Abstract
Purpose
This study was conducted in order to evaluate the image quality of 70 kVp and 25 mL contrast medium (CM) volume for head and neck computed tomographic angiography (CTA) and assess the diagnostic accuracy for arterial stenosis.
Methods
Fifty patients were prospectively divided into two groups randomly: group A (n = 25), 70 kVp with 25 mL CM, and group B (n = 25), 100 kVp with 40 mL CM. CT attenuation values, noise, signal-to-noise ratio (SNR), and contrast-to-noise ratio (CNR) of the shoulder, neck, and cerebral arteries were measured for objective image quality. Subjective image quality of the shoulder and cerebral arteries was also evaluated. For patients undergoing digital subtracted angiography (DSA), diagnostic accuracy of CTA was assessed with DSA as reference standard.
Results
The SNRs of the shoulder, neck, and cerebral arteries in group A were higher than those in group B (P < 0.05). The CNRs of the shoulder and neck arteries in group A were higher than those in group B (P < 0.05). There was no significant difference in subjective image quality of arteries between group A and group B (P > 0.05). The accuracy was noted as 94.0% (156/166) in group A and 97.1% (134/138) in group B for ≥ 50% stenosis. The accuracy of intracranial arterial stenosis was lower than that of extracranial arterial stenosis in group A. The radiation dose of group A was significantly decreased by 56% than that of group B.
Conclusion
Head and neck CTA at 70 kVp using 25 mL CM can obtain diagnostic image quality with lower radiation dose while maintaining high accuracy in detecting the arterial stenosis compared with the 100-kVp and 40-mL CM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In patients with symptoms of stroke or transient ischemic attack, computed tomography angiography (CTA) can swiftly and accurately assess the patency of the intracranial circulation, evaluate for carotid atherosclerosis as a potential embolic source, and provide information about vessel occlusion that is essential before treatment with thrombolysis or even embolectomy is considered [1, 2]. CTA also has high sensitivity and specificity for the detection of intracranial aneurysms and is, thus, used in the emergency setting to detect intracranial aneurysms in patients with subarachnoid hemorrhage [3, 4].
Exposure of the patient to a nephrotoxic contrast medium (CM) and carcinogenic radiation limits the usefulness of head and neck CT. Thus, strategies to reduce the radiation dose and CM associated with CTA are desirable. Several dose-reduction strategies have been investigated and lowering tube voltage appears to be the most effective, because radiation dose is proportional to the square of tube voltage. Meanwhile, the CT attenuation of enhanced vessels can be substantially increased by lowering tube voltage, and therefore, CM could be reduced.
In view of contrast enhancement, image acquisition at the lowest currently achievable tube voltage setting (70 kV) could be even more efficient. However, image quality at CT angiography performed with 70 kVp has been limited by the maximum tube current output (500 mA) in previous CT systems. This limitation might be overcome with the recent introduction of third-generation dual-source CT systems (SOMATOM Force, Siemens Healthineers, Forchheim, Germany), which are equipped with two 120-kW X-ray tubes that could generate tube current up to 1300 mA [5].
There are several published studies on strategies of 70-kVp setting to decrease the radiation dose or the volume of CM for cerebral angiography. Cho et al. demonstrated that 70 kVp cerebral CTA combined with 40 mL CM improved arterial enhancement and an effective dose was 10% lower than that of 120 kVp combined with 64 mL CM using a 128-slice CT system [6]. One recent study demonstrated cerebral CTA at 70 kVp with 30 mL of iodinated CM was feasible, and the radiation dose was 85% reduction in comparison to 120 kVp with 60 mL CM [7].
Except cerebral angiography, 70-kVp settings have been applied in coronary CTA [5, 8], lower extremity, pulmonary angiography [9], aorta [10], cerebral CT perfusion [11], liver perfusion [12], and so on. To our knowledge, the feasibility of 70-kVp CTA of cervical arteries or combined cranial and cervical arteries has not yet been investigated.
The hypothesis of our study was that a 70-kVp protocol with the advanced modeled iterative reconstruction (ADMIRE) would lower the amount of contrast medium and radiation exposure while maintaining or even improving vessel contrast, when performing CTA for both head and neck.
Materials and methods
Patient population
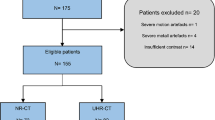
This prospective study was performed with institutional review board approval. Informed consent was obtained from all patients or their legal guardians. Between October 2015 and July 2016, consecutive patients suspected with arterial diseases of the head and neck and body weight less than 75 kg were enrolled in the study. All patients underwent CTA of the head and neck. Contraindications for head and neck CTA included known prior reactions to iodinated contrast agents, severe renal impairment, severe cardiac insufficiency, and pregnancy. Patients with moyamoya disease, arteriovenous fistula, intracranial clipping, or coiling were excluded from the study because these factors can affect CT measurements. Each patient’s age, body weight, and height were measured and recorded prior to the CT examination.
