Abstract
Objectives
To compare intraindividually two macrocyclic contrast agents - gadobutrol and gadoterate meglumine (Gd-DOTA) - for dynamic and quantitative assessment of relative enhancement (RE) in benign and malignant breast lesions.
Methods
This was an ethically approved, prospective, single-centre, randomized, crossover study in 52 women with suspected breast lesions referred for magnetic resonance imaging (MRI). Each patient underwent one examination with gadobutrol and one with Gd-DOTA (0.1 mmol/kg BW) on a 1.5 T system 1 – 7 days apart. Dynamic, T1-weighted, 3D gradient echo sequences were acquired under identical conditions. Quantitative evaluation with at least three regions of interest (ROI) per lesion was performed. Primary endpoint was RE during the initial postcontrast phase after the first and second dynamic acquisition, and peak RE. All lesions were histologically proven; differences between the examinations were evaluated.
Results
Forty-five patients with a total of 11 benign and 34 malignant lesions were assessed. Mean RE was significantly higher for gadobutrol than Gd-DOTA (p < 0.0001). Gadobutrol showed significantly less washout (64.4 %) than Gd-DOTA (75.4 %) in malignant lesions (p = 0.048)
Conclusions
Gadobutrol has higher RE values compared with Gd-DOTA, whereas Gd-DOTA shows more marked washout in malignant lesions. This might improve the detection of breast lesions and influence the specificity of breast MRI-imaging.
Key Points
• Contrast agents differ in terms of peak enhancement and postinitial contrast behaviour.
• Gadobutrol results in greater peak RE in lesions, likely due to relaxivity.
• Gd-DOTA shows more pronounced washout in postinitial contrast behaviour of malignant lesions.
• Further investigations of the differences among GD-CM may improve sensitivity and specificity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dynamic contrast-enhanced magnetic resonance mammography (CE-MRM) of the breast is now highly accepted as a diagnostic tool for detecting, assessing, staging, and managing breast cancer [1–3]. It has been shown to address some of the shortcomings of conventional mammography and has high sensitivity in detecting malignant lesions [1, 4–8].
CE-MRM is performed with a T1-weighted pulse sequence acquired before and at several time points after intravenous (IV) injection of a gadolinium-based, low-molecular-weight contrast medium (Gd-CM). Typically, serial acquisitions approximately 1 min in duration are performed at one precontrast time point and at least two postcontrast time points, one approximately after 2 min and one in the late phase after Gd-CM administration [9]. Mainly approx. 5 postcontrast time points until 5 – 8 min are used.
This basic principle has not changed much since CE-MRM was introduced in the late 1980s [10–13], apart from technical advances that have improved temporal and/or spatial resolution as well as signal-to-noise ratio (SNR). CE-MRM detects breast lesions with high sensitivity due to the high peak enhancement of most malignant and benign breast lesions. Different enhancement-time curves, acquired by manual ROI (region of interest) placement, have been identified to differentiate between malignant and benign breast lesions, which in conjunction with morphological imaging criteria, allows highly specific identification of malignant breast lesions [1–3, 9]. All this knowledge is based on studies using gadopentetate dimeglumine.
Gadopentetate dimeglumine (Magnevist®), a linear, ionic Gd-CM, was the first one used for CE-MRM on a larger scale [11]. Since then, several other linear or macrocyclic Gd-CM have been investigated and used for CE-MRM in clinical routine. These agents differ from gadopentetate dimeglumine and from each other with regard to physical and chemical properties. However, all Gd-CM share basic pharmacokinetic properties, namely distribution in the extracellular space, with one notable exception: gadobenate dimeglumine has weak protein binding and is excreted partially via the hepatobiliary route [14–17].
An important physical property that determines the diagnostic effectiveness of an MR contrast agent is T1-relaxivity [18, 19]. A higher T1-relaxivity has been shown to lead to higher peak enhancement of brain lesions and breast lesions, improving lesion conspicuity compared with contrast agents that have lower T1-relaxivity [20–23]. Another property in which Gd-CM differ from each other and which might influence their distribution in biological tissues is the electric charge of the contrast medium molecules. Ionic and nonionic Gd-CM differ in their electric charge since ionic agents have a negative charge and nonionic agents are neutral. Differences in the electric charge of Gd-CM molecules can alter the time course of signal intensities in tissues with a high content of negatively charged tissue components such as acidic mucopolysaccarides [24, 25]. It is known that malignant breast lesions have a high acidic mucopolysaccharide content, in other words, harbour negatively charged macromolecules, especially at the interface between malignant tissue and surrounding host tissue [26–28].
Gadobutrol is a nonionic Gd-CM with relatively high T1-relaxivity, while gadoterate meglumine is an ionic Gd-CM with lower T1-relaxivity [18, 19, 29]. Both agents consist of macrocyclic Gd complexes and are considered to be very stable.
