Abstract
Objectives
Feasibility studies have shown that contrast-enhanced spectral mammography (CESM) increases diagnostic accuracy of mammography. We studied diagnostic accuracy of CESM in patients referred from the breast cancer screening programme, who have a lower disease prevalence than previously published papers on CESM.
Methods
During 6 months, all women referred to our hospital were eligible for CESM. Two radiologists blinded to the final diagnosis provided BI-RADS classifications for conventional mammography and CESM. Statistical significance of differences between mammography and CESM was calculated using McNemar’s test. Receiver operating characteristic (ROC) curves were constructed for both imaging modalities.
Results
Of the 116 eligible women, 113 underwent CESM. CESM increased sensitivity to 100.0 % (+3.1 %), specificity to 87.7 % (+45.7 %), PPV to 76.2 % (+36.5 %) and NPV to 100.0 % (+2.9 %) as compared to mammography. Differences between conventional mammography and CESM were statistically significant (p < 0.0001). A similar trend was observed in the ROC curve. For conventional mammography, AUC was 0.779. With CESM, AUC increased to 0.976 (p < 0.0001). In addition, good agreement between tumour diameters measured using CESM, breast MRI and histopathology was observed.
Conclusion
CESM increases diagnostic performance of conventional mammography, even in lower prevalence patient populations such as referrals from breast cancer screening.
Key Points
• CESM is feasible in the workflow of referrals from routine breast screening.
• CESM is superior to mammography, even in low disease prevalence populations.
• CESM has an extremely high negative predictive value for breast cancer.
• CESM is comparable to MRI in assessment of breast cancer extent.
• CESM is comparable to histopathology in assessment of breast cancer extent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mammography remains the method of choice for breast imaging, despite the development and improvement of other imaging modalities in recent decades. In the field of mammography too, significant technical improvements were realized, mainly owing to the introduction of digital mammography. However, even though the diagnostic accuracy of full field digital mammography (FFDM) is good, it depends heavily on breast density [1, 2]. Abnormalities become more difficult to detect with increasing breast density, because of the latter’s masking effects. Indeed, Carney et al. showed that sensitivity of screening mammography in extremely dense breasts was 62.9 %, versus 87.0 % in fatty involution breasts. Specificity was 89.1 % in extremely dense breasts, versus 96.9 % in fatty involution breasts [2].
FFDM allows for new approaches not feasible in the analogue era, such as the use of iodine-based contrast agents. As is known from breast MRI, breast cancers show enhancement after administration of contrast agent, because tumoral microvessels form rapidly and consequently often have ‘leaky’ basement membranes. This makes the vessels permeable to contrast agent, resulting in tumour enhancement [3]. Contrast-enhanced spectral mammography (CESM) is based on this principle.
A recent summary review on contrast-enhanced mammography techniques found preliminary results encouraging [4]. The reviewed studies consisted mainly of small study populations with high breast cancer prevalence. Knowledge of the diagnostic accuracy of CESM in a (clinical) population with a relatively low pretest probability of breast cancer is currently lacking.
Our study aim was to evaluate the diagnostic performance of CESM as compared to conventional mammography in a true clinical population with the lowest disease prevalence reported thus far. For this reason, we chose to perform CESM in women referred from the Dutch breast cancer screening programme for a breast abnormality, for whom breast cancer prevalence is known to be approximately 31 % [5].
Materials and methods
Patients eligible for this study were women referred to our hospital from the breast cancer screening programme between 1 January and 1 July 2013. Patients with a known allergy for iodine-based contrast agents or at risk of developing contrast-induced nephropathy (as assessed by the guidelines provided by the Contrast Media Safety Committee of the European Society of Urogenital Radiology [6]) were excluded. The remaining patients were eligible to undergo CESM, the requirement for obtaining informed consent having been waived by our ethics committee.
In the Dutch screening programme, women between 50–75 years undergo mammography biannually. Women are referred for further analysis to a hospital of their choice when a breast abnormality is detected independently by two, or in case of discrepancies three, certified screening radiologists.