Head and neck CT angiography
Acquisition protocol
All head and neck CTA examinations were performed on a 192-slice third-generation dual-source CT system (SOMATOM Force, Siemens Healthineers, Forchheim, Germany). Patients were positioned supine on the CT table with both arms along the chest. The CM iodixanol (370 mg of iodine per milliliter, Ultravist, Bayer-Schering HealthCare, Germany) was injected intravenously through an 18-gauge needle, into the antecubital vein by using a high-power autoinjector.
Patients were randomly assigned to one of the 70-kVp (group A) or 100-kVp (group B) protocols. In group A, patients received 25 mL CM with a flow rate of 5 mL/s followed by 50 mL saline solution with the same flow rate. In group B, patients received 40 mL CM and 50 mL saline solution with a flow rate of 5 mL/s.
Automatic attenuation-based tube current modulation (CAREDose 4D, Siemens Healthcare) was used in either the 70- or 100-kVp protocols. Automated attenuation-based tube voltage (CAREkV, Siemens Healthcare) was off for the 70-kVp protocol, and 700 mAs of reference tube current was used according to theoretical need in the previous study [6]. Studies of 100 kVp were performed with semiautomated CAREkV with referenced 120 kVp and 84 mAs following the vendor’s recommendation.
CT acquisition was triggered by using a bolus tracking technique with the region of interest (ROI) placed in the ascending aorta. For the 70-kVp protocols, image acquisition was started 4 s after attenuation reached the predefined threshold of 80 HU in order to start earlier, pitch 1.55, gantry rotation time of 250 ms/rotation, and craniocaudal scan direction. For the 100-kVp protocol, image acquisition was started 2 s after attenuation reached the threshold of 100HU, and pitch 1.2, gantry rotation time of 280ms/rotation, caudocranial scan direction were used.
Image reconstruction
CT images at 100 kVp were reconstructed using a conventional filtered backprojection (FBP) algorithm. CT images of 70 kVp were reconstructed using ADMIRE at strength level 3 [13]. All image series were reconstructed with a 0.6-mm section thickness and 0.4-mm increment. The convolution kernels of all images for both groups were the “Bv36.”
Each image dataset was coded and randomized to enable double-blinded evaluation, also with anonymized patients’ information. All images were transferred to a dedicated workstation (syngo. Via VA30, Siemens Healthinners). Multiplanar reformation (MPR) and maximum intensity projections (MIP) images were reconstructed.
Quantitative image quality evaluation
Arteries with stent, very thin lumen, and occlusion were excluded. All measurements were performed by two radiologists (YZ and YC, with 3 and 8 years of experience in head and neck imaging, respectively). CT attenuation, standard deviation (SD), signal-to-noise ratio (SNR), and contrast-to-noise ratio (CNR) were calculated on three levels, and mean value of three measurements on the same vessel was calculated:
-
1.
On the shoulder level, CT attenuationAA, SDAA, CT attenuationSA, and SDSA were the mean HU value and noise of the aortic arteries (AA) and bilateral proximal segment of subclavian arteries (SA). CT attenuationshoulder and SDshoulder were the mean HU value and SD of the bilateral rhomboideus muscles at the same slice of SA.
-
2.
On the neck level, CT attenuationCCA, SDCCA, CT attenuationVA, and SDVA are the mean HU value of the bilateral bifurcation of CCA (CCAbi) and vertebral arteries (VA). CTattenuationneck and SDneck were the mean HU value and SD of the bilateral sternocleidomastoideus (SCM).
-
3.
On the brain level, CT attenuationMCA, SDMCA, CT attenuationBA, and SDBA are the mean HU value of the bilateral M1 segment of middle cerebral arteries (MCA) and middle segment of basilar artery (BA), respectively. CTattenuationbrain and SDbrain were the mean HU value and SD of the brain stem (BS) at the same slice of BA.
Arterial attenuation values ≥ 150 HU were considered as diagnosed and ≥ 200 HU as optimal for CTA [2, 14], and the number of arterial segments with attenuation values > 150 HU or > 200 HU was counted, respectively.