The aim of our study was to investigate whether gadobutrol and gadoterate meglumine differ not only with regard to peak enhancement, which is expected on the basis of their different T1-relaxivities, but also with regard to the time course of signal enhancement, which might be attributable to differences in electric charge. We, therefore, performed an intraindividual comparison of the signal intensity time courses after gadobutrol and gadoterate meglumine administration in women with different benign and malignant breast lesions.
Materials and methods
Overall study design
This was a prospective, single-centre, controlled, block randomized, intraindividual, crossover study comparing gadobutrol and Gd-DOTA for breast MR imaging. Fifty-two women were enrolled consecutively between January and July 2010.
The study was performed in accordance with the Declaration of Helsinki and was approved by the Federal Institute for Drugs and Medical Products and the local ethics committee (Clinical study phase: IIIb, EudraCT No.: 2009-013432-20).
All patients provided written informed consent prior to enrolment.
This investigator-initiated study was supported by a research grant from Bayer Healthcare and Siemens Healthcare. The investigators had exclusive control of all data in this study, manuscript drafting, and submission.
Parts of the study population have been included in an automated CAD analysis study [30].
Inclusion and exclusion criteria
Female patients aged 18–70 years old with histologically proven or biopsy-planned Breast Imaging Reporting and Data System [BI-RADS®] 4 or higher breast lesions diagnosed in mammography or ultrasound and with clinical indications for breast MR imaging were included consecutively in this study. Patients eligible for the study were willing to undergo all study procedures and provided informed consent.
Patients were excluded from the study if they were or have been suspected to be pregnant or breastfeeding; had contraindications to MR imaging, including history of anaphylactoid or anaphylactic reaction to any contrast media or impaired renal function due to chronic kidney disease stage 3 and higher (e.g., creatinine clearance <60 mL/min); had received any MRI contrast medium within 12 h prior to injection of contrast medium for the current study; were scheduled for breast surgery; or had a biopsy between the two breast MRI examinations.
MRI parameters
All contrast-enhanced examinations were performed on a 1.5 T MR system (Magnetom Avanto, Siemens, Erlangen, Germany) using a dedicated 4-channel breast coil. All patients were assessed in prone position without breast compression.
Each patient underwent two MRI examinations using an identical protocol consisting of a transverse T2-weighted (T2w) turbo spin echo (TSE) sequence and a transverse dynamic, contrast-enhanced, three-dimensional T1w gradient echo (GRE) sequence series (repetition time 7.5 ms, echo time 4.76 ms, flip angle 25°, field of view 320–360 mm, 512 × 512 matrix, imaging percentage 100 %, in-plane resolution 0.7 × 0.7 mm, slice thickness 2.0 mm, no intersectional gap). After the unenhanced T1w sequence, five contrast-enhanced image sets with no time gap were acquired, starting with a 20-s delay after starting the contrast injection. Depending on breast size, each acquisition took 59–77 s.
Contrast agents and administration
Gadobutrol (Gadovist®, Bayer-Healthcare, Germany) and Gd-DOTA (Dotarem®, Guerbet, France), each with a dose of 0.1 mmol/kg body weight gadolinium, were administered in a simple, randomized order. The dose of 0.1 mmol/kg was chosen as it was reported to be sufficient and because we did not want to apply more than the 0.2 mmol/kg Gd-CM recommended as maximum dose in the guidelines [9].
The two MRI examinations were separated by a minimum of 24 h to prevent any residual effects from the first agent, and a maximum of 7 days to reduce the potential of evolution and/or progression of disease. If possible, the examinations were performed between the seventh and 15th day of the menstrual cycle, with exceptions in patients with known malignant lesions in order to not postpone further treatment.
Both contrast agents were injected using an automated syringe at an injection speed of 2 mL/s (reflecting our standard injection protocol) as a single intravenous bolus by technicians non-blinded to the type of contrast agent, according to the recommendations of the European Society of Breast Imaging [9]. The total amount of injected volume was the same in both examinations due to an adapted saline flush of at least 30 mL after injection of the contrast medium.
Lesion biopsy and MR image analysis
All lesions were histologically proven. Core biopsy was performed using a sonographically, mammographically, or MRI-guided technique according to European guidelines. Pathological work-up was done by following the routine procedures of the hospital. The type of malignant and benign lesion, as well as the grading of malignant lesions have been categorized using the World Health Organization classification (grade 1, well differentiated; grade 2, moderately differentiated; grade 3, poorly differentiated). Twelve patients included in the analysis were biopsied after, 33 before the MRI exam.
