Abstract
Background
The high concentration of gadolinium in gadobutrol, which is widely used in Japan, helps visualize signal enhancement of neoplastic lesions, however, there was concern that high T1 relaxivity could decrease the contrast between the lesion and the background mammary gland. We evaluate the effect of gadobutrol on background parenchymal enhancement (BPE) and differential diagnosis between benign and malignant lesions in dynamic MRI of the breast.
Methods
Ninety-nine patients were enrolled prospectively. Measurements of the following signal intensities (SIs) were obtained: breast tissue on a pre-contrast image (SIpre) and an early-phase image (SIearly); and the SIs of breast cancer on a pre-contrast image (SIpre-cancer) and an early-phase image (SIearly-cancer). We calculated the BPE ratio, i.e., (SIearly − SIpre)/SIpre and the cancer/BPE ratio, i.e., (SIearly-cancer − SIpre-cancer)/(SIearly on the affected side − SIpre on the affected side). These quantitative assessments were compared with the data from the recently published multicenter study (reference study without use of gadobutrol). In addition, two radiologists reinterpreted each of the MR images, and a third radiologist set the ROIs in the lesions and performed kinetic analysis as a Reader 3.
Results
While there was no significant difference in the SI of breast cancer in the premenopausal patients between the two studies, that in postmenopausal patients was significantly higher in the present study than in the reference study (p = 0.002). Although there was no significant difference in the cancer/BPE ratio in the postmenopausal patients between the two studies, the cancer/BPE ratio in the premenopausal patients was significantly higher in the reference study than in the present study (p = 0.028). For differentiation between benign and malignant masses, the mass margin was found to be the most important term (p < 0.001). According to the data of Reader 3, visual washout was observed in all 18 patients in whom the interpretation was changed from “plateau” to “washout”.
Conclusions
Gadobutrol may decrease the contrast between breast cancer and background parenchyma in premenopausal patients, and it may have a characteristic that “washout” does not easily occur, leading to “plateau” in patients with breast cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gadobutrol is a macrocyclic gadolinium-based contrast agent (GBCA) formulated as a 1 mol/L solution, i.e., it contains twice the concentration of gadolinium as other currently licensed extracellular GBCAs [1]. The high paramagnetic effect of gadobutrol provides a higher relaxivity, associated with the image quality, as compared to other macrocyclic GBCAs [2, 3]. As a macrocyclic agent, the chemical structure of gadobutrol is more stable as compared to the chemical structures of linear GBCAs, which are associated with an increased risk of nephrogenic systemic fibrosis in patients with severely impaired renal function [4].
In the breast MRI field, it was expected that the higher concentration of gadolinium in gadobutrol as compared to that in other existing MRI contrast media would be useful to visualize the signal enhancement of neoplastic lesions. On the other hand, there was also the concern that the high T1 relaxivity may, conversely, cause a high signal intensity of the background mammary gland, thereby decreasing the contrast between the lesion and the background mammary gland. Furthermore, studies using gadobutrol and gadoterate meglumine (Gd-DOTA) in the same patients with breast tumors reported that the use of gadobutrol was associated with a reduced proportion of breast cancer patients showing a “washout” time-intensity curve (TIC) pattern [5, 6]; this could prove to be a pitfall in the ability of MRI using gadobutrol in the diagnosis of breast cancer.
We conducted this study to address the following two questions.
-
1.
Does the background parenchymal enhancement (BPE) on MRI with gadobutrol affect the visualization of breast cancer lesions? In regard to contrast-enhanced breast MRI, it has long been recognized that parenchymal enhancement is associated with the menstrual cycle, because the breast tissue structure is influenced by the cyclic hormonal changes occurring with the menstrual cycle [7, 8]. After a while, the Breast Imaging Reporting and Data System (BI-RADS) MRI lexicon, 5th edition [9], defined parenchymal enhancement on contrast-enhanced breast MRI as BPE. A recent multicenter study reported on the correlation between BPE and the menstrual cycle in the Japanese population [10]. Therefore, we planned to compare the data from this previous study [10] and our present study.
-
2.