To assess the mean signal intensity, the attenuation values in the ROI using a circular tool were measured. The ROI was drawn large enough to include as much of the contrast-filled vessel and to avoid vessel wall or small calcification. To avoid partial volume effects, the ROIs were placed individually suitable for MPR exactly perpendicular to the vessel lumen. Severe calcification or very thin parts of the arteries were avoided, and occluded vessels were excluded. SNR and CNR were calculated as the following formulas:
Where CT attenuationa and SDa indicated the mean HU and noise of target arteries. CT attenuationm and SDm indicated the mean HU and noise of surrounding muscles on the same level of target arteries.
Qualitative image quality evaluation
Image scoring of arteries was performed independently by two neuroradiologists (YC and YZ with 8 and 3 years of experience in head and neck imaging). Both of them were blinded to the patients and group information (i.e., tube voltage, reconstruction parameters). In case of disagreement between both readers, consensus was reached in a joint reading to determine the final image quality score.
SA contralateral to the side of injection (SAcontra), anterior cerebral arteries (ACA), MCA, and posterior cerebral arteries (PCA) underwent artery evaluation. MIP images of arteries were evaluated using a 4-point scale as follows (Fig. 1):
-
Sartery = 4 excellent, with very sharp edges and high subjective contrast-to-noise ratio
-
Sartery = 3 good, with restrictions due to minimal blurring or slightly suboptimal subjective contrast-to-noise ratio
-
Sartery = 2 moderate but still diagnostic, with considerable restrictions due to vessel edge blurring or markedly suboptimal subjective contrast-to-noise ratio
-
Sartery = 1 nondiagnostic, with unacceptable blurring or subjective contrast-to-noise ratio
Cases of arterial MIP reconstructed image quality evaluation. A 63-year-old man with 67-kg body weight in the 70-kVp group; the MIP (a) image of ACA was evaluated as score 3. A 62-year-old man with 70-kg body weight in the 70-kVp group; the MIP (b) image of ACA was evaluated as score 4. There is no image of scores 1 and 2 in this study
Score ≥ 2 was considered as diagnosed, and score ≥ 3 was considered as optimal for CTA.
Superior vena cava (SVC), brachiocephalic vein (BCV), and subclavian vein (SV) were observed for CM residues that could lead to artifacts on the angiography that were rated using a three-point scale as follows (Fig. 2):
-
Sresidues = 1 poor, artifacts present in the SVC, BCV, and SV.
-
Sresidues = 2 good, artifacts present in the BCV and SV, no artifacts are detected in the SVC.
-
Sresidues = 3 excellent, no artifacts appear in the SVC, BCV, and SV, or there were a little artifacts in the SV.
Examples of contrast medium residues. a A 67-year-old man with 65-kg body weight in the 70-kVp group. On the MIP image, only slight residues are seen in the subclavian vein (arrow) and evaluated as score 3. b A 62-year-old man with 70-kg body weight in the 70-kVp group. On the MIP image, moderate residues appeared in the right subclavian vein, parts of them went into the right brachiocephalic vein (arrow) and evaluated as score 2. c A 70-year-old man with 65-kg body weight in the 100-kVp group. On the MIP image, obvious residues were present in the superior vena cava (arrow) and brachiocephalic and subclavian veins and evaluated as score 1
Radiation dose estimation
With all protocols, the volume CT dose index (CTDIvol) was given based on the 32-cm CTDI phantom. CTDIvol and dose-length product (DLP) were recorded for every CT examination and effective dose (in millisieverts) estimated by multiplying DLP with a constant region-specific conversion coefficient of 0.0048 mSv/(mGy cm).
DSA
DSA was performed only if clinically indicated and was, thus, only performed in a subgroup of patients. The DSA technique included femoral catheterization by the Seldinger technique and used a biplane DSA unit with rotational capabilities (AXION Artis zeego, Siemens Medical Systems, Forchheim, Germany). Firstly, aortic and bilateral common carotid artery angiography was performed. Then, bilateral internal carotid and vertebral artery angiography was performed optionally. Standard anteroposterior, lateral, and oblique positions were performed. A single rotational (3D-DSA) acquisition was typically obtained before removing the catheter from each vessel if necessary. DSA data were sent to an adjacent 3D workstation (Lenoardo, Siemens Medical Solutions, Erlangen, Germany). A well-experienced neuroradiologist (XZ with 20 years of experience in head and neck imaging) performed DSA and evaluated the arterial stenosis and location of intracranial aneurysms in the DSA images.
Arterial stenosis
Two well-experienced neuroradiologist (YC and XZ) evaluated the CTA and DSA data. Both of them were blinded to the patients’ information and reached a consensus conclusion. Segments of arteries with stent in either CTA or DSA were excluded.