Only patients with two completed contrast-enhanced examinations were included in the analysis. Cases with severe motion artefacts or marked background enhancement that disabled the reader to identify clearly the same area and slice of the lesion for correct and comparable ROI-placement were excluded. MR images were anonymized and sent to a multi-monitor imaging workstation for analysis (syngo MultiModality Workplace, Siemens Healthcare, Erlangen, Germany). One radiologist with 10 years of experience in reading breast MR images evaluated both sets of MR images. The radiologist was fully blinded to the patients’ medical histories and to the administered contrast agent.
Breast density and background enhancement was assessed in accordance with the MRI-BI-RADS®-Lexicon.
Examinations from the same patient were viewed simultaneously in order to identify a comparable slice with nearly identical tumour parts to place a region of interest (ROI) for signal intensity (SI) measurement (Fig. 1).
Region of interest placement and relative SI enhancement for gadobutrol or gadoterate meglumine. Example illustrates placement of two ROIs in the most strongly enhancing parts of the breast lesion and shows the corresponding dynamic curves for gadobutrol and Gd-DOTA. Gd-DOTA = gadoterate meglumine; ROI = region of interest
At least three ROIs of a size of five pixels were placed per index lesion in the strongest and the most rapidly enhancing regions of the tumour (identified by comparing the subtracted and nonsubtracted images) avoiding fat-containing areas in accordance with the recommendations of the American College of Radiology Lexicon for placing ROIs [3].
If a patient had a biopsy before the MRI exam, the reader carefully avoided to place an ROI in a biopsy-altered part of the lesion like artefacts or biopsy channel (compare Fig. 1).
If a (larger) lesion had several strongly enhancing parts not visible in a single representative slice, the ROIs were placed in several slices showing the most intense tumour SI enhancement.
Placement of ROIs was based on the reader’s subjective visual assessment and on comparison with a wash-in map calculated by the system to minimize inter-examination variability [31]. Minor in-plane motion artefacts were compensated for by manual adaptation of the ROI in each series. Using the ‘mean curve’ application of the imaging workstation, SI measurements were carried out for all nonsubtracted dynamic images.
Image analysis
Relative SI enhancement (RE)
Relative SI enhancement was calculated as 100*([SIpost – SIpre]/SIpre).
It was the primary endpoint in the initial postcontrast phase of both examinations which is after the first and second dynamic measurement. In addition, RE was calculated for peak SI enhancement as well as for all other time points after contrast administration.
The ratio of the RE values for gadobutrol and Gd-DOTA at the first (T1) and second (T2) measurement time points after contrast administration and the peak SI enhancement time points (TPE) were compared.
Time to peak SI enhancement and postinitial contrast behaviour
In addition, the time to peak SI enhancement and the postinitial contrast behaviour as the difference in relative SI enhancement of the second postcontrast measurement time point minus the last time point (T2–TL), and peak SI enhancement minus the last time point (TPE–TL) were evaluated and compared between the two contrast agents investigated.
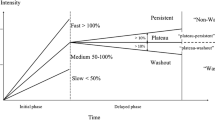
The curve type was evaluated using time points T2 and TL. The curve type ‘washout’ was defined as a relative loss of more than 10 %, a ‘plateau’ as a relative difference of no more than 10 %, and ‘progression’ as a relative increase of more than 10 % in SI, according to the BI-RADS® lexicon and the European guidelines [9, 32].
Statistical analysis
Data from up to four slices were available for each patient and each time point. In case of multiple regions of interest (ROIs) per slice, the arithmetic mean of the ROIs was used in the analysis. Continuous data are presented as means with 95 % confidence intervals (CIs) and categorical data as counts and frequencies. The analysis of continuous data was performed with mixed linear models, taking into account the multiple observations per patient based on the logarithmic transformed values, as the data were close to normal distribution just after transformation.
Time-to-peak analysis was performed using Poisson regression based on generalized estimation equations, which accounted for multiple measurements per patient. All analyses were performed on all lesions and the subgroups of benign and malignant lesions.
The dynamic SI enhancement-time curve analysis was performed using mixed linear models, which accounted for multiple observations per patient.
All tests were performed two-sided, assuming statistical significance for p values below 0.05. Calculations were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
The determination of sample size was based on the requirements of an intra-individual comparison test for non-inferiority for the primary variable. Pilot data of three patients were used to set the assumptions for the sample size calculation. In this pilot study, the peak enhancement was found to be 454 for Gadovist® and 470 for Dotarem® with a common standard deviation of 343. The difference in relative peak enhancement of Gadovist® and Dotarem® was calculated as -16 with a standard deviation of 73. The non-inferiority margin -Δ was based on the minimum clinically relevant difference for the peak enhancement. When the limit is above -47 (≅ -10 %), non-inferiority can be concluded.
To ensure that the required number of evaluable cases of the per-protocol population is available for this study a drop-out rate of about 10 % is assumed which leads to a planned total sample size of 52 patients.
