Abstract
Purpose
To determine the utility of stretched exponential diffusion model in characterisation of the water diffusion heterogeneity in different tumour stages of nasopharyngeal carcinoma (NPC).
Materials and methods
Fifty patients with newly diagnosed NPC were prospectively recruited. Diffusion-weighted MR imaging was performed using five b values (0–2,500 s/mm2). Respective stretched exponential parameters (DDC, distributed diffusion coefficient; and alpha (α), water heterogeneity) were calculated. Patients were stratified into low and high tumour stage groups based on the American Joint Committee on Cancer (AJCC) staging for determination of the predictive powers of DDC and α using t test and ROC curve analyses.
Results
The mean ± standard deviation values were DDC = 0.692 ± 0.199 (×10−3 mm2/s) for low stage group vs 0.794 ± 0.253 (×10−3 mm2/s) for high stage group; α = 0.792 ± 0.145 for low stage group vs 0.698 ± 0.155 for high stage group. α was significantly lower in the high stage group while DDC was negatively correlated. DDC and α were both reliable independent predictors (p < 0.001), with α being more powerful. Optimal cut-off values were (sensitivity, specificity, positive likelihood ratio, negative likelihood ratio) DDC = 0.692 × 10−3 mm2/s (94.4 %, 64.3 %, 2.64, 0.09), α = 0.720 (72.2 %, 100 %, −, 0.28).
Conclusion
The heterogeneity index α is robust and can potentially help in staging and grading prediction in NPC.
Key Points
• Stretched exponential diffusion models can help in tissue characterisation in nasopharyngeal carcinoma
• α and distributed diffusion coefficient (DDC) are negatively correlated
• α is a robust heterogeneity index marker
• α can potentially help in staging and grading prediction
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is endemic in Southeast Asia. Accurate staging is imperative for optimal treatment planning. Conventional magnetic resonance (MR) imaging has excellent diagnostic accuracy in the depiction and delineation of the tumour, but with lack of functional information. We then commonly rely on 18-fluoro-2-deoxy-glucose (18F-FDG) positron emission tomography with computed tomography (PET/CT) imaging but with the risk of radiation exposure. Diffusion-weighted (DW) MR imaging has been shown to be useful in the functional evaluation of NPC, allowing a noninvasive means of tumour characterisation [1]. In particular, the intravoxel incoherent motion (IVIM) biexponential diffusion model is one type of DW MR imaging which has shown promising results in tissue characterisation and treatment monitoring studies [2–4]. The IVIM biexponential model is advantageous over the conventional monoexponential model in that it allows separation of pure diffusion and perfusion properties, and provides their estimates [5]. While pure diffusion had been shown to be a reliable predictor with high reproducibility, the reliability and reproducibility of the perfusion estimates have been a major concern [6].
The stretched exponential diffusion model is an alternative model to characterise the diffusion signal in a multicompartment model using the Kohlrausch decay function [7–9]. It provides a measure of signal deviation from the monoexponential behaviour caused by pseudoperfusion effects, hence giving information on diffusion and intravoxel heterogeneity. It was thought to be a more suitable model for diffusion-weighted signal decay due to the heterogeneous environment of the spins [10]. In addition, the parameters derived had been shown to be more reliable and reproducible [11]. However, this type of diffusion model has yet to be properly tested in NPC so far.
In this study, we aimed to determine the potential utility of the stretched exponential diffusion model in the characterisation of the diffusion heterogeneity in different tumour stages of NPC, hence its ability in the prediction of tumour staging and prognostication.
Materials and methods
Patient selection
A prospective study was carried out after institutional review board approval with written informed consent was obtained. Patients with newly diagnosed biopsy-proven NPC seen in our institution from May 2013 to March 2014 were consecutively recruited. The main inclusion criterion was patients with first episode of newly diagnosed histologically proven NPC. Major exclusion criteria included (a) prior treatment of any form given already; (b) concurrent head and neck diseases or other tumours.
