Abstract
Objectives
MRI can detect early-stage nasopharyngeal carcinoma (NPC), but the detection is more challenging in early-stage NPCs because they must be distinguished from benign hyperplasia in the nasopharynx. This study aimed to determine whether intravoxel incoherent motion diffusion-weighted imaging (IVIM DWI) MRI could distinguish between these two entities.
Methods
Thirty-four subjects with early-stage NPC and 30 subjects with benign hyperplasia prospectively underwent IVIM DWI. The mean pure diffusion coefficient (D), pseudo-diffusion coefficient (D*), perfusion fraction (f) and apparent diffusion coefficient (ADC) values were calculated for all subjects and compared between the 2 groups using Student’s t test. Receiver operating characteristics with the area under the curve (AUC) was used to identify the optimal threshold for all significant parameters, and the corresponding diagnostic performance was calculated. A p value of < 0.05 was considered statistically significant.
Results
Compared with benign hyperplasia, early-stage NPC exhibited a significantly lower D mean (0.64 ± 0.06 vs 0.87 ± 0.11 × 10−3 mm2/s), ADC0–1000 mean (0.77 ± 0.08 vs 1.00 ± 0.13 × 10−3 mm2/s), ADC300–1000 (0.63 ± 0.05 vs 0.86 ± 0.10 × 10−3 mm2/s) and a higher D* mean (32.66 ± 4.79 vs 21.96 ± 5.21 × 10−3 mm2/s) (all p < 0.001). No significant difference in the f mean was observed between the two groups (p = 0.216). The D and ADC300–1000 mean had the highest AUC of 0.985 and 0.988, respectively, and the D mean of < 0.75 × 10−3 mm2/s yielded the highest sensitivity, specificity and accuracy (100%, 93.3% and 96.9%, respectively) in distinguishing early-stage NPC from benign hyperplasia.
Conclusion
DWI has potential to distinguish early-stage NPC from benign hyperplasia and D and ADC300–1000 mean were the most promising parameters.
Key Points
• Diffusion-weighted imaging has potential to distinguish early-stage nasopharyngeal carcinoma from benign hyperplasia in the nasopharynx.
• The pure diffusion coefficient, pseudo-diffusion coefficient from intravoxel incoherent motion model and apparent diffusion coefficient from conventional diffusion-weighted imaging were significant parameters for distinguishing these two entities in the nasopharynx.
• The pure diffusion coefficient, followed by apparent diffusion coefficient, may be the most promising parameters to be used in screening studies to help detect early-stage nasopharyngeal carcinoma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Benign hyperplasia causes generalised thickening of the mucosa/submucosa along the nasopharyngeal walls and enlargement of the adenoid and is a common incidental finding on MRI of the adult head and neck, as well as on brain imaging [1]. Benign hyperplasia may need to be distinguished from early-stage nasopharyngeal carcinoma (NPC) confined to the nasopharynx. This is especially relevant in NPC endemic regions because the current trend of using plasma Epstein–Barr virus (EBV) DNA to screen asymptomatic subjects for NPC has led to an increase in the number of tumours detected at an early stage [2]. In most cases, these entities can be distinguished using anatomical images; lymphoid hyperplasia causes diffuse wall thickening symmetrically involving both sides of the nasopharynx, and when there is hyperplasia of the adenoid, the adenoidal stripes tend to be preserved, whereas early-stage NPC tends to cause a focal mass and loss or distortion of the adenoidal stripes [1, 3, 4]. However, in a small number of patients, lymphoid hyperplasia may cause asymmetrical thickening and the adenoidal stripes may not be identified, especially if the scan is not a dedicated scan of the nasopharynx. Furthermore, we have shown recently that 12% of stage T1 NPCs cause diffuse symmetrical thickening of the nasopharyngeal walls similar to that in benign hyperplasia [4].
The ability of MRI to accurately distinguish benign hyperplasia from NPC is also becoming more important because MRI is used more frequently to detect the 10% of NPCs that are occult on the endoscopic examination [3]. The detection of NPC by MRI is even more challenging in the screening setting, wherein plasma markers such as EBV DNA are used to identify early-stage NPCs in asymptomatic subjects [2].