Results
Patients
A total of 52 women consented to participate in the study: gadobutrol was administered first in 26 patients, and Gd-DOTA in the other 26 patients. Histology was available for 51 patients; one patient refused biopsy after MR imaging showed no signs of malignancy. This lesion did not change within a 2-year follow-up and was classified as a fibroadenoma. Seven of the 52 patients were excluded from further analysis. Reasons for exclusion were withdrawal of consent owing to claustrophobia after the first examination (n = 2) and inability to identify clearly the lesion on both examinations due to marked background enhancement in a blinded reading (n = 2) or severe motion artefacts (n = 3). All of the motion occurred after admission, one time in the first and two times in the second exam. None of the patients had an adverse event.
A total of 45 patients received both contrast agents and were included in the analysis. The overall sample consisted of 115 slices from 45 patients. For three patients, data on one slice each was reported, for 20 patients on two slices, for 16 patients on three slices, and for six patients on four slices. ROIs were placed in 90 slices of malignant and 25 slices of benign lesions.
The 45 patients included in the analysis had a mean age of 49.1 years (range, 30–70 years) and a mean weight of 67.5 kg (±12.6 standard deviations [SD]; range 42–94 kg). Twenty-two women were postmenopausal and 23 premenopausal. The mean time between the two MR examinations was 2.3 days with a range of 24 h to 7 days. Data on menstrual cycle status at the time of MRI of the premenopausal women and intervals between the two examinations are presented in Table 1.
Histology identified 34 malignant (mean tumour size, 27.1 mm; range, 7–58 mm; SD, ±14.3 mm) and 11 benign lesions. The distribution of the different histology results are presented in Table 2.
Breast density of the analyzed patients was almost entirely fatty in three, scattered fibroglandular tissue in 22, heterogeneous fibroglandular tissue in 12, and extremely fibroglandular tissue in eight cases. Eighteen patients revealed a minimal background enhancement; in 14 it was mild, 12 showed moderate enhancement and in one patient it was marked.
Early relative SI enhancement
Relative SI enhancement, for all lesions, in the initial phase was significantly higher after gadobutrol administration than after Gd-DOTA. Mean relative SI enhancement at the first postcontrast time point was 273.5 % (95 % CI: 237.2, 315.3 %) and 225.1 % (95 % CI: 195.3, 259.5 %) for gadobutrol- and for Gd-DOTA-enhanced examinations, respectively. In most cases, RE values were higher at the second postcontrast time point than at the first time point, resulting in increased mean RE values for the second postcontrast images: 302.7 % (95 % CI: 269.5, 340 %) and 249.2 % (95 % CI: 221.8 %, 279.9 %) for gadobutrol and Gd-DOTA, respectively. Mean peak relative SI enhancement for gadobutrol and Gd-DOTA was 330.6 % (95 % CI: 295.2, 370.2 %) and 276. 7 % (95 % CI: 247.1, 309.9 %), respectively.
The ratio of gadobutrol/Gd-DOTA RE differed significantly at the first time point (1.22; 95 % CI: 1.11, 1.33, p = 0.0001) and second time point (1.22; 95 % CI: 1.12, 1.32, p < 0.0001) and for peak SI enhancement (1.19, 95 % CI: 1.10, 1.30, p < 0.0001).
The ratio of gadobutrol/Gd-DOTA with regard to relative SI enhancement was also analyzed for the benign and malignant subgroups (Table 3). The difference was significant for all subgroups and all time points, with the exception of nonductal malignant lesions, for which the ratio was not significantly different.
Time-to-peak SI enhancement
Results for time-to-peak relative SI enhancement are summarized in Table 3. No differences were found between gadobutrol and Gd-DOTA for time-to-peak RE for all lesions and for the individual lesion subgroups. Benign lesions and nonductal invasive lesions showed later peak RE for both contrast agents compared with ductal malignant invasive lesions (Table 4).
Postinitial contrast behaviour
The curve types ‘washout’, ‘plateau’, and ‘progression’ were found in benign and malignant lesions; however, the percentage of washout curves was highest in malignant and lowest in benign lesions (Table 5). There was no significant difference in the postinitial behaviour of the two contrast agents with respect to postinitial RE in benign lesions after 2 min (Fig. 2); however, the malignant lesions differed significantly after 2 min, as Gd-DOTA resulted in a higher percentage of washout in this group. This difference was mainly attributable to a difference for nonductal carcinomas, while invasive ductal carcinomas showed no difference (Fig. 3a–d), Fig. 4. Nevertheless, invasive ductal carcinomas also tended to show a higher percentage of washout using Gd-DOTA (53.15 % vs. 46.85 %).