Standard reference procedures used to assess true disease status
When referred to our hospital, women undergo repetition of the bilateral mammogram according to our national guideline [7]. True disease status (i.e. standard reference procedure or gold standard [8]) is assessed by one of the following strategies: if the breast abnormality is caused by superposition of normal fibroglandular tissue, at least one additional view of the ipsilateral breast next to the repeated mammogram is taken, in combination with targeted ultrasound. In agreement with the NHS Breast Screening Programme (NHSBSP) Clinical Guidelines for Breast Cancer Screening Assessment, women are discharged from further evaluation if all imaging is negative [9]. The safety of this approach was confirmed by an internal institutional quality control (see “Discussion”). If the breast abnormality is caused by cyst(s), an additional targeted ultrasound is performed to confirm the diagnosis, together with cyst aspiration to prove its non-solid state. If the breast abnormality is caused by microcalcifications or a solid mass, the repeated mammogram is combined with stereotactic or ultrasound-guided large core biopsies for histopathological diagnosis.
In some cases of breast cancer, an additional (preoperative) breast MRI is performed according to national and European indications [7, 10]. These cases enabled us to carry out a sub-analysis of tumour diameter agreement using CESM, breast MRI and histopathology. All breast MRI exams were performed on a 1.5-T system (Intera, Philips Healthcare, Best, the Netherlands) using a dedicated 16-channel breast coil. The protocol consisted of a transverse T2w sequence and diffusion-weighted imaging (DWI), combined with a dynamic, contrast-enhanced T1w sequence in the transverse plane (consisting of 1.0-mm isotropic voxels and a temporal resolution of 93 s). Tumour diameter measurements were performed on the first dynamic MRI after contrast administration, i.e. at peak enhancement of the tumour, using a dedicated work station (Dynacad, Invivo International, Best, the Netherlands).
Imaging and image analysis
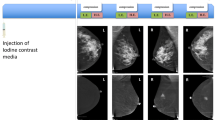
CESM was recently introduced in our hospital as part of the breast imaging modalities. All referred cases underwent CESM (SenoBright*, GE Healthcare, UK), in which a non-ionic monomeric low-osmolar contrast agent (iopromide; Ultravist® 300, Bayer Healthcare, Germany) was administered intravenously (1.5 mL/kg body weight, flow rate 3 mL/s, followed by saline flush). The imaging protocol of CESM itself is explained in more detail elsewhere [4, 11]. Following contrast administration, CESM acquires a set of low and high energy images in quick succession while the breast remains compressed. The low energy image is comparable to a regular mammogram. In post-processing, both low and high energy images are used to create a so-called recombined image, in which contrast enhancement is visible (Fig. 1).
Typical example of CESM, showing low energy images in the top row (i.e. regular mammography) and the recombined images (showing enhancement of lesions) in the bottom row. In this case, a 54-year-old patient was referred because of a new irregular density in the craniocaudal view of the right breast (arrow, top row). CESM demonstrated the true extent of the disease as well as the multifocal nature of this breast cancer (bottom row), which was confirmed by histopathological analysis of the surgical specimen
In order to avoid influencing our standard care protocol and the indication for the performance of additional breast MRI, preliminary diagnosis was based solely on low energy images (i.e. regular mammography). Recombined images (with enhancement information) were subsequently examined to detect additional lesions of interest.
To assess the diagnostic performance of CESM as compared to regular mammography, two dedicated breast radiologists (blinded to the final diagnosis; both viewing over 12,000 mammograms annually) retrospectively reviewed all cases by first viewing the low energy images. Similar to normal clinical practice, they were allowed to view the letter from the screening institution in which the referred breast abnormality was indicated in a drawing, together with the screening radiologists’ BI-RADS classification. In this re-evaluation, the radiologists provided a BI-RADS classification for conventional mammography [12]. Subsequently, the recombined images were viewed and the radiologists were allowed to up- or downgrade their BI-RADS classification. In case of discrepancies between the two radiologists, a third breast radiologist provided consensus opinion.