A total of 53 patients with newly diagnosed histologically proven undifferentiated NPC were initially recruited. Other histological subtypes of NPC were not identified in our cohort. Three patients were later excluded because of small tumour volumes (<30.00 mm3) or motion artefacts with resultant reduced signal-to-noise ratio (SNR) in region-of-interest (ROI) analysis of DW images. Therefore, a total of 50 patients (40 male, 10 female; mean age 53.04 ± 12.61 years, range 26–83 years) were subsequently included. American Joint Committee on Cancer (AJCC) and TNM stages for all patients were then devised by three independent clinical oncologists subspecialized in NPC, based on available clinical and imaging findings (MR imaging of the nasopharynx and neck, computed tomography scan of the thorax and abdomen or whole body 18F-FDG PET/CT). Any discrepancy was resolved after subsequent consensus. The clinical demographic data and distribution of the AJCC and TNM stages of all patients are listed in Table 1.
Conventional MR imaging techniques
All MR imaging was performed with a 3.0-T MR scanner (Achieva 3.0T, Philips Healthcare, Best, the Netherlands), utilizing a 16-channel neurovascular coil. Four standard sequences were performed: (a) axial T1-weighted turbo spin echo (TSE) [repetition time/echo time (TR/TE), 454/9.2 ms; turbo factor, 3; FOV, 230 × 230 mm; matrix, 672 × 672; slice number, 32; slice thickness, 3 mm; intersection gap, 0.3 mm]; (b) axial T2-weighted short TI inversion recovery (STIR) [TR/TE, 4,644/60 ms; FOV, 230 × 230 mm; matrix, 672 × 672; slice number, 32; slice thickness, 3 mm; intersection gap, 0.3 mm]; (c) coronal T2-weighted STIR [TR/TE, 4,644/60 ms; FOV, 230 × 230 mm; matrix, 480 × 480; slice number, 32; slice thickness, 3 mm; intersection gap, 0.3 mm]; (d) 3D T1-weighted turbo-field-echo (TFE) post-contrast scan [TR/TE, 4.8/2.4 ms; flip angle, 10°; FOV, 230 × 230 mm; matrix, 640 × 640; slice number, 319; slice thickness, 0.72 mm]. Intravenous bolus injection of gadopentetate dimeglumine (0.1 mmol/kg body weight) was administered at 1.5 mL/s for post-contrast acquisition after the DW MR sequence was performed.
Diffusion-weighted MR imaging
DW MR imaging was performed utilizing a single-shot spin-echo echo-planar imaging sequence, with the following parameters: TR/TE, 7,996/43 ms; echo-planar imaging factor, 35; sensitivity encoding factor, 3.5; FOV, 230 × 230 mm; slice thickness, 3 mm; intersection gap, 0.3 mm; matrix, 256 × 256; receiver bandwidth, 2,735.7 Hz/pixel; motion probing gradients in three orthogonal axes; number of signal averages, 3; parallel imaging (SENSitivity Encoding [SENSE]) factor, 3; spectral presaturation inversion recovery (SPIR) fat suppression. Image acquisition was performed in the axial plane covering the nasopharynx, with 18 slices obtained. A total of five b values were used: 0, 500, 1,000, 2,000 and 2,500 s/mm2. The scanning time was about 7 min.
Image and data analysis
Individual DW images for the three directions were automatically averaged, and the averaged DW images were then included for subsequent analysis. The stretched exponential model was introduced by Bennett et al. [7, 8] and described by the following formula:
where S(b) is the signal magnitude with diffusion weighting b, S 0 is the signal magnitude with no diffusion weighting, b is the b value, α is the intravoxel water diffusion heterogeneity (ranging between 0 and 1) and DDC is the distributed diffusion coefficient reflecting the mean intravoxel diffusion rate. α is a relatively new parameter and corresponds to the heterogeneity index. When α = 1, it points towards low intravoxel diffusion heterogeneity; when α = 0, it signifies high intravoxel diffusion heterogeneity related to multiexponential decay.