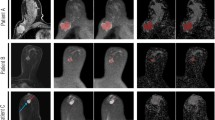
Breast lesion enhancement with gadobutrol (a, c) and gadoterate meglumine (b, d). Corresponding maximum intensity projection and first non-subtracted T1 image of a bifocal G3 invasive ductal carcinoma 12 and 6 mm in size within a distance of 3 mm. Artifact overlay completed for both images; however, lesions are clearly visible with gadobutrol only because of the higher enhancement levels
Influence of menopausal status and examination time point
Regarding the absolute RE related to the menopausal status no significant differences could be found for the groups postmenopausal, premenopausal-correct time point (examined at recommended time of cycle), and premenopausal-incorrect time point using gadobutrol or gadoterate meglumine at 1 min, 2 min or at the peak enhancement time point (Table 6).
Again gadobutrol was significantly higher in absolute RE than gadoterate meglumine for all subgroups (p < 0.04).
Safety
Twelve (23.1 %) patients experienced minor adverse events following administration of either contrast agent. They included headache (n = 4 for gadobutrol, 2 for Gd-DOTA), nausea (n = 2 for gadobutrol), rash (n = 2 for gadobutrol), paresthesia (n = 2 for Gd-DOTA), dizziness (n = 2 for gadobutrol), and palpitations (n = 1 for Gd-DOTA).
Discussion
Contrast-enhanced MR mammography using linear Gd-CM has been shown to be an effective diagnostic breast imaging technique with regard to both the detection and characterization of breast lesions [9, 33–35].
Dynamic contrast-enhanced MR imaging of the breast detects solid breast lesions with high sensitivity based on peak enhancement, while their differentiation relies on the evolution of enhancement over time. In a first approximation, we may assume that all nonspecific, low-molecular-weight, gadolinium-based contrast agents have similar pharmacodynamics and, therefore, produce similar enhancement patterns over time when used for dynamic contrast-enhanced breast MRI. On the other hand, MR contrast agents are known to have different T1 relaxivity [18, 19]. In several studies, use of contrast agents with higher T1 relaxivity has resulted in stronger contrast enhancement compared to agents with lower T1 relaxivity [20, 22, 23, 36, 37]. For some clinical applications, the stronger enhancement has been shown to be associated with a clinically relevant diagnostic gain [20–23, 38].
In the present study, we intraindividually compared gadobutrol and Gd-DOTA with T1 relaxivities of 5.2 and 3.6 L/mmol s–1, respectively (at 1.5 T in plasma at 37 °C), in terms of signal enhancement of breast lesions. Our results show that gadobutrol produces significantly higher peak RE in both benign and malignant breast lesions. The ratio of mean peak RE roughly corresponds to the ratio of relaxivities. These findings are in agreement with the results of the studies of Attenberger et al. comparing gadobutrol and Gd-DOTA, which have demonstrated superiority of gadobutrol in MR imaging of brain lesions [20]. The stronger relative SI enhancement of gadobutrol can possibly be explained by its higher relaxivity compared with Gd-DOTA. Interestingly, this effect did not differ between pre- and postmenopausal woman independently from the timing of examination. Indeed, previous breast MR imaging studies have shown that GBCAs with higher relaxivities are associated with higher SI enhancement levels, which in turn have been shown to improve sensitivity of lesion detection [21–23, 36].
It has been shown by Pediconi et al. and Knopp et al. that the detection of lesions in MR mammography can be increased by the use of linear contrast agents with higher relaxivity than gadopentetate dimeglumine [21, 23, 36]. The influence on the shape of the SI enhancement curves, however, was not assessed in detail in these studies.
In addition, gadolinium-based contrast agents also differ in terms of the electrical charge of the active molecules. Regarding the two contrast agents investigated here, gadobutrol is electrically neutral, while Gd-DOTA has a negative charge. Studies investigating the distribution of gadolinium-based contrast media in cartilage have revealed that ionic and nonionic agents differ in terms of uptake into cartilage. Cartilage is rich in negatively charge acidic mucopolysaccharides, resulting in lower contrast when an ionic, negatively charged contrast medium is used. This effect has been attributed to electrostatic repulsion of the contrast agent by the negatively charged mucopolysaccharides. Increased enhancement results when the cartilage examined has a reduced mucopolysaccharide content [25, 28, 39]. In light of this mechanism, the second result of our study appears to be of interest, namely that Gd-DOTA tended to show a more marked washout from breast lesions. Since breast lesions have been shown to have an increased content of acidic mucopolysaccharides, especially in the periphery [26–28], this observation might be attributable to repulsion of the negatively charged Gd-DOTA, resulting in more rapid washout from the lesion, especially the periphery, following initial inflow due to lesion vascularization. In contrast to the postinitial phase, in which the temporal development might be influenced by the charge of the tissue, the signal enhancement during the initial phase is dominated by vascularization.
This finding is in line with a previous study comparing the described contrast agents in analysis using a fully automatic computer-assisted diagnosis (CAD) technique also showing a more marked washout for Gd-DOTA in malignant lesions [30]. However, our results are acquired via manual ROI placement reflecting common clinic practice.