How does use of gadobutrol affect the differential diagnosis between benign and malignant breast lesions? We constructed a diagnostic tree for mass lesions using BI-RADS categories, to investigate the effect of kinetic analyses on the diagnostic tree. In particular, we investigated whether the use of gadobutrol decreases the proportion of breast cancer patients showing “washout” and its effects on the diagnostic ability of the MRI.
Materials and methods
Patients
We conducted a prospective multicenter study in Japan, with the participation of 5 hospitals. One of the institutions participated in the previous multicenter study [10]. The study was conducted with the approval of the Ethical Review Board on Clinical Studies at each of the participating institutions. Written informed consent for participation was obtained from all the participating patients. The inclusion criteria were: (1) female patients who had undergone dynamic contrast-enhanced breast MRI for closer examination of a breast lesion; (2) age 20–69 years; (3) patients for whom information about the menstrual cycle could be obtained by interview. The exclusion criteria were: (1) history of anaphylactoid or anaphylactic reaction to any contrast media; (2) impaired renal function (e.g., estimated glomerular filtration rate (eGFR) < 30 mL/min./1.73 m2); (3) pregnant or breastfeeding women; (4) patients with a previous history of treatment for breast cancer; (5) patients undergoing drug therapy for breast cancer; (6) patients judged for any reason as being ineligible for participation in this study by the investigator.
Between May 2017 and July 2018, 99 patients (age range 30–69 years, mean 53 years) from the 5 centers were enrolled. The patients included 55 postmenopausal patients and 44 premenopausal patients (Table 1). Five patients were examined in the menstrual phase (days 1–4), 10 in the proliferative phase (days 5–14), and 12 in the secretory phase (days 15–30) of the menstrual cycle; the remaining 17 patients had irregular menstrual cycles. Patients with regular menstrual cycles were defined as those in whom the self-reported menstrual cycles were regular, with a cycle length of ≤ 30 days, and the interval from the first day of the last menstrual period to the date of MRI examination was ≤ 30 days.
MRI protocols
MRI examinations were performed with a 1.5 or 3 T system. Axial- or coronal-view 3D fast gradient echo (GRE) T1-weighted images with fat suppression of the whole breasts of either side were acquired with the patients lying in the prone position. No restrictions were set in regard to the repetition time (TR), echo time (TE), or flip angle (FA). The prescribed slice thickness was 1–2 mm.
Gadobutrol (Gadovist®, Bayer-Healthcare, Germany) was administered at the dose of 0.1 mmol/kg body weight of gadolinium. Dynamic MR images were acquired before and three times after bolus injection of the contrast medium, followed by a 20-mL saline flush using an automatic injector. The injection rates for both contrast medium and saline were 1 mL/s. Both breasts were examined in the first-, second-, and third-phase dynamic imaging, at 1, 2, and 5 min, respectively, after gadobutrol injection. The scanning timing of dynamic MRI was the same as the reference study [10].
Image analysis
Evaluation 1: quantitative assessment
-
1.
BPE ratio on the unaffected side and affected side
BPE generally means non-specific enhancements such as spotty, patchy, regional and diffuse, but strictly speaking, it is difficult to distinguish it from the high signal intensity (SI) of the background mammary gland. Therefore, BPE was defined in this study as s high SI of the background mammary gland including non-specific enhancements, similar to the reference study [10].
Regions of interest (ROIs) were manually set by one radiologist at each facility for the unaffected side and the affected side. One slice distant from the lesion was selected and ROIs of the same size were set for each of the both breasts as to include the fibroglandular breast tissue. Visible cystic areas, fatty tissue, and nodular enhancing areas were avoided. The size of ROI was not specified. The SIs of the breast tissue were measured in the pre-contrast image (SIpre) and early-phase images obtained after the contrast injection (early post-contrast images, or SIearly). We then calculated the BPE ratio for each patient, as follows: (SIearly − SIpre)/SIpre.
-
2.
SI of the breast cancer and the cancer/BPE ratio
The same radiologist at each facility measured the SIs of the breast cancer in the pre-contrast images (SIpre-cancer) and early-phase post-contrast images (SIearly-cancer). We then calculated the SI of the breast cancer [(SIearly-cancer − SIpre-cancer)/SIpre-cancer] and the cancer/BPE ratio [(SIearly-cancer − SIpre-cancer)/(SIearly on the affected side—SIpre on the affected side)] for each patient.
