Abstract
Rationale
We aimed to test the interscan variation of semi-automatic volumetry of subsolid nodules (SSNs), as growth evaluation is important for SSN management.
Methods
From a lung cancer screening trial all SSNs that were stable over at least 3 months were included (N = 44). SSNs were quantified on the baseline CT by two observers using semi-automatic volumetry software for effective diameter, volume, and mass. One observer also measured the SSNs on the second CT 3 months later. Interscan variation was evaluated using Bland-Altman plots. Observer agreement was calculated as intraclass correlation coefficient (ICC). Data are presented as mean (± standard deviation) or median and interquartile range (IQR). A Mann-Whitney U test was used for the analysis of the influence of adjustments on the measurements.
Results
Semi-automatic measurements were feasible in all 44 SSNs. The interscan limits of agreement ranged from -12.0 % to 9.7 % for diameter, -35.4 % to 28.6 % for volume and -27.6 % to 30.8 % for mass. Agreement between observers was good with intraclass correlation coefficients of 0.978, 0.957, and 0.968 for diameter, volume, and mass, respectively.
Conclusion
Our data suggest that when using our software an increase in mass of 30 % can be regarded as significant growth.
Key Points
• Recently, recommendations regarding subsolid nodules have stressed the importance of growth quantification.
• Volumetric measurement of subsolid nodules is feasible with good interscan agreement.
• Increase of mass of 30 % can be regarded as significant growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lung cancer screening with computed tomography (CT) has increased the awareness of a specific subtype of pulmonary nodules, the subsolid nodule (SSN). An SSN is defined as a circumscribed area of increased lung attenuation with preservation of the bronchial and vascular margins. It is also referred to as a ground glass opacity [1]. An SSN can be part-solid (part of the nodule completely obscures the underlying lung parenchyma) or pure nonsolid. Persistent SSNs have a high likelihood of malignancy. The ELCAP study [2] reported a malignancy rate of 34 % for all SSNs, 18 % for nonsolid nodules and 63 % for part-solid SSNs. Others reported even higher malignancy rates for part-solid lesions up to 75 % [3].
Recently, a statement from the Fleischner Society with recommendations for the management of SSNs detected at computed tomography (CT) was published [4]. It was recommended that because most nonsolid solitary SSNs prove either to be benign or to represent isolated foci of atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), or minimal invasive adenocarcinoma (MIA), close monitoring of persistent nonsolid nodules is appropriate. A monitoring strategy can obviate unnecessary surgery and potentially avoid overdiagnosis in cases in which no change is identified. Close monitoring should also allow identification of growing lesions that prove to be adenocarcinomas manifesting as SSNs. For close monitoring reproducible measurements are mandatory and knowledge on the interscan error is required in order to define growth.
For that purpose, volumetric and automated measurements are preferable to diameter and manual measurements [5]. In a previous study, two observers manually segmented SSNs in order to assess their volume and mass, which took about 10 min per nodule [5]. Recently, software has become available for semi-automatic segmentation of SSNs in which these nodules are segmented within a few seconds [6].
The current data on interscan variability of SSNs are limited [7, 8]. Park et al [7] reported on the interscan variability of volume and attenuation of SSNs, but included only nonsolid SSNs. Kim et al [8] reported on measurement variability of volume and mass in nonsolid and part-solid nodules, but they excluded part-solid nodules with a solid component greater than 5 mm.
The aim of the current study was to the test interscan variation and interobserver agreement of SSN nodule of semi-automated measurements of diameter, volume, and mass in order to establish the percentage of change indicative of significant growth.
Methods
Study participants
This is an ancillary study of the Dutch-Belgian Lung Cancer Screening Trial (NELSON trial; ISRCTN63545820). The NELSON trial was approved by the Dutch Ministries of Health and by the ethical review board of the participating hospitals. Written informed consent was obtained from each participant. The trial population comprised current or former smokers between 50 and 75 years old at time of inclusion with a smoking history of at >15 cigarettes/day during >25 years or >10 cigarettes/day during >30 years. Former smokers were included only if they quit smoking ≤10 years before the start of the study. Exclusion criteria for participating in the lung cancer screening trial were self-reported moderate or poor health status and/or inability to climb two flights of stairs, a chest CT in the 12 months before inclusion, and body weight ≥140 kg. Other exclusion criteria were a current or previous history of cancer in the last 5 years, a history of melanomas, breast cancer, or hypernephroma anytime, or a previous pneumonectomy.
Participants were randomized to the screening arm (screening with low-dose CT) or the control arm (no imaging).