Clinical significance
Several factors are known to affect the signal intensity in contrast-enhanced MR imaging, such as magnetic field strength, sequence type, and relaxivity of contrast agents, which may have impact on diagnosis and lesion characterization [18, 40, 41]. As such, the thresholds of relative SI enhancement levels should be individually adjusted to provide optimal diagnostic performance for a given contrast agent and a specific imaging protocol [42]. Thus, the thresholds of a slow SI enhancement of 50 %, intermediate SI enhancement of 50–100 %, and rapid SI enhancement of more than 100 % – established to distinguish benign and malignant breast lesions using gadopentetate dimeglumine and two dimensional imaging sequences – are not appropriate for macrocyclic GBCAs. This is supported by the high RE data obtained for benign lesions, suggesting that these thresholds may have to be adjusted for macrocyclic GBCAs for the human reader and for any computerized assisting system. Adjusting the thresholds may increase the specificity, accuracy, and predictive value [42].
The slightly higher peak relative enhancement produced by gadobutrol compared with Gd-DOTA might improve the detection of breast lesions, especially when they are small.
Further studies are needed to investigate whether this improvement in lesion detection translates into relevant benefits for patient management. Especially in premenopausal women, it would be interesting to analyse in a bigger study population if the higher enhancement translates in an increased sensitivity and/or specificity in patients with moderate or marked background enhancement and adjustment of the examination time point can be neglected to avoid the postponing of treatment.
Since the postinitial course of relative SI enhancement does not differ much between gadobutrol and Gd-DOTA in average, we may assume that these two gadolinium-based contrast agents do not differ in their ability to characterize breast lesions. Nevertheless, the type of contrast agent used has to be taken into account when judging signal intensity time curves, as there have been significant differences in malignant lesions. This might influence the specificity as well.
The clinical use of macrocyclic GBCAs in breast MR imaging is desirable, as they are associated with a lower propensity to release gadolinium ions compared with linear GBCAs. This is particularly important for renally impaired patients, where the use of linear GBCAs is contraindicated owing to their association with a high/medium risk of nephrogenic systemic fibrosis (NSF) [43]. This may be of particular benefit for older women – where the incidence of breast cancer is greater – who are more likely to have compromised renal function. The use of macrocyclic GBCAs could, therefore, also benefit the screening of high-risk patients with breast cancer who require repeated follow-up examinations by reducing gadolinium exposure/burden.
Limitations
Our study is limited by using a 1.5 T system with a 4-channel coil only, as it is known that field strength and coil design influence the signal intensity of enhancing breast lesions. Therefore, the results are not directly transferrable to different field strength or breast coils.
The ROI placement was done by one single reader only.
Inclusion of patients before and after biopsy could affect the measured signal intensities. Careful ROI placement avoiding the biopsied parts of the breast was chosen to keep this influence minimal. Nevertheless, imaging after biopsy represents in some parts clinical practice, when MRI is used for assessment of the extend of a malignant lesion before operation.
Another potential limitation is the different molarity of the contrast agents injected, as the concentration for Gd-DOTA and gadobutrol are 0.5 mmol/L and 1.0 mmol/L, respectively. The injection protocol was in accordance with the recommendations of the European Society of Breast Imaging, and hence comparable with the conditions likely to be used in daily routine [9, 44]. Moreover, we did not expect that the difference in molarity would result in differences in concentration in the blood after lung passage and, therefore, would not have affected the results significantly. Also, the images in this study were acquired with a relatively low temporal resolution, most likely not able to detect the first pass of the contrast agents.
Only routine histological stains were performed for workup of the breast lesions investigated. No specific staining for acidic mucopolysaccharides was done. Therefore, it was not possible to directly correlate the patterns of signal enhancement with histology.
Finally, interpretation of the subgroup analyses should be made with care, owing to the low number of patients within the subgroup populations analyzed.