Diffusion-weighted imaging (DWI) can distinguish benign from malignant head and neck tumours [5] and intravoxel incoherent motion (IVIM) DWI may be superior to conventional DWI for tissue characterisation, as the former can distinguish the process of pure diffusion from microcirculation in the capillary network. Indeed, an early study reported that IVIM DWI could distinguish NPC from benign adenoids in the nasopharynx [6]. If IVIM DWI is useful in the screening setting, it would not only improve the diagnostic performance of the current MRI protocol but potentially could replace the T1 contrast-enhanced scan which could expand the use of MRI.
Therefore, this preliminary study hypothesised that DWI has the potential to distinguish benign hyperplasia from early-stage NPC. This study was designed to examine whether IVIM-derived parameters, together with apparent diffusion coefficient (ADC) obtained from conventional DWI, are able to distinguish benign hyperplasia from early-stage NPC. The second part of the study compared diagnostic performance of these DW parameters to examine whether IVIM DWI had advantages over the conventional DWI in distinguishing between these two entities in the nasopharynx.
Methods
Patients
This prospective study was performed under approval from the local institutional review board. The patients were ethnically Chinese who were recruited between March 2015 and July 2018, and written informed consent was obtained from all patients.
Patients with early-stage NPC
Consecutive symptomatic and asymptomatic patients who met the following criteria were included: (1) newly diagnosed, biopsy-proven undifferentiated NPC; (2) primary tumour confined to the nasopharynx (stage T1) on MRI without superficial spread to the nasal cavity or oropharynx or deep spread within the nasopharynx; and (3) a primary tumour size > 5 mm.
Patients with benign hyperplasia
Consecutive asymptomatic patients who were undergoing follow-up investigations for benign hyperplasia were recruited from a previous NPC detection study with persistently raised plasma EBV DNA [2] who met the following criteria: (1) did not have NPC after the initial endoscopic examination and contrast-enhanced MRI and showed no change suggestive of NPC on the follow-up endoscopic examination and non-contrast-enhanced MRI two years later; (2) follow-up MRI examination showed benign hyperplasia > 5 mm in thickness.
MRI acquisition
MRI was performed using a Philips Achieva TX 3.0-T scanner (Philips Healthcare) with a body coil for radiofrequency transmission and a 16-channel Philips neurovascular phased-array coil for reception. IVIM DWI was acquired using a fat-suppressed, single-shot, spin-echo, echo-planar imaging sequence (number of slices: 9, repetition time/echo time, 2000/50 ms; field of view, 230 mm × 230 mm; section thickness, 4 mm; echo train length, 55; sensitivity encoding factor, 2; number of signals acquired, 4) and 14 b-values (0, 20, 40, 50, 60, 80, 100, 200, 300, 400, 500, 600, 800 and 1000 s/mm2). The total IVIM DWI scan time was 2 min, 30 s.
Anatomical MRI in all patients included a fat-suppressed T2-weigthed turbo-spin-echo sequence and a T1-weighted turbo-spin-echo sequence. The patients with early-stage NPC were also scanned using a T1-weighted turbo-spin-echo sequence following a bolus injection of 0.1 mmol of gadoteric acid (Dotarem; Guerbet) per kilogramme of body weight.
Imaging analysis
Olea Sphere (version 3.0; Olea Medical SA) was used for all the post-processing steps by implementing a Bayesian probability–based algorithm using all 14 b-values to fit a bi-exponential diffusion model to calculate the pure diffusion coefficient (D); the pseudo-diffusion coefficient (D*), which reflects blood microcirculation in the capillary networks; and the perfusion volume fraction (f), which correlates with capillary geometry and blood velocity [7], and using six b-values (0, 200, 400, 600, 800, 1000 s/mm2) to fit a mono-exponential diffusion model to calculate the conventional ADC (ADC0–1000). In addition, b-values of 300, 400, 600, 800 and 1000 s/mm2 were used fit a mono-exponential diffusion model for the purpose of comparing conventional ADC300–1000 with D in the IVIM model. On the ADC map, the region of interest was manually drawn around the whole primary tumour or benign hyperplasia with reference to the corresponding anatomical images by a radiologist with more than 20 years of experience with specialisation in the head and neck region and a researcher with 4 years of experience of treating NP disease. The region of interest on the ADC map was then fitted automatically to the other diffusion maps. Necrotic and cystic regions were excluded from the analysis during the delineation phase. The total volume of the abnormalities in the nasopharynx was obtained by manually outlining the cross-sectional area on each slice, summating the cross-sectional areas, and multiplying by the slice thickness of 4 mm.