Arteries were divided into 43 segments, including aortic arch (AA), brachiocephalic trunk artery (BTA), bilateral S1–3 segments of SA, bilateral external carotid arteries (ECA) and branches, bilateral CCA, bilateral bifurcation of CCA (CCAbi), bilateral C1–7 segments of the ICA, bilateral ACA, bilateral MCA, bilateral PCA, and bilateral V1–4 segments of the VA and BA [15]. AA, BTA, SA1–3, CCA, CCAbi, ECA, C1, V1, and V2 belong to extracranial arteries. C2–7, V3–4, BA, ACA, MCA, and PCA belong to intracranial arteries.
The presence of steno-occlusive disease in arteries was calculated according to the North American Symptomatic Carotid Endarterectomy Trial criteria and categorized into mild (<50%), moderate (50–69%), severe stenosis (70–99%), and complete occlusion (100%) [16, 17]. The accuracy, sensibility, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the 70- and 100-kVp groups were calculated.
Statistical analysis
Statistical analysis was performed using SPSS software (IBM SPSS, version 17, SPSS Inc. Chicago, IL, USA). Quantitative variables were expressed as mean ± SD. Age, height, weight, BMI, scan time, CT values, SD, SNR, CNR, and radiation dose of the two protocols were compared using t test, after normal distribution was proved by a Kolmogorov-Smirnov test. The nonparametric Mann-Whitney test was used to analyze differences between qualitative image quality of the two protocols. P values < 0.05 were considered to indicate statistically significant differences.
Results
Patient population and radiation dose
Fifty-five patients met the inclusion criteria. Five patients were ineligible because of moyamoya disease (n = 1), arteriovenous fistula (n = 2), and intracranial clipping or coiling (n = 2). Thus, 50 patients were included in the final analysis. Group A included 23 men and 2 women with a mean age of (64 ± 8). Group B included 21 men and 4 women with a mean age of (62 ± 7). The mean estimated effective radiation dose of group A was 0.40 ± 0.03 mSv and was 56% lower than that of group B (0.92 ± 0.08) mSv. Patient population and scan characteristics are displayed in Table 1.
Quantitative image quality evaluation
In group A, 1 SA and 1 CCAbi with stent and 9 occluded arteries (1SA, 6 V2, 2 MCA) were excluded. Therefore, 239 arteries underwent image quality evaluation. In group B, 1 SA with stent and 16 occluded arteries (2 SA, 1 CCAbi, 8 V2, 3 MCA, 2 BA) were excluded. Therefore, 233 arteries underwent image quality evaluation.
All the arteries had attenuation > 200 HU in group A and group B. The CT attenuation of AA in group A was lower than that of group B (P = 0.002). The SDs of AA and CCAbi in group A were lower than those in group B (P < 0.05). The SNRs of SA, CCAbi, V2, and MCA in group A were higher than those in group B (P < 0.05). The CNRs of SA, CCAbi, and V2 in group A were higher than those in group B (P < 0.05). The other quantitative values of group A and group B were not significantly different (P > 0.05) (Table 2).
Qualitative image quality evaluation
Except 1 occluded ACA and 1 occluded MCA, 98 arteries underwent qualitative image quality evaluation in group A and all of them had a score ≥ 3. Except 1 occluded SA, 99 arteries underwent qualitative image quality evaluation in group B and all of them (100%) had a score ≥ 3. There was no significant difference between group A and group B (P > 0.05) (Table 3).
When evaluating the CM residues, 16 of 25 (60%) patients had scores of 2 (n = 13) and 3 (n = 3) in group A, and all the 25 patients (100%) had a score of 1 in group B. The image quality of CM residues of group A was better than that of group B (P < 0.001) (Table 3).
Diagnostic accuracy of stenosis
With invasive DSA results as reference, 70- and 100-kVp CTA were assessed both by patient-based and vessel-based intracranial and extracranial analyses of arterial stenosis. DSA was performed in 9 patients (n = 5 for the 70-kVp group, n = 4 for the 100-kVp group) and 306 arterial segments (n = 167 for the 70-kVp group, n = 139 for the 100-kVp group) in this study. It is expected that each of the two groups had one arterial segment with stent, therefore 166 of the 70-kVp group (77 extracranial arterial segments, 89 intracranial arterial segments) and 138 of the 100-kVp group (68 extracranial arterial segments, 70 intracranial arterial segments) underwent stenosis evaluation (Tables 4 and 5).