References
Kuhl CK, Mielcareck P, Klaschik S et al (1999) Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology 211:101–110
Montemurro F, Martincich L, Sarotto I et al (2007) Relationship between DCE-MRI morphological and functional features and histopathological characteristics of breast cancer. Eur Radiol 17:1490–1497
Morris EA, Harms S (2004) ACR practice guideline for the performance of magnetic resonance imaging (MRI) of the breast. American College of Radiology, Reston (VA)
Heywang-Kobrunner SH, Viehweg P, Heinig A, Kuchler CH (1997) Contrast-enhanced MRI of the breast: accuracy, value, controversies, solutions. Eur J Radiol 24:94–108
Lehman CD, Isaacs C, Schnall MD et al (2007) Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology 244:381–388
Saslow D, Boetes C, Burke W et al (2007) American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography. CA Cancer J Clin 57:75–89
Schelfout K, Van Goethem M, Kersschot E et al (2004) Preoperative breast MRI in patients with invasive lobular breast cancer. Eur Radiol 14:1209–1216
Van Goethem M, Schelfout K, Dijckmans L et al (2004) MR mammography in the pre-operative staging of breast cancer in patients with dense breast tissue: comparison with mammography and ultrasound. Eur Radiol 14:809–816
Mann RM, Kuhl CK, Kinkel K, Boetes C (2008) Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol 18:1307–1318
Flickinger FW, Allison JD, Sherry RM, Wright JC (1993) Differentiation of benign from malignant breast masses by time-intensity evaluation of contrast enhanced MRI. Magn Reson Imaging 11:617–620
Heywang SH, Hahn D, Schmidt H et al (1986) MR imaging of the breast using gadolinium-DTPA. J Comput Assist Tomogr 10:199–204
Kaiser WA, Zeitler E (1989) MR imaging of the breast: fast imaging sequences with and without Gd-DTPA. Preliminary Observations. Radiology 170:681–686
Stack JP, Redmond OM, Codd MB, Dervan PA, Ennis JT (1990) Breast disease: tissue characterization with Gd-DTPA enhancement profiles. Radiology 174:491–494
Weinmann HJ, Laniado M, Mutzel W (1984) Pharmacokinetics of GdDTPA/dimeglumine after intravenous injection into healthy volunteers. Physiol Chem Phys Med NMR 16:167–172
Allard M, Doucet D, Kien P, Bonnemain B, Caille JM (1988) Experimental study of DOTA-gadolinium. Pharmacokinetics and pharmacologic properties. Investig Radiol 23:S271–S274
Le Mignon MM, Chambon C, Warrington S, Davies R, Bonnemain B (1990) Gd-DOTA. Pharmacokinetics and tolerability after intravenous injection into healthy volunteers. Investig Radiol 25:933–937
Bourasset F, Dencausse A, Bourrinet P, Ducret M, Corot C (2001) Comparison of plasma and peritoneal concentrations of various categories of MRI blood pool agents in a murine experimental pharmacokinetic model. MAGMA 12:82–87
Pintaske J, Martirosian P, Graf H et al (2006) Relaxivity of Gadopentetate Dimeglumine (Magnevist), Gadobutrol (Gadovist), and Gadobenate Dimeglumine (MultiHance) in human blood plasma at 0.2, 1.5, and 3 Tesla. Investig Radiol 41:213–221
Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann H-J (2005) Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Investig Radiol 40:715–724
Attenberger UI, Runge VM, Morelli JN, Williams J, Jackson CB, Michaely HJ (2010) Evaluation of gadobutrol, a macrocyclic, nonionic gadolinium chelate in a brain glioma model: comparison with gadoterate meglumine and gadopentetate dimeglumine at 1.5T, combined with an assessment of field strength dependence, specifically 1.5 versus 3T. J Magn Reson Imaging 31:549–555
Knopp MV, Bourne MW, Sardanelli F et al (2003) Gadobenate dimeglumine-enhanced MRI of the breast: analysis of dose response and comparison with gadopentetate dimeglumine. AJR Am J Roentgenol 181:663–676
Martincich L, Faivre-Pierret M, Zechmann CM et al (2011) Multicenter, double-blind, randomized, intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for Breast MR imaging (DETECT Trial). Radiology 258:396–408
Pediconi F, Catalano C, Padula S et al (2008) Contrast-enhanced MR mammography: improved lesion detection and differentiation with gadobenate dimeglumine. AJR Am J Roentgenol 191:1339–1346
Gillis A, Gray M, Burstein D (2002) Relaxivity and diffusion of gadolinium agents in cartilage. Magn Reson Med 48:1068–1071
Wiener E, Woertler K, Weirich G, Rummeny EJ, Settles M (2007) Contrast enhanced cartilage imaging: Comparison of ionic and non-ionic contrast agents. Eur J Radiol 63:110–119
Marotta M, D'Armiento FP, Martino G et al (1985) Glycosaminoglycans in human breast cancer: morphological and biochemical study. Appl Pathol 3:164–169
Olsen EB, Trier K, Eldov K, Ammitzboll T (1988) Glycosaminoglycans in human breast cancer. Acta Obstet Gynecol Scand 67:539–542
Takeuchi JSM, Sato E, Shamoto M, Miura K, Nakagaki S (1976) Variation in Glycosaminoglycan Components of Breast Tumors. Cancer Res 36:2133–2139
van der Molen AJ, Bellin MF (2008) Extracellular gadolinium-based contrast media: differences in diagnostic efficacy. Eur J Radiol 66:168–174