Statistical analysis
BI-RADS classifications 1 to 3 were considered ‘benign’, and 4 and 5 ‘malignant’. On the basis of this cut-off value, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for regular mammography and CESM. Differences in diagnostic parameters between regular mammography and CESM were calculated with corresponding 95 % confidence intervals (CI) for paired proportions. The differences were tested for statistical significance using McNemar’s test for paired proportions. Receiver operating characteristic (ROC) curves were constructed for both imaging modalities and the areas under the curve (AUC) with corresponding 95 % CI were calculated and compared according to the method described elsewhere [8, 13].
Agreement between maximum lesion diameter based on CESM and MRI, or CESM and surgical specimen was expressed in Bland–Altman plots for a subgroup of patients [14]. Measurement of maximum lesion diameter was performed on the entire CESM exam (i.e. viewing low and high energy images together) or on the first post-contrast dynamic scan on MRI (approximately 93 s after contrast administration). For the comparison of maximum lesion diameter found using CESM and pathology, surrounding ductal carcinoma in situ (DCIS) was included in the final maximum diameter measurement. In case of multifocal breast cancer, the maximum diameter of the largest invasive tumour (i.e. primary index tumour) was assessed. Patients that underwent neoadjuvant chemotherapy (n = 3) were not included in the comparison with histopathological results, because good or pathological complete response might render the latter inaccurate.
All statistical analyses were performed using SPSS Statistics (version 20.0, IBM, Armonk, USA), STATA (StataCorp LP, College Station, USA) and MedCalc (version 12.7.8, MedCalc Software, Ostend, Belgium). P values of 0.05 or less were considered statistically significant.
Results
In our study period, 7,703 women were invited to participate in the regional breast cancer screening programme and 6,384 women attended (82.9 %) (Fig. 2). From these, 116 women (1.8 %) were referred to our hospital. One patient refused contrast administration. Two patients did not undergo CESM because of known allergy to an iodine-based contrast agent (n = 1) and renal insufficiency (estimated glomerular filtration rate of 33 mL/min/1.73 m2) (n = 1). Consequently, 98.3 % (113/115) of eligible patients underwent CESM (median age 54.0 years, mean 57.2 years, range 49–73 years). Only one minor reaction to contrast administration was observed in the form of a few urticaria which resolved spontaneously.
Masses or densities were the most frequent cause of referrals (76.1 %), followed by microcalcifications (15.0 %), asymmetry (6.2 %) and architectural distortions (2.7 %). The standard reference procedures resulted in the following final diagnoses: superposition densities (24.8 %), simple cysts (21.2 %), invasive ductal carcinoma (20.4 %), fibroadenoma (7.1 %), invasive lobular carcinoma (4.4 %), ductal carcinoma in situ (3.5 %, all grade 2 or 3) and other benign causes (18.6 %). Breast cancer prevalence was 28.3 %. A detailed overview of patient characteristics is presented in Table 1.
Diagnostic accuracy parameters for conventional mammography were (absolute numbers given in parentheses) sensitivity 96.9 % (31/32), specificity 42.0 % (34/81), PPV 39.7 % (31/78) and NPV 97.1 % (34/35). After subsequent CESM, diagnostic accuracy parameters changed to sensitivity 100.0 % (32/32), specificity 87.7 % (71/81), PPV 76.2 % (32/42) and NPV 100.0 % (71/71). The 95 % confidence intervals are summarized in Table 2. In short, sensitivity remained stable when using CESM, but interestingly, specificity increased by a factor of more than 2. The observed differences were statistically significant (Fig. 3, p < 0.0001). A similar trend was observed in the ROC curve: for conventional mammography, the AUC was 0.779: for CESM, the AUC increased to 0.976 (Table 2 and Fig. 4, p < 0.0001).