The above four quantitative parameters were compared with those reported from the reference study [10] conducted in Japanese people. This comparative study was conducted with the approval of the Ethical Review Board on Clinical Studies at the representative institution for the reference study [10].
Evaluation 2: inter-observer variability for the 5th-edition BI-RADS MRI descriptors
Two radiologists with 18 and 25 years of experience in breast MRI reinterpreted each of the MR images using the 5th-edition BI-RADS MRI lexicon [9]. The two readers were from different institutions and were blinded to the original breast MRI interpretations as well as to each other’s interpretations.
The readers independently evaluated the BPE level (minimal, mild, moderate, and marked), and delayed-phase kinetic patterns (“persistent,” “plateau,” “washout”) for each lesion. When they were reinterpreting the MR images, Reader 1 and Reader 2 were not presented with the TIC created in advance. In addition, no other special rules were set for the kinetic assessment.
For masses, the shape (round, oval, and irregular), margin (circumscribed, spiculated, and irregular), and internal enhancement characteristics (homogeneous, heterogeneous, rim enhancement, and dark internal septations) were recorded. For non-mass enhancement (NME), the distribution (focal, linear, segmental, regional, multiple regions, and diffuse) and internal enhancement patterns (homogeneous, heterogeneous, clumped, and clustered ring) were recorded.
The readers then provided a final BI-RADS category assessment (scale 1–5; 1 = negative; 2 = benign findings; 3 = probably benign; 4 = suspicious for malignancy; and 5 = highly suspicious for malignancy). For the purposes of our analysis, BI-RADS categories 1–3 were classified as benign lesions and categories 4/5 as malignant lesions.
Evaluation 3: verification of the usefulness of the TIC for interpreting a mass lesion
In addition to the above two radiologists, a third radiologist (M.T) with 26 years’ experience in breast radiology who was not involved in the patient enrollment set the ROIs in the lesions and performed kinetic analysis as Reader 3. ROIs were set for the masses, but not for NME. This is because it is difficult to set the ROI and reproducibility is not guaranteed. A ROI larger than 3 pixels was placed on the most suspicious region of the enhancement within a mass lesion. Suspicious regions were defined as areas that exhibit a washout in the delayed-phase or a rapid rise in the early phase and were usually located at the margin of the tumor.
As for the TIC pattern, the enhancement in the initial phase was determined by comparing the enhancement intensity in the first post-contrast image with the intensity in the pre-contrast image. An intensity increase of < 50% is classified as slow, 50–100% is classified as medium, and > 100% enhancement is classified as fast. For the delayed-phase, “persistent” is SI increase ≥ 10% of the initial enhancement; “washout” is SI decrease ≥ 10% of the initial enhancement; and “plateau” is between “persistent” and “washout”.
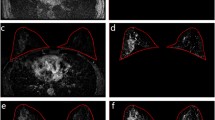
A diagnostic tree for mass lesions based on the evaluation 2 was constructed and the usefulness of including the quantitative TIC analysis on the diagnostic capability of the diagnostic tree was evaluated. Furthermore, we subclassified the term “plateau” into “plateau-persistent” and “plateau-washout” (Fig. 1). “plateau-persistent” means SI increase < 10% and “plateau-washout” means SI decrease < 10%. “Non-washout” includes SI increase = 0% + “plateau-persistent” + persistent, and “Washout” includes “plateau-washout” + washout. We evaluated whether inclusion of these TIC patterns in the revised assessment of the delayed-phase images affected the diagnostic capability of the diagnostic tree or not.
A revised assessment of the delayed-phase images of TIC patterns. We subclassified the term “plateau” into “plateau-persistent” and “plateau-washout”. “plateau-persistent” means SI increase < 10% and “plateau-washout” means SI decrease < 10%. “Non-washout” includes SI increase = 0% + “plateau-persistent” + persistent, and “Washout” includes “plateau-washout” + washout
Statistical analyses
Results of comparative studies of the quantitative assessment (BPE ratio, SI of breast cancer and the cancer/BPE ratio) were compared using the Mann–Whitney U test. Inter-observer variability for each BI-RADS MRI descriptor of mass and non-mass lesions was calculated by determining the kappa coefficient, and for the BPE level, kinetic parameters, and final category assessment was calculated by determining the weighted kappa coefficient. A kappa statistic of less than 0.4 was defined as poor agreement, that of 0.40–0.59 as moderate agreement, that of 0.60–0.79 as good agreement, and that of 0.80 or higher as excellent agreement.