All image analysis were performed on in-house software developed in IDL 6.3 (ITT Visual Information Solutions, Boulder, CO) and fitted on a pixel-by-pixel basis using a nonlinear least-squares routine to create isotropic α and DDC maps [7–9]. Matlab (The MathWorks, Natick, Massachusetts) was used to label voxels with low SNR as “not-a-number” and they were removed from subsequent analysis. Only pixels with signals safely above background noises were included for calculation.
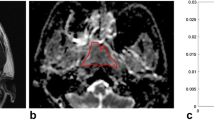
An ROI was manually drawn by a radiologist (specialized in head and neck radiology) to contour the border of NPC on each slice in order to obtain the total tumour volume. The total tumour volume was then calculated on the basis of the segmentation method. Each ROI was contoured on STIR T2W images (Fig. 1a) and co-registered to DW images (Fig. 1b) for analysis using Image J (NIH, Bethesda, MD). Each lesion was measured twice in two separate sessions at 2 weeks apart to ensure reproducibility. The obtained parameters for each lesion were then calculated on a pixel-by-pixel basis and expressed as means of the whole volume analysed.
Statistical analysis
Patients were stratified into two groups for statistical analysis by combining AJCC stage I and II (n = 14) into a low stage group, and AJCC stage III and IV (n = 36) into a high stage group. This was because of the small patient numbers in stage I (n = 5) and stage II (n = 9) diseases.
Univariate analysis using Student’s t test was carried out to compare α and DDC between the low and high stage groups. Box–whisker plots were obtained to illustrate the group-wise differences. Receiver operating characteristic (ROC) curves were generated with respective cut-off values determined to accommodate the best diagnostic accuracy based on the Youden index. All statistical analyses were performed by SPSS version 21 for windows. A p value of less than 0.05 was considered statistically significant.
Results
The intraobserver agreement was good with a kappa value (κ) for α and DDC of 0.81 and 0.77 respectively. The mean tumour volume was 1,334.29 ± 1,978.01 mm3 (53.2–10,098.60). The mean values of α and DDC in the low and high stage groups for AJCC staging are summarized in Table 2 and represented in respective box plots (Fig. 2a and b). Their respective optimal cut-off values upon ROC curve analyses are summarized in Table 3 and shown in Fig. 3.
DDC was statistically significantly higher in the high stage group while α was statistically lower in the high stage group as compared with the low stage group. The mean ± standard deviation (SD) values were as follows: DDC = 0.692 ± 0.199 (×10−3 mm2/s) for the low stage group vs 0.794 ± 0.253 (×10−3 mm2/s) for high stage group (p < 0.001); α = 0.792 ± 0.145 for the low stage group vs 0.698 ± 0.155 for the high stage group (p < 0.001). On the basis of the ROC curve analysis (Fig. 3), their optimal cut-off values (with respective sensitivity, specificity, positive likelihood ratio, negative likelihood ratio and area under curve) were DDC = 0.692 × 10−3 mm2/s (94.4 %, 64.3 %, 2.64, 0.09, 0.833); α = 0.720 (72.2 %, 100 %, −, 0.28, 0.911). Stepwise regression analysis revealed that both the DDC (p < 0.001) and α (p < 0.001) were reliable independent predictors. On the basis of the area under curve (AUC), α was the more powerful parameter, though no statistical significance could be reached on direct comparison between DDC and α (p = 0.125).
Discussion
DW MR imaging has received much attraction in the evaluation and characterisation of tumour behaviours with regard to its water diffusivity, showing promising results, in particular with various different types of head and neck tumours especially NPC. Different diffusion models had been devised to better account for the complicated non-Gaussian diffusion behaviour of biological tissues, as well as providing additional information on different aspects of tissue properties [12–14]. IVIM diffusion MR imaging had been shown to be useful in the functional assessment and characterisation of NPC, providing one with the pure diffusion and perfusion properties. The stretched exponential diffusion model is an alternate method that can provide us with the tissue heterogeneity and diffusion simultaneously. It was found to be more reliable and reproducible than the monoexponential and biexponential models [11] and was shown to be successful in tissue characterisation in brain and prostate tumours [11, 15]. In particular, Kwee et al. [15] found that high grade glioma had a significantly lower α value than that of the normal brain structure, suggesting its usefulness in the assessment of histological heterogeneity and hence potential grading of tumours. This type of diffusion model, however, has received little attention in head and neck tumours and has not been properly assessed in NPC so far.