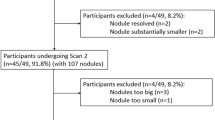
For the present study, we investigated all 94 participants who had a stable SSN >5 mm for a maximum period of 12 months recorded in the Nelson Management System from the CT screening arm from the Dutch centers (University Medical Center Groningen, University Medical Center Utrecht and Kennemer Gasthuis, Haarlem, the Netherlands). All CTs were carefully reviewed by two experienced reviewers, both with more than 10 years of experience in chest CT, to confirm the stable character of the nodule visually. If one or both of the reviewers had any doubt about the perfect stability of the nodule, the participant was excluded from this study. This procedure resulted in 44 included cases with 44 SSNs.
CT imaging and reading protocol
The NELSON protocol included a low-dose CT examination. Participants were imaged using a 16-detector row CT system (Somatom Sensation 16, Siemens Medical Solutions, Mx8000 IDT or Brilliance-16, Philips Medical Systems, Cleveland, OH, USA ) in helical mode with 16 × 0.75-mm collimation and 15-mm table feed per rotation (pitch 1.3). CT acquisition was done in full inspiration. No intravenous contrast was injected.
Depending on the body weight (<50, 50—80, and >80 kg), the kVp settings were 80—90, 120, and 140 kVp. To achieve a CTDIvol of 0.8, 1.6, and 3.2 mGy, respectively, the mAs settings were adjusted accordingly depending on the machine used. Axial images of 1.0-mm thickness were reconstructed in 0.7-mm increments with a 512 × 512 matrix, using a moderately soft reconstruction kernel and the smallest field of view (FOV) that included both lungs. The CT exams were evaluated by double reading with a consensus reading in case of discrepant results. All CT examinations were read for nodules, and detected nodules were characterized as solid nodule or subsolid nodule, either nonsolid or part-solid.
Subsolid nodule evaluation and measurements
For the current analysis, we retrospectively measured volume and mass of all 44 nodules with dedicated software, based on an established method for solid lesion segmentation [6] adapted for segmentation and volumetry of SSNs (CIRRUS Lung, Diagnostic Image Analysis Group, Nijmegen, the Netherlands, Fraunhofer MEVIS, Bremen, Germany).
The user can either click a center point or draw a stroke on the largest diameter of the nodule as an input to the algorithm. Based on this input, a volume of interest (VOI) is automatically defined around the nodule. An initial segmentation is acquired by region growing using thresholds applicable to subsolid nodules. The default value for the lower threshold is -750 HU and for the higher threshold -150 HU. Two parameters, density threshold value and roundness versus irregularity, can be adjusted by the user. Finally, a sequence of morphological operations is used to remove the chest wall and adjacent vessels (Fig. 1).
Observer 1 (over 10 years of experience with chest CT) measured the nodules semi-automatically in this way on the first and the second CT. To investigate the interobserver variation, a second reader (1 year of experience with chest CT) measured the nodules on the first CT independently.
Data analysis
Data are presented as percentage (%) or mean and standard deviation (SD) for data with a normal distribution or as median and InterQuartile Range (IQR) for non-normally distributed data. Diameter, volume, and mass of the SSNs were compared with Bland-Altman plots. Observer agreement was calculated as intraclass correlation coefficient. P-values < 0.05 were considered significant.
A Mann-Whitney U test was used for the analysis of the influence of adjustments on the measurements.
Results
Subjects and nodule characteristics
Ninety-four subjects had stable SSNs according to the database. Fifty of them were excluded, because on critical review by two experienced observers, the stable character of the lesion could not be confirmed, leaving 44 subjects with 44 SSNs available for analysis. Thirty-three (75 %) subjects were male. Mean age was 62.9 (SD 6.5) years, the median number of pack-years was 34.2 (IQR 28.0 to 44.3), and 24 (54.5 %) were current smokers. The semi-automatic program was successful in all nodules. Of the 44 nodules, 24 were nonsolid and 20 were part-solid. Mean diameter was 12.2 mm (SD 6.4 mm) (Table 1). Observer 1 did not make any adjustments to the measurements in 61 of the 88 (69 %) measurements, in 18 (20 %) the density cut-off was adjusted, and in 11 (13 %) the roundness was adjusted. Semi-automatic measurements involved a few seconds.
Observer 2 did not make any adjustment to the semi-automatic measurements in 68 of the 88 (77 %) measurements, in 18 (20 %) the density cut-off was adjusted, and in 4 (5 %) measurements the roundness was adjusted.
Interscan agreement: comparison of first and second CT
Mean measured diameter, volume, and mass in the first CT were 12.2 mm, 1,892.0 mm3, and 922 mg.
Mean difference between the first and the second CT was 0.2 mm, 167.4 mm3, and 55.8 mg for diameter, volume, and mass, respectively. On average, measurements were 2 %, 9 %, and 6 % greater on the second CT for diameter, volume, and mass, respectively.