Using conditional inference trees as the algorithm for decision tree learning, we attempted to build classification trees that distinguished between benign and malignant lesions based on the BI-RADS MRI descriptors. p values of < 0.05 were considered as being indicative of significance. The statistical analyses were performed using SPSS Statistics ver. 26 (IBM, Chicago, IL), R version 3. 6. 3 (http://www.r-project.org) and the ctree function (package partykit version 1.2-7).
Results
Table 1 shows an overview of the results of the present study and the reference study [10]. Gadobutrol was used as the contrast medium in all the patients in the present study, while some other contrast medium had been used in all the patients in the reference study. The mean age of breast cancer patients, menopausal status, menstrual cycle and rate of benign and malignant lesions were relatively similar in both studies. However, the present study had a higher proportion of 3 T scanners compared to the reference study (81% vs. 39%).
Evaluation 1: quantitative assessment
Table 2 shows the results of the four quantitative assessments. There was no significant difference in the BPE ratio on either the unaffected side or the affected side between the present study and the reference study.
The SI of breast cancer in postmenopausal patients was significantly higher in the present study than in the reference study (p = 0.002). However, no significant difference in the SI of breast cancer in premenopausal patients was found between the two studies. A significant difference in the SI of breast cancer was found between the two studies in the overall subject population (p < 0.001).
The cancer/BPE ratio in premenopausal patients was significantly higher in the reference study than in the present study (p = 0.028). However, no significant difference in the cancer/BPE ratio between the two studies were found in the postmenopausal patients or the overall subject population.
Evaluation 2: inter-observer variability for the 5th-edition BI-RADS MRI descriptors
Table 3 shows the inter-observer variability between the two radiologists in this study. The agreement rate was highest for the BPE level (ĸ = 0.74), followed by that for the BI-RADS category assessment (scale 1–5) and BI-RADS category assessment (1–3 vs. 4/5) (ĸ = 0.69 and ĸ = 0.66, respectively). The results of assessment of the mass margin also showed good agreement, with ĸ = 0.65. The agreement rate was poor for all the other descriptors.
The agreement rate was particularly low for the kinetic parameters (ĸ = 0.17), as shown in detail in Table 4. There were 10 cases in which Reader 1 judged to be difficult to evaluate, and 7 cases in which Reader 2 judged to be difficult to evaluate. The analysis was performed in 89 cases excluding these 10 cases. Of the 10 cases excluded, 8 were benign and 2 were malignant.
Evaluation 3: verification of the usefulness of inclusion of the TIC pattern in the diagnostic tree for interpretation of mass lesions
The number of cases determined to be a mass by Reader 1, Reader 2 and Reader 3 was 64, 77 and 68, respectively. The frequency of benign and malignant lesions was 10 and 54 for Reader 1, 12 and 65 for Reader 2, and 13 and 55 for Reader 3, respectively.
On the basis of the results of image interpretation by Reader 1 and Reader 2, a diagnostic tree for the interpretation of masses was constructed (Fig. 2a, b). For differentiation between benign and malignant masses, the mass margin was found to be an important term (p < 0.001), whereas none of the other terms or the TIC pattern was found to be useful. Furthermore, inclusion of the revised delayed-phase assessment in the diagnostic tree was not found to be useful. The sensitivity, specificity, accuracy, positive predictive value (PPV) for malignancy, and negative predictive value (NPV) for the correct diagnosis were 94% (51/54), 80% (8/10), 92% (59/64), 96% (51/53), and 73% (8/11), respectively, for Reader 1, and 89% (58/65), 67% (8/12), 86% (66/77), 94% (58/62), and 53% (8/15), respectively, for Reader 2.