Statistical analysis
Distributions of DW parameters in the early-stage NPC and benign hyperplasia groups were examined using Kolmogorov–Smirnov test. Differences in the DW parameters between the two groups were compared using Student’s t test (data normally distributed) or Mann–Whitney U test (data not normally distributed). A receiver-operating characteristic curve analysis and subsequent area under the curve (AUC) calculation were used to identify the optimal thresholds for the significant DW parameters by maximising the sensitivity plus specificity, and the diagnostic performance of these thresholds was calculated and the significance of these thresholds was re-evaluated with the chi-square test. The AUCs of significant DW parameters were compared using the Delong test. The above analyses used the average values of the parameters obtained from the 2 observers. Intra-class correlation coefficients (ICCs) with 95% confidence intervals (95% CIs) were calculated to assess the inter-observer agreement regarding the IVIM-derived parameters and ADC0–1000. ICC was also calculated to assess the agreement between D from the IVIM model and ADC300–1000.
All of the statistical tests were 2-sided, and a p value < 0.05 was considered to indicate a statistically significant difference. Analyses were performed using the statistical analysis software SPSS (version 25.0; IBM) and MedCalc Statistical Software (version 14.8.1; MedCalc Software).
Results
Patients
Thirty-nine patients with early-stage NPC confined to the nasopharynx and 32 patients with benign hyperplasia were recruited. Of these, 7 were excluded because of degraded images (NPC = 5 and benign hyperplasia = 2). The characteristics of the remaining 34 patients with NPC and 30 patients with benign hyperplasia are shown in Table 1.
DW parameters
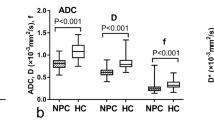
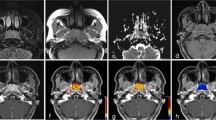
The DW parameters of subjects with early-stage NPC and benign hyperplasia are shown in Table 2. DW parameters in each group was normally distributed (p = 0.072–0.200), and compared with the subjects with benign hyperplasia; those with early-stage NPC had significantly lower D mean, ADC0–1000 mean (Fig. 1) and ADC300–1000 mean values (Fig. 2a–c, Table 2) and a higher D* mean value (Fig. 2d, Table 2). However, no significant inter-group difference was observed in the f mean values (Fig. 2e, Table 2).
Pure diffusion (D) and apparent diffusion coefficient0–1000 (ADC0–1000) maps and histograms of a representative subject with early-stage nasopharyngeal carcinoma (NPC) (a–d) and a representative subject with benign hyperplasia in the nasopharynx (e–h). The respective D mean and ADC0–1000 mean values were 0.59 × 10−3 mm2/s (b) and 0.82 × 10−3 mm2/s (d) for the subject with early-stage NPC and 0.76 × 10−3 mm2/s (f) and 0.93 × 10−3 mm2/s (h) for the subject with benign hyperplasia
Side-by-side box plots of the mean true diffusion coefficient (D), apparent diffusion coefficient0–1000 (ADC0–1000), ADC300–1000, pseudo-diffusion (perfusion) coefficient (D*) and perfusion volume fraction (f) values in subjects with early-stage nasopharyngeal carcinoma (NPC) and benign hyperplasia. Patients with early-stage NPC had a significant lower D mean (a), ADC0–1000 mean (b) and ADC300–1000 (c), and a significantly higher D* mean (d), compared to patients with benign hyperplasia. The difference in the f mean (e) between the two groups was not significant. The optimal thresholds (grey dotted lines) of D mean, ADC0–1000 mean, ADC300–1000 and D* mean for distinguishing early-stage NPC from benign hyperplasia were 0.75 × 10−3 mm2/s, 0.74 × 10−3 mm2/s, 0.86 × 10−3 mm2/s and 27.4 × 10−3 mm2/s, respectively
Table 3 and Fig. 3 present the diagnostic performance of the optimal thresholds for the above-listed significant DW parameters, as well as the AUCs of these parameters for distinguishing early-stage NPC from benign hyperplasia. Notably, the D mean was best able to distinguish these two entities in the nasopharynx, although when compared to ADC0–1000 mean and D* mean, there were no significant differences between the AUCs. Analysis of the inter-observer agreement yielded the highest ICC values for the D mean and ADC0–1000 mean (0.963 (95% CI, 0.940–0.978)), followed by the D* mean (0.953 (95% CI, 0.922–0.971)), and f mean (0.920 (95% CI, 0.868–0.951)) (all p < 0.001). The ADC300–1000 mean had a similar threshold and AUC (Table 3 and Fig. 3) (p = 0.823) to the D mean for distinguishing early-stage NPC from benign hyperplasia with a high ICC of 0.968 (95% CI, 0.948–0.981).