Patient-based 70- and 100-kVp CTA results are shown in Table 6. Excellent PPV and NPV of 70 kVp were noted 100% (4/4) and 100% (1/1) for ≥ 50% arterial stenosis. Excellent PPV and NPV of 100 kVp were also noted 100% (3/3) and 100% (1/1) for ≥ 50% arterial stenosis.
Vessel-based 70- and 100-kVp CTA results are shown in Table 7. The accuracy, sensibility, specificity, PPV, and NPV of the 70-kVp group were noted as 94.0% (156/166), 91% (10/11), 94.2% (146/155), 52.6% (10/19), and 99.3% (146/147) for ≥ 50% stenosis and 98.2% (163/166), 83.3% (5/6), 98.8% (158/160), 71.4% (5/7), and 99.4% (158/159) for ≥ 70% stenosis (Figs. 3 and 4).
The accuracy, sensibility, specificity, PPV, and NPV of the 100-kVp group were noted as 97.1% (134/138), 91.6% (11/12), 97.6% (123/126), 78.6% (11/14), and 99.1% (123/124) for ≥ 50% stenosis and 97.1% (134/138), 76.9% (10/13), 99.2% (124/125), 91% (10/11), and 97.6% (124/127) for ≥ 70% stenosis (Fig. 5).
One 66-year-old female with true-positive severe stenosis of the left internal carotid artery (ICA) in the 100-kVp group. a Curved multiplanar projection reconstruction shows severe stenosis of the left ICA (arrow). b Maximum intensity projection shows obvious calcification which affects the observation of lumen (arrow). c Digital subtraction angiography confirms the stenosis (arrow)
Excellent PPV of 70-kVp CTA was noted as 100% (7/7) for ≥ 50% extracranial arterial stenosis, while poor PPV was noted as 25% (3/12) for intracranial arterial stenosis. Excellent NPVs of 70-kVp CTA were noted as 100% (70/77) and 98.7% (76/77) for both extracranial and intracranial ≥ 50% arterial stenosis. The diagnostic accuracy of extracranial arterial stenosis in 70-kVp CTA was higher than that of 100-kVp CTA (77 of 77, 100% vs 66 of 68, 97.0%). The diagnostic accuracy of intracranial arterial stenosis in 70-kVp CTA was lower than that of 100-kVp CTA (79 of 89, 88.8% vs 68 of 70, 97.1%).
Discussion
In this study, we confirmed that for patients with body weight lower than 75 kg, 70 kVp improved arterial enhancement and maintained 100% optimal enhancement, and subjective image quality reduces the volume of CM by 37.5% and lowers the effective dose by 56%, compared with the 100-kVp protocol.
Low tube voltage acquisition CT has been widely investigated in different body regions as a technique to reduce radiation exposure. May et al. showed an equivalent good image quality in 100 kV contrast-enhanced neck CT on a second-generation DSCT using automated tube voltage adaptation compared to standard 120 kV examination [18]. Scholtz et al. demonstrated that third-generation DSCT allows tube voltage selection in steps of 10 kV between 70 and 150 kV compared to steps of 20 kV between 80 and 140 kV with second-generation DSCT in contrast-enhanced neck CT [19]. In previous studies, 100-kVp setting had been demonstrated feasible to cerebral CTA. Luo et al. [20] reported that 100 kVp combined with 30 mL CM with 300 mg iodine/mL had the mean attenuation of enhanced intracranial arteries varied from 294 to 337 HU and the radiation dose was decreased by 45%, compared with the 120-kVp setting. In our study, the mean arterial attenuation was 434 HU in the 100-kVp protocol when 40 mL CM with 370 mg iodine/mL was administered. All the arterial subjective image quality in the two body weight groups had a score ≥ 3 with the 100-kVp setting. Furthermore, the average CTDIvol and DLP of 100 kVp were 1.94 mGy and 178.6 mGy cm with the scan range covering the head and neck, which was extremely lower than the previous study (14.4 mGy and 286 mGy cm) with scan range just covering the cerebral arteries [20]. Therefore, 100 kVp with 40 mL CM could be recommended as the routine protocol of head and neck CTA for unselected patients with body weight lower than 75 kg, when using the third-generation dual source CT. Thus, we used the 100-kVp setting as the control group also as a clinical routine in our department.