Mean relative difference between the first and the second CT for the diameter was -1.1 %, (CI: -12.0 % to 9.7 %), for volume -3.4 %, (CI: -35.4 % to 28.6 %), and for mass -1.6 % (CI: -27.6 % to 30.8 %) (Fig. 2 a-c).
a-f – Bland-Altman plots of relative differences between the first and second measurement of one observer (a-c) and between the two observers (d-f) on CT for diameter, volume, and mass of subsolid nodules. The X-axis is on a logarithmic scale. a Interscanvariation diameter. b Interscanvariation volume. c Interscanvariation mass. d Interobservariation diameter. e Interobservariation volume. f Interobservariation mass
Influence of adjustments of density cut-off and roundness on the measurements
For nodules in which no adjustments of the measurements were made, the mean measured diameter, volume, and mass in the first CT were 10.5 mm, 1,147.2 mm3, and 501.9 mg.
Mean relative difference between the first and the second CT for the diameter was -1.1 %, (CI: -12.1 % to 9.9 %), for volume -3.2 % (CI: -35.9 % to 29.5 %), and for mass 0.2 % (CI: -29.0 % to 29.5 %).
In cases in which either density cut-off or roundness were adjusted, mean measured diameter, volume, and mass in the first CT were 16.6 mm, 3,877.9 mm3, and 2,043.9 mg.
Mean relative difference between the first and the second CT for the diameter was -1.1 %, (CI: -12.1 % to 9.9 %), for volume -3.2 %, (CI: -35.9 % to 29.5 %), and for mass -6.5 % (CI: -34.7 % to 21.8 %).
These relative differences between measurements of the first and the second CT with and without adjustment were not significant for diameter (p = 0.98), volume (p = 0.98), and diameter (p = 0.28).
Observer agreement
Mean difference between the first and the second observer was 0.0 mm, 190.6 mm3, and 32.0 mg for diameter, volume, and mass, respectively. On average measurements were 0 %, 10 % greater, and 3 % smaller for the second reader for diameter, volume, and mass respectively.
Mean relative interobserver variation for diameter was 1.4 % (CI: -22.2 % to 25.0 %), for volume 3.8 % (CI: -64.4 % to 72.1 %), and mass 5.0 % (CI: -43.4 % to 53.4 %) (Fig. 2d-f).
The intraclass correlation between the observers was best for diameter measurements with an intraclass correlation coefficient of 0.978, and good with intraclass correlation coefficients of 0.957 and 0.968 for volume and mass, respectively.
Discussion
Our results show that semi-automatic measurements of SSN are feasible with good interscan agreement. Correction of density cut-off and roundness, mainly used to correct segmentation imperfection on larger lesion, did not influence the interscan error significantly. These results are promising as the importance of precise SSN quantification is increasingly recognized and manual measurements are impractical due to the time involved in the segmentation.
SSNs are a major challenge, both clinically [9] and in lung cancer screening trials [2], because these nodules are relatively rare, slow growing, often multiple, and bear a high malignancy rate. Although transient SSNs can represent a large range of benign diseases, persistent SSNs have a high likelihood of malignancy, with reported malignancy rates ranging from 18 % to 75 % [2, 3]. In the first round of the Dutch-Belgian lung cancer screening trial, only 2.0 % of the total of 8,673 nodules found in 7,557 participants were nonsolid nodules or part-solid nodules [10]. In this trial, for solid nodules, volumetry software was used to measure nodule dimensions because volumetry had been proven to be superior to diameter measurements in terms of accuracy and reproducibility [5]. SSN were assessed visually and by manual diameter measurements as no volumetry software for SSNs was available at the start of the trial. For SSN, accurate measurement is becoming increasingly important, as differentiation of benign and malignant nodules is largely based on change in size or on the development of a solid component. Recently, a statement from the Fleischner Society with recommendations for the management of SSNs detected at CT was published [4]. For solitary nonsolid nodules and for solitary part-solid GGNs, in which the solid component is smaller than 5 mm, it was recommended that close monitoring is appropriate to enable early detection of even subtle interval change in their appearance. Such close monitoring requires precise measurements in order to detect early changes. So far, data on measurement variability of subsolid nodules are very limited. Park et al [7] included only nonsolid SSNs.
Kim et al [8] were the first to investigate the measurement variability volume and mass in pure and part-solid nodules with a solid portion less than or equal to 5 mm. They reported a variability of mass measurements from -17.7 % to 18.6 % and a similar variation of volume measurements of -17.3 % to 18.5 %. No significant correlation was found between the presence or the size of a solid portion and measurement variability.
The 95 % confidence interval for difference in volume in our series was -35.4 % to 28.6 % (mean difference -3.4 %) and for mass -30.8 % to 27.6 % (mean difference -1.6 %). The discrepancy of our results with those of Kim et al remains unclear.