Diagnostic trees for the interpretation of masses by Reader 1 (a) and Reader 2 (b). The mass margin was found to be an important term (p < 0.001), whereas none of the other terms or the TIC pattern was found to be useful. Initial-phase and revised delayed-phase are derived from the quantitative assessment of kinetics by Reader 3 and delayed-phase mean visual assessment of kinetics in Evaluation 2
Table 5 shows the detailed results of use of the revised assessment of the delayed-phase images. The percentage of patients with a plateau pattern varied among the three readers: 39% (25/64), 87% (67/77) and 47% (32/68) for Reader 1, Reader 2 and Reader 3, respectively. The percentages of patients with a “persistent” and “washout” pattern were 27% (17/64) and 34% (22/64), respectively, according to Reader 1, and 8% (6/77) and 5% (4/77), respectively, according to Reader 2, while in the revised assessment of the delayed-phase images, the results were similar between the two readers: 53% (34/64) and 47% (30/64), respectively, for Reader 1 and 56% (43/77) and 43% (33/77), respectively, for Reader 2. The percentages of patients showing the “persistent” and “washout” patterns according to the revised assessment of the delayed-phase images by Reader 3 (57% [39/68] and 43% [29/68], respectively) were also similar to those reported by Reader 1 and Reader 2. Data from Reader 2 included one patient in whom kinetic analysis was not performed, because Reader 3 considered that this patient had NME.
According to the data of Reader 3, visual washout was observed in all 18 patients (17 with malignant lesions and 1 with a benign lesion) in whom the interpretation was changed from “plateau” to “washout” (revised assessment of delayed-phase images) and the ROI were set in these areas (Fig. 3).
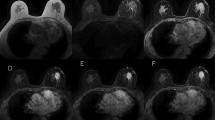
A 50-year-old female with invasive ductal carcinoma. Coronal a pre-contrast, b early-phase and c delayed-phase images clearly demonstrate an enhancing mass in upper inner region of right breast. Reader 3 set the region of interest in the areas with visual “washout” in the kinetics analysis. Signal intensities of d pre-contrast, e early-phase and f delayed-phase images are 144, 375, and 339, respectively. Therefore, this case was judged as showing the “plateau” pattern in the measurement according to the BI-RADS definitions
Discussion
One of the characteristics of this study was that the data from this study were compared with those from a multicenter study conducted previously to determine the correlation between BPE and the menstrual cycle in the Japanese population [10]. While gadobutrol was used as the contrast agent in all the patients in this study, it was not used as the contrast agent in any of the patients in the reference study. No significant difference was found in the BPE ratio on either the unaffected or the affected side between the present study and the reference study. While there was no significant difference in the SI of breast cancer in the premenopausal patients between the two studies, that in postmenopausal patients was significantly higher in the present study than in the reference study (p = 0.002). There was also a significant difference in the SI of breast cancer in the overall subject population (p < 0.001). The results are considered to reflect the high T1 relaxivity, one of the characteristics, of gadobutrol, and were similar to those of studies performed using gadobutrol and Gd-DOTA in the same patients with breast tumors [5, 6]. Although there was no significant difference in the cancer/BPE ratio in the postmenopausal patients between the two studies, the cancer/BPE ratio in the premenopausal patients was significantly higher in the reference study than in the present study (p = 0.028). In the premenopausal patients, the high T1 relaxivity of gadobutrol was suggested as being the cause of the high SI of the background mammary gland and the consequent decrease in the contrast between the breast cancer lesions and the background mammary gland.
Next, we investigated whether the reduced contrast between the breast cancer lesions and the background mammary gland influenced the accuracy of the differential diagnosis between benign and malignant lesions by the two radiologists. The agreement rates between the two radiologists were very low for all the BI-RADS MRI descriptors of mass and non-mass lesions, except the mass margin (ĸ = 0.65). Grimm et al. [11] also reported the highest agreement rate for the mass margin (ĸ = 0.78) among the BI-RADS MRI descriptors of mass and non-mass lesions. However, the agreement rate was high for the BI-RADS category assessment (scale 1–5) and BI-RADS category assessment (1–3 vs. 4/5) (ĸ = 0.69 and ĸ = 0.66, respectively). This is considered as being because although the MRI descriptor terms used vary among individual radiologists, the differential diagnosis between benign and malignant lesions is made in a comprehensive manner, which eventually led to the good agreement rate between Reader 1 and Reader 2, both of whom were well-experienced in breast MRI interpretation. Furthermore, the mass margin was the most useful for the differential diagnosis between benign and malignant masses, as shown in the diagnostic tree (Fig. 2), and the high agreement rate for mass margin between Reader 1 and Reader 2 is another possible reason for the high agreement rate between the two on the final BI-RADS category assessment.