Compared with the 100-kVp protocol, the 70-kVp setting further reduced the radiation dose. Several studies showed that tube voltage lower than 100 kVp can effectively reduce the radiation dose while maintaining diagnostic image quality. Luo et al. [20] found that when the tube voltage was reduced from 100 to 80 kVp on the first dual-source CT system (Somatom Definition, Siemens Healthineers, Forchheim, Germany), the radiation dose decreased by 50%. Chen et al. [7] found that when the tube voltage was reduced from 120 to 70 kVp on the second dual-source CT system (Somatom Definition Flash, Siemens Healthineers, Forchheim, Germany), the radiation dose decreased by 85%. We demonstrated that when the tube voltage was reduced from 100 to70 kVp on the third dual-source CT system, the radiation dose decreased by 56%. What is more, as well as we know, the 70-kVp setting is not used for CTA combining the head and neck, because of the potential obvious artifact in the shoulder area. The 70-kVp setting in the study of Chen et al. was used for intracranial CTA and DLP was 118 mGy cm. In our study, 70-kVp setting was used for both head and neck area, and DLP was 84.2 mGy cm.
Lowering tube voltage can significantly increase intravascular CT attenuation. This is due to the characteristic absorption spectrum of iodine with markedly higher attenuation at lower photon energies, where the X-ray energy approaches the K-edge of iodine [5]. Chen et al. [7] demonstrated that 30 mL (300 mg I/mL, total iodine load of 9 g) of iodinated contrast agent allowed satisfactory diagnostic image quality of cerebral arteries using the 70-kVp setting. In our study, a total of 9.25 g iodine (25 mL, 370 mg I/mL) for body weight lower than 75 kg using the 70-kVp setting and satisfactory image quality was obtained. Compared with the 100-kVp CTA protocol, our results suggested that head and neck CTA protocol can thus reduce the contrast agent volume by nearly half with 70 kVp.
Iterative reconstruction (IR) can substantially improve image quality by reducing image noise, compared with traditional FBP. SNR and CNR are more comprehensive measures of radiological image quality than signal intensity and contrast alone. In the third-generation dual-source CT system, the new IR technique, ADMIRE, performs detailed modeling in the projection data domain, resulting in less noise [21]. Scholtz et al. demonstrated that subjective image quality and image contrast of ADMIRE 3 and 5 were consistently rated better than those for filtered backprojection (all P < 0.001) for contrast-enhanced neck CT [21]. Therefore, we chose ADMIRE 3 for the 70-kVp protocols, and image noise was comparable or improved compared with 100 kVp. Combined with increased vessel opacification in image sets with lower kVp, the SNR and CNR of 70 kVp were also equal or higher than those of 100 kVp.
Objective image quality showed score of 3 or 4 for all patients in the 70- and 100-kVp groups (Fig. 1 and Table 3). There is no image with scores of 1 and 2 in this study. Therefore, the image quality was optimal for both the 70- and 100-kVp groups.
To synchronize data acquisition with optimal arterial enhancement, it has been recommended that scan direction during CTA should be in the direction of the contrast medium flow; however, with modern technology and faster scan times, changes in scan direction no longer compromise vascular attenuation. Several studies have been recommended that scan direction which is contrary to contrast medium flow during CTA with lower contrast volume will improve the vascular attenuation [22, 23]. For head and neck CTA, craudocranial scan direction is the direction of contrast flow; therefore, it was used for the 100-kVp group with longer scan time and more contrast volume, while craniocaudal scan direction was used for the 70-kVp group with shorter scan time and fewer contrast volume. Our study demonstrated that the 70-kVp group has comparable or higher arterial attenuation than the 100-kVp group.
In the subgroup of patients with a clinically indicated DSA available as the reference standard, we further found that both 70- and 100-kVp CTA had high diagnostic accuracy and excellent NPV for ≥ 50 and ≥ 70% arterial stenosis. The diagnostic accuracy of 70-kVp CTA for intracranial arterial stenosis was lower than that of extracranial arterial stenosis. It was because one patient’s internal carotid artery with severe calcification in CTA showed more severe stenosis than DSA. Therefore, the diagnostic accuracy of 70-kVp CTA improved when using patient-based analysis. Taken together, these findings indicated that head and neck CTA at 70 kVp is feasible using 25 mL of contrast media volume and can be safely used in clinical routine and might be used as a screening method before DSA.
Our study has several limitations. Firstly, only a subgroup of patients underwent DSA examinations and few of them received 3D DSA, which induced to the inadequate assessment of stenosis for some patients. Secondly, only a few patients received DSA; therefore, the PPV of both 70 and 100 kVp was lower based on vessel analysis and higher based on patient analysis. Studies with a larger cohort need to be performed to confirm our findings regarding the diagnostic accuracy. Thirdly, we did not evaluate the different ADMIRE strength settings, to determine whether the higher ADMIRE strength will improve the image quality further.
In conclusion, the results of our study demonstrate that head and neck CTA at 70 kVp is feasible with CM volume reduced to 25 mL when patients’ body weight was lower than 75 kg. Image quality and diagnostic accuracy can be obtained using this “double low” CTA technique.