Our results showed that the stretched exponential diffusion model with utilization of higher b values was feasible in the evaluation of NPC. It was also advantageous in that it provided with us the new parameter α, reflecting the tissue heterogeneity, which characterised deviation of the signal attenuation from monoexponential form. α was found to be statistically significantly lower in the high stage group of NPC when compared to the low stage group of NPC. This signifies considerable tumoral or histological heterogeneity in high stage tumours, which is likely related to a higher degree of cellular pleomorphism, presence of intravoxel microscopic necrotic foci, and intravoxel heterogeneity in vascular structures [15]. On the contrary, DDC was found to be statistically significantly higher in the high stage group of NPC and negatively correlated with α. This is in concordance with past studies showing an inverse correlation between tumour cellularity and tumour diffusion coefficient [15, 16]. Nevertheless, this inverse relationship warrants further larger cohort study for confirmation. Histopathological correlation would also be helpful in understanding their relationship. α was a more reliable independent predictor and with a cut-off value of 0.72 it was able to differentiate between low and high stage groups (sensitivity 72.2 %, specificity 100 %).
One major limitation of this study was the lack of histopathological correlation with the heterogeneity index in both the low and high stage groups of NPC. Inclusion of other histological subtypes of NPC would be of great interest to test for the ability of the heterogeneity index in their differentiation. Data reproducibility is another major concern [17]. The use of the very high b values required in this stretched exponential diffusion model could lead to impaired SNR. The highest b value (2,500 s/mm2) chosen in our study was based on the adequate and satisfactorily obtained SNR above background noise in the region of the nasopharynx. Because of the poor SNR, NPC with small tumour volume had to be excluded because of inadequate SNR within the small fraction of voxel. In the present study, the evaluation of reproducibility was limited to repeated measurements within one single acquisition only. Further study focusing on the reproducibility from different acquisitions within the short term should be carried out to establish its clinical utility as a quantitative tool. A larger cohort study would be helpful to further consolidate our preliminary findings. Despite these drawbacks, our promising results have shed light on this stretched exponential diffusion model as a potential new method in the evaluation of NPC. In addition, it can also potentially help in treatment monitoring and in post-treatment assessment by studying serial changes in both DDC and α.
In conclusion, the stretched exponential diffusion model is feasible and useful in the noninvasive tissue characterisation of NPC. The heterogeneity index α is robust and can potentially help in staging and grading prediction.
Abbreviations
- α:
-
Alpha (intravoxel water diffusion heterogeneity)
- AJCC:
-
American Joint Committee on Cancer
- AUC:
-
Area under curve
- DDC:
-
Distributed diffusion coefficient
- DW:
-
Diffusion-weighted
- F-FDG:
-
18-fluoro-2-deoxyglucose
- IVIM:
-
Intravoxel incoherent motion
- MR:
-
Magnetic resonance
- NPC:
-
Nasopharyngeal carcinoma
- PET/CT:
-
Positron emission tomography with computed tomography
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region-of-interest
- SD:
-
Standard deviation
- SNR:
-
Signal-to-noise ratio
- SPIR:
-
Spectral presaturation inversion recovery
- STIR:
-
Short TI inversion recovery
- TFE:
-
Turbo-field-echo
- TR/TE:
-
Repetition time/echo time
- TSE:
-
Turbo spin echo
References
Lai V, Khong PL (2014) Updates on MR imaging and 18F-FDG PET/CT imaging in nasopharyngeal carcinoma. Oral Oncol 50:539–548