On the other hand, the agreement rate was lowest for the kinetic pattern (“persistent,” “plateau,” “washout”) (ĸ = 0.17). Grimm et al. [11] also reported a clearly lower agreement rate for the kinetic patterns (ĸ = 0.19) than for the BI-RADS MRI descriptors of mass and non-mass lesions, consistent with the results of the present study. In the present study, when they were interpreting the images, Reader 1 and Reader 2 were not presented with the TIC patterns constructed in advance. Namely, Reader 1 and Reader 2 performed the assessment of the kinetic patterns without having information on the TIC patterns. Grimm et al. [11] reported that the agreement rate was lower for the “plateau” pattern (ĸ = 0.07) than for the “persistent” (ĸ = 0.27) or “washout” pattern (ĸ = 0.15) in the kinetic analysis (ĸ = 0.19). Namely, the definition of “plateau” itself is suggested as being of no special significance for the differential diagnosis between benign and malignant lesions.
One of the characteristics of this study was that the “plateau” pattern was subclassified into the “plateau-persistent” and “plateau-washout” patterns, and additional analysis was performed. The percentage of cases judged as showing the “washout” pattern differed greatly between Reader 1 and Reader 2 [34% (22/64) and 5% (4/77), respectively], while in the revised interpretation of the delayed-phase images, the percentages were similar between the two readers [47% (30/64) and 43% (33/77), respectively]. In addition, Reader 3 set the ROI in the areas with visual “washout” in the kinetics analysis, and “washout” was observed visually in all 18 patients (17 with malignant lesions and 1 with a benign lesion) in whom the interpretation was changed by Reader 3 from “plateau” to “washout” (revised interpretation of delayed-phase images), but these cases were judged as showing the “plateau” pattern in the measurement (according to the BI-RADS definitions) (Fig. 3). The differences in interpretation between Reader 1 and Reader 2 are suggested to arise from the fact that cases that could be visually interpreted as showing the “washout” pattern could be interpreted as “plateau” pattern according to the BI-RADS definitions.
Renz et al. [5], reported that “the higher rate of continuous increases accompanied by fewer washout curves can pharmacokinetically be explained that gadobutrol accumulates to a higher extent in the extravascular extracellular space of breast carcinomas than gadoteric acid.” However, it remains doubtful as to whether breast cancers showing a clear “washout” pattern when other contrast media are used, i.e., breast cancer with abundant cellular components and a small amount of desmoplastic changes and scirrhous component [12], could be evaluated as “persistent” when gadobutrol is used, due to pooling of the contrast medium in the tumor. Rather, it may be a characteristic of gadobutrol that “washout” of the contrast medium does not easily occur, leading to a “plateau” pattern in many patients with breast cancer. If this hypothesis is correct, some patients may be visually evaluable as “washout” even if they are classified as showing a “plateau” pattern according to the BI-RADS definitions.
There were some limitations of this study. First, it is scientifically desirable to directly compare images of the same patient using different contrast agents at short intervals. However, because it is not ethically feasible, this study compared data from a previously reported multicenter study [10] as a feasible option. As a result, the proportion of 3 T scanners in this study was higher than in the reference study (81% vs. 39%). This fact can affect the results of quantitative and statistical analysis of SI. In the future, comparative studies using MRI equipment with the same magnetic field strength will be required. Second, the number of patients was very small: 76 patients with breast cancer and 20 women with regular menstrual cycles in this study. Third, a diagnostic tree for mass lesions was constructed on the basis of BI-RADS categories, but the number of benign lesions was very small (10 patients as assessed by Reader 1 and 12 patients as assessed by Reader 2). In the present study, approximately half of the benign lesions, which accounted for 11–13% of all cases (11/99 or 13/99), were not used in the construction of the diagnostic tree. Therefore, the clinical effect of “a possible lower frequency of “washout” in malignant lesions when gadobutrol was used as compared to other contrast media” could not be fully elucidated. However, we found that if delayed-phase images showed “washout”, many patients could be judged as showing “visual washout” even if they had been classified as “plateau” according to the BI-RADS definitions. This may be useful in examining patients showing the “plateau” pattern in future studies.