Abbreviations
- CM:
-
Contrast medium
- CTA:
-
Computed tomographic angiography
- ADMIRE:
-
Advanced modeled iterative reconstruction
- FBP:
-
Filtered back projection
- SNR:
-
Signal-to-noise ratio
- CNR:
-
Contrast-to-noise ratio
- AA:
-
Aortic arch
- BTA:
-
Brachiocephalic trunk artery
- SA:
-
Subclavian artery
- CCA:
-
Common carotid artery
- VA:
-
Vertebral artery
- MCA:
-
Middle cerebral artery
- BA:
-
Basilar artery
- ED:
-
Effective dose
- DSA:
-
Digital subtracted angiography
- ROI:
-
Region of interest
- MPR:
-
Multiplanar reformation
- MIP:
-
Maximum intensity projections
- SD:
-
Standard deviation
- SCM:
-
Sternocleidomastoideus
- BS:
-
Brain stem
- ACA:
-
Anterior cerebral arteries
- PCA:
-
Posterior cerebral arteries
- SVC:
-
Superior vena cava
- SV:
-
Subclavian vein
- CTDIvol :
-
Volume CT dose index
- DLP:
-
Dose-length product
- ECA:
-
External carotid arteries
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- CCAbi:
-
Bifurcation of CCA
- IR:
-
Iterative reconstruction
References
Eswaradass P, Appireddy R, Evans J, Tham C, Dey S, Najm M, Menon BK (2016) Imaging in acute stroke. Expert Rev Cardiovasc Ther 14:963–975. doi:10.1080/14779072.2016.1196134
Kim JJ, Dillon WP, Glastonbury CM, Provenzale JM, Wintermark M (2010) Sixty-four-section multidetector CT angiography of carotid arteries: a systematic analysis of image quality and artifacts. AJNR Am J Neuroradiol 31:91–99. doi:10.3174/ajnr.A1768
Hsu CC, Kwan GN, Singh D, Pratap J, Watkins TW (2016) Principles and clinical application of dual-energy computed tomography in the evaluation of cerebrovascular disease. J Clin Imaging Sci 6:27. doi:10.4103/2156-7514.185003
Chen GZ, Zhang LJ, Schoepf UJ, Wichmann JL, Milliken CM, Zhou CS, Qi L, Luo S, Lu GM (2015) Radiation dose and image quality of 70 kVp cerebral CT angiography with optimized sinogram-affirmed iterative reconstruction: comparison with 120 kVp cerebral CT angiography. Eur Radiol 25:1453–1463. doi:10.1007/s00330-014-3533-y
Meyer M, Haubenreisser H, Schoepf UJ et al (2014) Closing in on the K edge: coronary CT angiography at 100, 80, and 70 kV-initial comparison of a second- versus a third-generation dual-source CT system. Radiology 273:373–382. doi:10.1148/radiol.14140244
Cho ES, Chung TS, Ahn SJ, Chong K, Baek JH, Suh SH (2015) Cerebral computed tomography angiography using a 70 kVp protocol: improved vascular enhancement with a reduced volume of contrast medium and radiation dose. Eur Radiol 25:1421–1430. doi:10.1007/s00330-014-3540-z
Chen GZ, Fang XK, Zhou CS, Zhang LJ, Lu GM (2017) Cerebral CT angiography with iterative reconstruction at 70 kVp and 30 mL iodinated contrast agent: initial experience. Eur J Radiol 88:102–108. doi:10.1016/j.ejrad.2016.12.037
Meyer M, Haubenreisser H, Schoepf UJ, Vliegenthart R, Ong MM, Doesch C, Sudarski S, Borggrefe M, Schoenberg SO, Henzler T (2016) Radiation dose levels of retrospectively ECG-gated coronary CT angiography using 70-kVp tube voltage in patients with high or irregular heart rates. Acad Radiol. doi:10.1016/j.acra.2016.08.004
Boos J, Kröpil P, Lanzman RS, Aissa J, Schleich C, Heusch P, Sawicki LM, Antoch G, Thomas C (2016) CT pulmonary angiography: simultaneous low-pitch dual-source acquisition mode with 70 kVp and 40 ml of contrast medium and comparison with high-pitch spiral dual-source acquisition with automated tube potential selection. Br J Radiol 89:20151059
Higashigaito K, Schmid T, Puippe G, Morsbach F, Lachat M, Seifert B, Pfammatter T, Alkadhi H, Husarik DB (2016) CT angiography of the aorta: prospective evaluation of individualized low-volume contrast media protocols. Radiology 280:960–968. doi:10.1148/radiol.2016151982