Analysis of areas under the curves (AUCs) of the mean values of the true diffusion coefficient (D), pseudo-diffusion coefficient (D*), apparent diffusion coefficient0–1000 (ADC0–1000) and ADC300–1000 to determine the ability to distinguish early-stage nasopharyngeal carcinoma from benign hyperplasia. The D mean and ADC300–1000 had the highest AUCs of 0.985 and 0.988, respectively (blue curve and black curve, respectively), followed by the ADC0–1000 mean (0.948; green curve) and D* mean (0.926; red curve). This suggests that the D mean and ADC300–1000 were best able to distinguish early-stage nasopharyngeal carcinoma from benign hyperplasia
Discussion
MRI has been established as the imaging modality of choice for the staging of biopsy-proven NPC [8, 9]. However, the role of this imaging modality is expanding to include the detection of endoscopically occult tumours [3, 10]. As a result of the more widespread use of plasma markers to screen for early-stage NPC, there is increasing pressure on MRI to distinguish benign hyperplasia from early-stage cancers which do not have other features such as deep spread or nodal metastases to suggest malignancy on MRI. Therefore, an MRI sequence that could improve the diagnostic performance of anatomical imaging and even replace contrast-enhanced images would be advantageous in the screening setting.
In this study, the mean values of the IVIM-derived parameters D and D*, but not f, could successfully distinguish early-stage NPC from benign hyperplasia. D mean was significantly lower and D* significantly higher in NPC than in benign hyperplasia suggesting NPC has hyper-cellularity, smaller extra-cellular space and more vascularity than benign hyperplasia. Of these two parameters, the D mean yielded the best diagnostic performance and reproducibility, but ADC0–1000 from a conventional DWI was also useful. Similar to D, subjects with early-stage NPC had a significantly lower ADC0–1000 than those with benign hyperplasia, suggesting that pure diffusion, compared with microcirculation, more strongly influences the ADC0–1000 value. Although the accuracy of ADC0–1000 mean was slightly lower than that of D, conventional DWI is still an acceptable alternative to IVIM DWI for patients, especially for those patients unable to tolerate the longer time scan. Scan time could be further reduced by limiting the conventional DWI to only those b-values between 300 and 1000 s/mm2 to acquire the ADC300–1000 which we found provided similar results to the D mean from the IVIM model. Our DWI results suggest that biopsies targeted to sites with a low D mean or low ADC300–1000 mean should have a high sensitivity for NPC, although a small number will be false positive because of an overlap in the mean values with benign hyperplasia. In addition, a turbo-spin-echo sequence, rather than an echo-planar sequence, can be used during conventional DW image acquisition. This is advantageous because the turbo-spin-echo sequence reduces susceptibility artefacts in the nasopharynx [11, 12].
DWI aids for the MRI-based characterisation of tissues in the head and neck. Most studies have reported lower ADC values in malignant tumours relative to benign processes, which reflects the more restricted diffusion in the former lesions [5]. More recently, IVIM studies in the head and neck have reported lower D values in malignant tumours relative to benign pathologies [6, 13,14,15,16,17,18,19], including comparisons of NPC with benign hyperplasia [6], which are consistent with our findings. The previously reported values for D in NPC [6, 18,19,20,21,22,23,24] and benign hyperplasia [6, 25] were also similar to those in our study. This similarity is encouraging given that our prospective study of consecutive patients in a screening setting included those with small volumes of NPC and benign hyperplasia. The other two IVIM parameters, D* and f, are likely less robust, which is supported by the greater range of values reported in studies of the head and neck [26], including NPC [6, 18,19,20,21,22,23,24].