In conclusion, the high T1 relaxivity of gadobutrol is considered to significantly increase the SI of breast cancer in postmenopausal patients. In premenopausal patients, gadobutrol may increase SI of the background mammary gland, thereby decreasing the contrast between breast cancer lesions and the background mammary gland. When using gadobutrol for breast MRI, it is necessary to make a diagnosis in consideration that it affects the TIC used for differential diagnosis between benign and malignant lesions. In addition to the BI-RADS definition, “visual washout” should be useful in the diagnosis of breast cancer.
References
Huppertz A, Rohrer M. Gadobutrol, a highly concentrated MR-imaging contrast agent: its physicochemical characteristics and the basis for its use in contrast-enhanced MR angiography and perfusion imaging. Eur Radiol. 2004;14(Suppl 5):M12–8.
Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715–24.
Shen Y, Goerner FL, Snyder C, Morelli JN, Hao D, Hu D, et al. T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 T. Invest Radiol. 2015;50:330–8.
Kuwatsuru R, Takahashi S, Umeoka S, Sugihara R, Zeng M, Huan Y, et al. A multicenter, randomized, controlled, single-blind comparison phase III study to determine the efficacy and safety of gadobutrol 1.0 M versus gadopentetate dimeglumine following single injection in patients referred for contrast-enhanced MRI of the body regions or extremities. J Magn Reson Imaging. 2015;41:404–13.
Renz DM, Durmus T, Böttcher J, Taupitz M, Diekmann F, Huppertz A, et al. Comparison of gadoteric acid and gadobutrol for detection as well as morphologic and dynamic characterization of lesions on breast dynamic contrast-enhanced magnetic resonance imaging. Invest Radiol. 2014;49:474–84.
Fallenberg EM, Renz DM, Karle B, Schwenke C, Ingod-Heppner B, Reles A, et al. Intraindividual, randomized comparison of the macrocyclic contrast agents gadobutrol and gadoterate meglumine in breast magnetic resonance imaging. Eur Radiol. 2015;25:837–49.
Kuhl CK, Bieling HB, Gieseke J, Kreft BP, Sommer T, Lutterbey G, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology. 1997;203:137–44.
Müller-Schimpfle M, Ohmenhaüser K, Stoll P, Dietz K, Claussen CD. Menstrual cycle and age: influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology. 1997;203:145–9.
ACR BI-RADS®Atlas Breast imaging reporting and data system, 5thed, American College of Radiology, Reston, 2013.
Kamitani T, Yabuuchi H, Kanemaki Y, Tozaki M, Sonomura T, Mizukoshi W, et al. Effects of menstrual cycle on background parenchymal enhancement and detectability of breast cancer on dynamic contrast-enhanced breast MRI: a multicenter study of an Asian population. Eur J Radiol. 2019;110:130–5.
Grimm LJ, Anderson AL, Baker JA, Johnson KS, Walsh R, Yoon SC, et al. Interobserver variability between breast imagers using the fifth edition of the BI-RADS MRI Lexicon. AJR Am J Roentgenol. 2015;204:1120–4.
Tozaki M, Fukuda K, Suzuki M. Dynamic high-spatial-resolution MR imaging of invasive ductal carcinoma: influence of histological scirrhous component on MR descriptors. Magn Reson Med Sci. 2006;5:137–46.
Acknowledgements
This work was supported by a Grant from Bayer Yakuhin Ltd. (Osaka, Japan). Part of the statistical analyses was conducted by Kondo Photo Process Co. Ltd. (Osaka, Japan).
Funding
This study was supported by Bayer Yakuhin Ltd. (Osaka, Japan), which had no role regarding the study performance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Tozaki, M., Yabuuchi, H., Goto, M. et al. Effects of gadobutrol on background parenchymal enhancement and differential diagnosis between benign and malignant lesions in dynamic magnetic resonance imaging of the breast. Breast Cancer 28, 927–936 (2021). https://doi.org/10.1007/s12282-021-01229-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-021-01229-w