Fang XK, Ni QQ, Schoepf UJ, Zhou CS, Chen GZ, Luo S, Fuller SR, De Cecco CN, Zhang LJ, Lu GM (2016) Image quality, radiation dose and diagnostic accuracy of 70 kVp whole brain volumetric CT perfusion imaging: a preliminary study. Eur Radiol 26:4184–4193. doi:10.1007/s00330-016-4225-6
Gawlitza J, Haubenreisser H, Meyer M, Hagelstein C, Sudarski S, Schoenberg SO, Henzler T (2016) Comparison of organ-specific-radiation dose levels between 70 kVp perfusion CT and standard tri-phasic liver CT in patients with hepatocellular carcinoma using a Monte-Carlo-simulation-based analysis platform. Eur J Radiol Open 3:95–99. doi:10.1016/j.ejro.2016.04.003
Sabel BO, Buric K, Karara N, Thierfelder KM, Dinkel J, Sommer WH, Meinel FG (2016) High-pitch CT pulmonary angiography in third generation dual-source CT: image quality in an unselected patient population. PLoS One 11:e0146949. doi:10.1371/journal.pone.0146949
Mohan S, Agarwal M, Pukenas B (2016) Computed tomography angiography of the neurovascular circulation. Radiol Clin N Am 54:147–162. doi:10.1016/j.rcl.2015.09.001
Chen Y, Xue H, Jin ZY et al (2013) 128-Slice acceletated-pitch dual energy CT angiography of the head and neck: comparison of different low contrast medium volumes. PLoS One 8:e80939. doi:10.1371/journal.pone.0080939
Barnett HJ, Taylor DW, Eliasziw M et al (1998) Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 339:1415–1425. doi:10.1056/NEJM199811123392002
Huang J, Degnan AJ, Liu Q, Teng Z, Yue CS, Gillard JH, Lu JP (2012) Comparison of NASCET and WASID criteria for the measurement of intracranial stenosis using digital subtraction and computed tomography angiography of the middle cerebral artery. J Neuroradiol 39:342–345. doi:10.1016/j.neurad.2011.11.005
May MS, Kramer MR, Eller A, Wuest W, Scharf M, Brand M, Saake M, Schmidt B, Uder M, Lell MM (2014) Automated tube voltage adaptation in head and neck computed tomography between 120 and 100 kV: effects on image quality and radiation dose. Neuroradiology 56:797–803. doi:10.1007/s00234-014-1393-4
Scholtz JE, Wichmann JL, Hüsers K, Albrecht MH, Beeres M, Bauer RW, Vogl TJ, Bodelle B (2016) Third-generation dual-source CT of the neck using automated tube voltage adaptation in combination with advanced modeled iterative reconstruction: evaluation of image quality and radiation dose. Eur Radiol 26:2623–2631. doi:10.1007/s00330-015-4099-z
Luo S, Zhang LJ, Meinel FG, Zhou CS, Qi L, McQuiston AD, Schoepf UJ, Lu GM (2014) Low tube voltage and low contrast material volume cerebral CT angiography. Eur Radiol 24:1677–1685. doi:10.1007/s00330-014-3184-z
Scholtz JE, Kaup M, Hüsers K et al (2016) Advanced modeled iterative reconstruction in low-tube-voltage contrast-enhanced neck CT: evaluation of objective and subjective image quality. AJNR Am J Neuroradiol 37:143–150. doi:10.3174/ajnr.A4502
Saade C, El-Merhi F, El-Achkar B, Kerek R, Vogl TJ, Maroun GG, Jamjoom L, Al-Mohiy H, Naffaa L (2016) 256 Slice multi-detector computed tomography thoracic aorta computed tomography angiography: improved luminal opacification using a patient-specific contrast protocol and caudocranial scan acquisition. J Comput Assist Tomogr 40:964–970. doi:10.1097/RCT.0000000000000456
Saade C, Bourne R, Wilkinson M, Evanoff M, Brennan PC (2013) Caudocranial scan direction and patient-specific injection protocols optimize ECG-gated and non-gated thoracic CTA. J Comput Assist Tomogr 37:725–731. doi:10.1097/RCT.0b013e31829e02b9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Chen, Y., Zhang, X., Xue, H. et al. Head and neck angiography at 70 kVp with a third-generation dual-source CT system in patients: comparison with 100 kVp. Neuroradiology 59, 1071–1081 (2017). https://doi.org/10.1007/s00234-017-1901-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1901-4