This study had several limitations. Firstly, we were unable to assess whether there are differences in IVIM parameters between the two patterns of contrast enhancement in benign hyperplasia, because at the time of this IVIM study we only obtained plain scans in our patients who were undergoing follow-up MRI. Secondly, although the D mean yielded a superior diagnostic performance relative to the ADC0–1000 mean and D* mean, these differences did not reach statistical significance, likely due to the small sample size. Thirdly, the optimal thresholds obtained in this preliminary study will require validation in an independent data set before implementation in clinical practice.
Conclusion
In conclusion, the mean IVIM values of D and D* but not f, together with the mean ADC0–1000 and ADC300–1000 values obtained from conventional DWI, were identified as potential DW parameters that could distinguish early-stage NPC from benign hyperplasia in the nasopharynx. NPC was associated with a lower D, ADC0–1000, ADC300–1000 and a higher D* compared with benign hyperplasia, with the D and ADC300–1000 being the most promising parameters for use in the screening setting.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- D :
-
Pure diffusion coefficient
- D*:
-
Pseudo-diffusion coefficient
- DWI:
-
Diffusion-weighted imaging
- EBV:
-
Epstein–Barr virus
- f :
-
Perfusion volume fraction
- ICC:
-
Intra-class correlation coefficient
- IVIM:
-
Intravoxel incoherent motion
- NPC:
-
Nasopharyngeal carcinoma
References
Bhatia KSS, King AD, Vlantis AC et al (2012) Nasopharyngeal mucosa and adenoids: appearance at MR imaging. Radiology 263:437–443
Chan KCA, Woo JKS, King A et al (2017) Analysis of plasma Epstein–Barr virus DNA to screen for nasopharyngeal Cancer. N Engl J Med 377:513–522
King AD, Vlantis AC, Bhatia KSS et al (2011) Primary nasopharyngeal carcinoma : diagnostic accuracy of MR imaging versus that of endoscopy and endoscopic biopsy. Radiology 258:531–537
King AD, Wong LYS, Law BKH et al (2018) MR imaging criteria for the detection of nasopharyngeal carcinoma: discrimination of early-stage primary tumors from benign hyperplasia. AJNR Am J Neuroradiol 39:515–523
Thoeny HC, De Keyzer F, King AD (2012) Diffusion-weighted MR imaging in the head and neck. Radiology 263:19–32
Zhang SX, Jia QJ, Zhang ZP et al (2014) Intravoxel incoherent motion MRI: emerging applications for nasopharyngeal carcinoma at the primary site. Eur Radiol 24:1998–2004
Le Bihan D, Breton E, Lallemand D et al (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497–505
King AD, Lam WWM, Leung SF et al (1999) MRI of local disease in nasopharyngeal carcinoma: tumour extent vs tumour stage. Br J Radiol 72:734–741
King AD, Bhatia KSS (2010) Magnetic resonance imaging staging of nasopharyngeal carcinoma in the head and neck. World J Radiol 2:159
King AD, Vlantis AC, Yuen TWC et al (2015) Detection of nasopharyngeal carcinoma by MR imaging: diagnostic accuracy of MRI compared with endoscopy and endoscopic biopsy based on long-term follow-up. AJNR Am J Neuroradiol 36:2380–2385
Yoshida T, Urikura A, Shirata K et al (2016) Image quality assessment of single-shot turbo spin echo diffusion-weighted imaging with parallel imaging technique: a phantom study. Br J Radiol 89:20160512
Mikayama R, Yabuuchi H, Sonoda S et al (2018) Comparison of intravoxel incoherent motion diffusion-weighted imaging between turbo spin-echo and echo-planar imaging of the head and neck. Eur Radiol 26:316–324
Sakamoto J, Imaizumi A, Sasaki Y et al (2014) Comparison of accuracy of intravoxel incoherent motion and apparent diffusion coefficient techniques for predicting malignancy of head and neck tumors using half-Fourier single-shot turbo spin-echo diffusion-weighted imaging. Magn Reson Imaging 32:860–866
