae-Sub Chung. The authors of this manuscript declare relationships with the following companies: KyoungHoon Chong, an employee of Siemens Healthcare, provided us with important information about the CT scanner and protocol. The other authors, who are not employees of Siemens Healthcare, had control of the inclusion of any data and information that might present a conflict of interest. This study received funding (6-2012-0118) from Yonsei University College of Medicine. Jong Hyeung Lee (Biostatistics consulting laboratory, Gangnam Severance Hospital Medical Research Center, Yonsei College of Medicine) kindly provided statistical advice for this manuscript. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (volunteers) in this study. No study subjects or cohorts have been previously reported. Methodology: prospective diagnostic or prognostic study, performed at one institution.
We thank R.T. Jeong Hui Seo for vital assistance in initiating this study. We thank Jong Hyeung Lee, Biostatistics consulting laboratory, Gangnam Severance Hospital Medical Research Center, Yonsei College of Medicine, Seoul, Korea, for statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, ES., Chung, TS., Ahn, S.J. et al. Cerebral computed tomography angiography using a 70 kVp protocol: improved vascular enhancement with a reduced volume of contrast medium and radiation dose. Eur Radiol 25, 1421–1430 (2015). https://doi.org/10.1007/s00330-014-3540-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3540-z