Lai V, Li X, Lee VHF, Lam KO, Chan Q, Khong PL (2013) Intravoxel incoherent motion MR imaging: comparison of diffusion and perfusion characteristics between nasopharyngeal carcinoma and post-chemoradiation fibrosis. Eur Radiol 23:2793–2801
Lai V, Li X, Lee VHF et al (2014) Nasopharyngeal carcinoma: comparison of diffusion and perfusion characteristics between different tumour stages using intravoxel incoherent motion MR imaging. Eur Radiol 24:176–183
Hauser T, Essig M, Jensen A et al (2013) Characterization and therapy monitoring of head and neck carcinomas using diffusion-imaging-base intravoxel incoherent motion parameters – preliminary results. Neuroradiology 55:527–536
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497–505
Riches SF, Hawtin K, Charles-Edwards EM, de Souza NM (2009) Diffusion-weighted imaging of the prostate and rectal wall: comparison of biexponential and monoexponential modeled diffusion and associated perfusion coefficients. NMR Biomed 22:318–325
Bennett KM, Schmainda KM, Bennett RT, Rowe DB, Lu H, Hyde JS (2003) Characterization of continuously distributed cortical water diffusion rates with a stretched-exponential model. Magn Reson Med 50:727–734
Bennett KM, Hyde JS, Rand SD et al (2004) Intravoxel distribution of DWI decay rates reveals C6 glioma invasion in rat brain. Magn Reson Med 52:994–1004
Bennett KM, Hyde JS, Schmainda KM (2006) Water diffusion heterogeneity index in the human brain is insensitive to the orientation of applied magnetic field gradients. Magn Reson Med 56:235–239
Hall MG, Barrick TR (2008) From diffusion-weighted MRI to anomalous diffusion imaging. Magn Reson Med 59:447–455
Mazaheri Y, Afaq A, Rowe DB, Lu Y, Shukla-Dave A, Grover J (2012) Diffusion-weighted magnetic resonance imaging of the prostate: improved robustness with stretched exponential modeling. J Comput Assist Tomogr 36:695–703
Lu Y, Jansen JF, Mazaheri Y, Stambuk HE, Koutcher JA, Shukla-Dave A (2012) Extension of the intravoxel incoherent motion model to non-gaussian diffusion in head and neck cancer. J Magn Reson Imaging 36:1088–1096
Jansen JF, Stambuk HE, Koutcher JA, Shukla-Dave A (2010) Non-gaussian analysis of diffusion-weighted MR imaging in head and neck squamous cell carcinoma: a feasibility study. AJNR Am J Neuroradiol 31:741–748
Vandecaveye V, De Keyzer F, Dirix P, Lambrecht M, Nuyts S, Hermans R (2010) Applications of diffusion-weighted magnetic resonance imaging in head and neck squamous cell carcinoma. Neuroradiology 52:773–784
Kwee T, Galban CJ, Tsien C et al (2010) Intravoxel water diffusion heterogeneity imaging of human high-grade gliomas. NMR Biomed 23:179–187
Provenzale JM, Mukundan S, Barboriak DP (2006) Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology 239:632–649
Braithwaite AC, Dale BM, Boll DT, Merkle EM (2009) Short- and midterm reproducibility of apparent diffusion coefficient measurements at 3.0-T diffusion-weighted imaging of the abdomen. Radiology 250:459–465
Acknowledgments
The scientific guarantor of this publication is Prof. Khong Pek Lan. The authors of this manuscript declare relationships with the following companies: Dr. Q Chan is currently employed by Philips Medial Systems. This study has received funding by University Grants Council (UGC) seed funding from The University of Hong Kong, project no. 201112159010.
No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Approval from the institutional animal care committee was not required because this study did not involve animals. Study subjects or cohorts have not been previously reported.
Methodology: prospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, V., Lee, V.H.F., Lam, K.O. et al. Intravoxel water diffusion heterogeneity MR imaging of nasopharyngeal carcinoma using stretched exponential diffusion model. Eur Radiol 25, 1708–1713 (2015). https://doi.org/10.1007/s00330-014-3535-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3535-9