Renz DM, Durmus T, Bottcher J et al (2014) Comparison of Gadoteric Acid and Gadobutrol for Detection as Well as Morphologic and Dynamic Characterization of Lesions on Breast Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Investig Radiol. doi:10.1097/RLI.0000000000000039
Mussurakis S, Buckley DL, Coady AM, Turnbull LW, Horsman A (1996) Observer variability in the interpretation of contrast enhanced MRI of the breast. Br J Radiol 69:1009–1016
Committee ACoRB-R, Radiology ACo (2003) Breast imaging reporting and data system. American College of Radiology
Li SP, Padhani AR, Taylor NJ et al (2011) Vascular characterisation of triple negative breast carcinomas using dynamic MRI. Eur Radiol 21:1364–1373
Veltman J, Mann R, Kok T et al (2008) Breast tumor characteristics of BRCA1 and BRCA2 gene mutation carriers on MRI. Eur Radiol 18:931–938
Schmitz AC, Peters NH, Veldhuis WB et al (2008) Contrast-enhanced 3.0-T breast MRI for characterization of breast lesions: increased specificity by using vascular maps. Eur Radiol 18:355–364
Pediconi F, Catalano C, Occhiato R et al (2005) Breast lesion detection and characterization at contrast-enhanced MR mammography: gadobenate dimeglumine versus gadopentetate dimeglumine. Radiology 237:45–56
Pediconi F, Kubik-Huch R, Chilla B, Schwenke C, Kinkel K (2013) Intra-individual randomised comparison of gadobutrol 1.0 M versus gadobenate dimeglumine 0.5 M in patients scheduled for preoperative breast MRI. Eur Radiol 23:84–92
Anzalone N, Gerevini S, Scotti R, Vezzulli P, Picozzi P (2009) Detection of cerebral metastases on magnetic resonance imaging: intraindividual comparison of gadobutrol with gadopentetate dimeglumine. Acta Radiol 50:933–940
Ibrahim MA, Haughton VM, Hyde JS (1995) Effect of disk maturation on diffusion of low-molecular-weight gadolinium complexes: an experimental study in rabbits. AJNR Am J Neuroradiol 16:1307–1311
Jansen SA, Shimauchi A, Zak L et al (2009) Kinetic curves of malignant lesions are not consistent across MRI systems: need for improved standardization of breast dynamic contrast-enhanced MRI acquisition. AJR Am J Roentgenol 193:832–839
Pabst T, Kenn W, Kaiser WA, Hahn D (2001) Understanding why contrast enhancement in dynamic MRI is not reproducible: illustration with a simple phantom. Breast J 7:166–170
Sardanelli F, Fausto A, Esseridou A, Di Leo G, Kirchin MA (2008) Gadobenate dimeglumine as a contrast agent for dynamic breast magnetic resonance imaging: effect of higher initial enhancement thresholds on diagnostic performance. Investig Radiol 43:236–242
Prince MR, Zhang HL, Roditi GH, Leiner T, Kucharczyk W (2009) Risk factors for NSF: a literature review. J Magn Reson Imaging 30:1298–1308
Sardanelli F, Boetes C, Borisch B et al (2010) Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 46:1296–1316
Acknowledgments
The scientific guarantor of this publication is Prof. Bernd Hamm. The authors of this manuscript declare relationships with the following companies: Prof. B. Hamm nearly all medical companies. Dr. A. Huppertz, Bayer Healthcare, Siemens Healthcare. Dr. E.M. Fallenberg, travel support and speaker’s fee by Bayer Healthcare, Guerbet, Siemens Healthcare, GE Healthcare. This study has received funding from Bayer Healthcare and Siemens Healthcare. The investigators had exclusive control of all data, manu- script drafting, and submission of this study. One of the authors has significant statistical expertise. Institutional review board approval was not required because the study was approved by the Federal Institute for Drugs and Medical Products and the local ethics committee (Clinical study phase: IIIb, EudraCT No.: 2009-013432-20). Written informed consent was obtained from all subjects (patients) in this study. Some study subjects or cohorts have been previously reported in Investigative Radiology. Renz DM, Durmus T, Bottcher J, Taupitz M, Diekmann F, Huppertz A, Pfeil A, Maurer MH, Streitparth F, Bick U, Hamm B, Fallenberg EM (2014) Comparison of Gadoteric Acid and Gadobutrol for Detection as Well as Morphologic and Dynamic Characterization of Lesions on Breast Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Investigative Radiology. Methodology: prospective, randomised controlled trial, performed at one institution. We are grateful to Nikola Bangemann, MD, Tahir Durmus, MD, Elke Keil, MD, Christiane Richter-Ehrenstein, MD, Achim Schneider, MD and Klaus- Jürgen Winzer MD for their contribution in the patient recruitment and inclusion. We are thankful to Bettina Herwig for editorial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fallenberg, E.M., Renz, D.M., Karle, B. et al. Intraindividual, randomized comparison of the macrocyclic contrast agents gadobutrol and gadoterate meglumine in breast magnetic resonance imaging. Eur Radiol 25, 837–849 (2015). https://doi.org/10.1007/s00330-014-3426-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3426-0