In a previous study, SSNs were manually segmented with two observers, which took about 10 min per nodule. Recently, semi-automatic software has become available for segmentation of SSNs in which nodules are segmented in a few seconds. This way, volumetry and mass measurements of SSNs become feasible in clinical practice. The semi-automatic software has previously been tested in an anthropomorphic phantom study [11] and was further compared to manual measurements. In the phantom study, the semi-automatic measurements compared closely to the true values without systematic errors. In the comparison with manual measurements, semi-automatic measurement of SSN showed a good observer agreement [12].
Our study is limited by the relatively small number of nodules in the study that precluded the separate analysis of pure- and part-solid SSNs. Another limitation is the time lapse between the two measurements due to the retrospective nature of our study. Average measurements of diameter volume and mass were respectively 2 %, 9 %, and 6 % greater on the second CT. It, therefore, cannot be excluded that some growth occurred in the period between the two CTs that was not evident on critical review by two experienced observers.
Furthermore, interobserver variation might be influenced by the difference in experience of the two observers. Another limitation is the lack of a true ‘gold’ standard, as we have no pathological confirmation of our data. However, to assess change in size, precise measurements are more important than accurate measurement.
Conclusion
In conclusion, our study shows that semi-automatic measurement of the diameter, volume, and mass of subsolid nodules is feasible within seconds with good interscan agreement. To minimize the interscan variation it is advisable to have the measurements done by the same observer for successive studies.
References
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J (2008) Fleischner society: glossary of terms for thoracic imaging. Radiology 246:697–722
Henschke CI, Yankelevitz DF, Mirtcheva R et al (2002) CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 178:1053–1057
Kim HY, Shim YM, Lee KS, Han J, Yi CA, Kim YK (2007) Persistent pulmonary nodular ground-glass opacity at thin-section ct: histopathologic comparisons. Radiology 245:267–275
Naidich DP, Bankier AA, Macmahon H et al (2013) Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 266:304–317
de Hoop B, Gietema H, van de Vorst S, Murphy K, van Klaveren RJ, Prokop M (2010) Pulmonary ground-glass nodules: increase in mass as an early indicator of growth. Radiology 255:199–206
Kuhnigk JM, Dicken V, Bornemann L et al (2006) Morphological segmentation and partial volume analysis for volumetry of solid pulmonary lesions in thoracic CT scans IEEE Trans. Med Imaging 25:417–434
Park CM, Goo JM, Lee HJ et al (2010) Persistent pure ground-glass nodules in the lung: interscan variability of semiautomated volume and attenuation measurements. AJR Am J Roentgenol 195:408–414
Kim H, Park CM, Woo S et al (2013) Pure and part-solid pulmonary ground-glass nodules: measurement variability of volume and mass in nodules with a solid portion less than or equal to 5 mm. Radiology 269:585–593
Godoy MC, Naidich DP (2012) Overview and strategic management of subsolid pulmonary nodules. J Thorac Imaging 27:240–248
van Klaveren RJ, Oudkerk M, Prokop M et al (2009) Management of lung nodules detected by volume CT scanning. N Engl J Med 361:2221–2229
Scholten ET, Jacobs C, van Ginneken B et al (2013) Computer aided segmentation and volumetry of artificial ground glass nodules on chest computed tomography. AJR Am J Roentgenol 201:295–300
Scholten ET, de Hoop B, Jacobs C et al (2013) Semi-automatic quantification of subsolid pulmonary nodules: comparison with manual Measurements. PLoS One 21:8(11)
Acknowledgements
The scientific guarantor of this publication is Prof. Dr. W.P.Th.M.Mali. The authors of this manuscript declare relationships with the following companies: Prof. Dr. B. van Ginneken is affiliated with Fraunhofer MeVis, Bremen, Germany. The NELSON trial has received funding from Zorg Onderzoek Nederland-Medische Wetenschappen (ZonMw), KWF Kankerbestrijding, Stichting Centraal Fonds Reserves van Voormalig Vrijwillige Ziekenfondsverzekeringen (RvvZ), G. Ph. Verhagen Foundation, Rotterdam Oncologic Thoracic Study Group (ROTS) and Erasmus Trust Fund, Stichting tegen Kanker, Vlaamse Liga tegen Kanker, and LOGO Leuven and Hageland. One of the authors has significant statistical expertise. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: retrospective, observational, multicenter study/performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial Registration
Dutch-Belgian lung cancer screening trial (NELSON; ISRCTN63545820).
Rights and permissions
About this article
Cite this article
Scholten, E.T., de Jong, P.A., Jacobs, C. et al. Interscan variation of semi-automated volumetry of subsolid pulmonary nodules. Eur Radiol 25, 1040–1047 (2015). https://doi.org/10.1007/s00330-014-3478-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3478-1