Liang L, Luo X, Lian Z et al (2017) Lymph node metastasis in head and neck squamous carcinoma: efficacy of intravoxel incoherent motion magnetic resonance imaging for the differential diagnosis. Eur J Radiol 90:159–165
Sumi M, Van Cauteren M, Sumi T et al (2012) Salivary gland tumors: use of Intravoxel incoherent motion MR imaging for assessment of diffusion and perfusion for the differentiation of benign from malignant tumors. Radiology 263:770–777
Tan H, Chen J, Zhao Y et al (2018) Feasibility of intravoxel incoherent motion for differentiating benign and malignant thyroid nodules. Acad Radiol. https://doi.org/10.1016/j.acra.2018.05.011
Shen J, Xu X, Su G, Hu H (2018) Intravoxel incoherent motion magnetic resonance imaging of the normal-appearing parotid glands in patients with differentiated thyroid cancer after radioiodine therapy. Acta Radiol 59:204–211
Lai V, Li X, Ho V et al (2013) Intravoxel incoherent motion MR imaging : comparison of diffusion and perfusion characteristics between nasopharyngeal carcinoma and post-chemoradiation fibrosis. Eur Radiol 23:2793–2801
Mao J, Shen J, Yang Q (2016) Intravoxel incoherent motion MRI in differentiation between recurrent carcinoma and postchemoradiation fibrosis of the skull base in patients with nasopharyngeal carcinoma. J Magn Reson Imaging 44:1556–1564
Jia QJ, Zhang SX, Chen WB et al (2014) Initial experience of correlating parameters of intravoxel incoherent motion and dynamic contrast-enhanced magnetic resonance imaging at 3.0 T in nasopharyngeal carcinoma. Eur Radiol 24:3076–3087
Lai V, Li X, Lee VHF et al (2014) Nasopharyngeal carcinoma: comparison of diffusion and perfusion characteristics between different tumour stages using intravoxel incoherent motion MR imaging. Eur Radiol 24:176–183
Xiao Y, Pan J, Chen Y et al (2015) Intravoxel incoherent motionmagnetic resonance imaging as an early predictor of treatment response to neoadjuvant chemotherapy in locoregionally advanced nasopharyngeal carcinoma. Medicine (Baltimore) 94:e973
Xiao-Ping Y, Jing H, Fei-Ping L et al (2016) Intravoxel incoherent motion MRI for predicting early response to induction chemotherapy and chemoradiotherapy in patients with nasopharyngeal carcinoma. J Magn Reson Imaging 43:1179–1190
Yu X, Hou J, Li F et al (2016) Quantitative dynamic contrast-enhanced and diffusion-weighted MRI for differentiation between nasopharyngeal carcinoma and lymphoma at the primary site. Dentomaxillofac Radiol 45:20150317
Surov A, Ryl I, Bartel-Friedrich S et al (2015) Diffusion weighted imaging of nasopharyngeal adenoid hypertrophy. Acta Radiol 56:587–591
Noij DP, Martens RM, Marcus JT et al (2017) Intravoxel incoherent motion magnetic resonance imaging in head and neck cancer: a systematic review of the diagnostic and prognostic value. Oral Oncol 68:81–91
Funding
This study has received funding by the Research Grant Council of Hong Kong. The work described in this paper was partially supported by grants from the Research Grants council of the Hong Kong Special Administrative Region, China (Project No. 14107216 and SEG_CUHK02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ann D King.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in New England Journal of Medicine entitled “Analysis of Plasma Epstein-Barr Virus DNA to Screen for Nasopharyngeal Cancer” (doi: https://doi.org/10.1056/NEJMoa1701717).
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ai, QY., King, A.D., Chan, J.S.M. et al. Distinguishing early-stage nasopharyngeal carcinoma from benign hyperplasia using intravoxel incoherent motion diffusion-weighted MRI. Eur Radiol 29, 5627–5634 (2019). https://doi.org/10.1007/s00330-019-06133-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06133-8