Analysis of agreement between CESM and breast MRI was possible for 24 breast cancer cases. For the assessment of agreement between CESM and histopathology 30 cases were available. Bland–Altman plots showed a mean difference of 1.0 mm with 95 % limits of agreement of 8.3 mm in tumour diameter measured using CESM and breast MRI. A mean difference of 1.4 mm was observed between tumour diameters assessed using CESM and histopathology, but with larger 95 % limits of agreement: 25.0 mm (Fig. 5).
Bland–Altman plots of agreements between a tumour diameter measured using CESM and breast MRI, and b CESM and pathological examination. Solid lines represent the mean of the differences between measurements, dotted lines represent upper and lower limits of 1.96 times the standard deviations of differences
In this population, we coincidently encountered five false-positives based on CESM (enhancement) information only: low energy images did not show any suspicious lesions, whereas recombined CESM images showed an enhanced lesion (Fig. 6). In all five cases, a solid mass was the cause of enhancement, and subsequent biopsy showed four fibroadenomas and one hamartoma.
False-positive finding owing to CESM use. Top row shows low energy images, bottom row shows recombined images (only left side shown). No abnormalities were detected on the low energy images. However, a small enhancing round mass was detected only on the recombined images (arrows). Biopsy results showed fibroadenoma
Discussion
Contrast-enhanced spectral mammography (CESM) is a new imaging technique, which uses mammography in combination with iodine-based contrast agents to increase diagnostic performance. A recent review on contrast-enhanced mammography techniques found encouraging preliminary results, with a mean sensitivity of 85.2 % (range 62.0–96.0 %) and a mean specificity of 66.1 % (range 50.0–83.3 %) [4]. These studies, consisting mainly of small study populations (mean size of 47 patients, range 20–120), were enriched with breast cancer cases (mean disease prevalence was 64.5 %, range 37.5–100.0 %), whereas breast cancer pretest probability in routine clinical practice is much lower (e.g. patients referred from the national breast cancer screening programme). Evaluating diagnostic performance of CESM in a clinical population with lower breast cancer pretest probability than previously published is the next logical step in the evaluation of CESM’s imaging efficacy. To our knowledge, ours is the first study to do so.
CESM is logistically feasible in clinical practice, as is demonstrated by the high percentage of eligible patients who underwent CESM (98 %). In addition, our study population was not enriched with cancer cases, reflecting a true clinical population. Breast cancer population prevalence was 28 %, which is in accordance with previously published findings (31 %) [5], and much lower than other previously published studies (mean 64. %, lower limit 37.5 %) [4]. We found the diagnostic accuracy of CESM to be excellent and superior to conventional mammography alone. Although sensitivity and NPV were found to be high for conventional mammography, both reached 100 % upon using CESM. More importantly, specificity and PPV were found to be low for conventional mammography, and to significantly increase upon using CESM.
The increased sensitivity of mammography owing to CESM was recently demonstrated by Jochelson et al. In their study, sensitivity of mammography was increased from 81 % to 96 % owing to CESM [15]. Similar observations were recently published by Fallenberg et al., who showed that mammography’s sensitivity (82.5 %) increased to 100.0 % owing to CESM [16]. The increased specificity found in our study underscores the contribution CESM may make towards eliminating the false-positives observed in conventional mammography. The NPV of 100 % found in this study population suggests that a negative CESM exam can rule out breast cancer.
Whilst both conventional mammography and CESM have excellent cancer detection scores, CESM may be recommended even if breast cancer is highly suspected from the screening mammography. CESM might theoretically replace breast MRI in the preoperative evaluation of breast cancer patients, both techniques being based on the same principle. Although still under debate, preoperative evaluation of disease extent is one of the most frequent indications for breast MRI [12]. Jochelson et al. were the first to compare CESM with MRI in a study of 52 index tumours and found that CESM and MRI were equally accurate in the detection of breast cancer, detecting 96 % of tumours [15]. A well-known limitation of breast MRI is the occurrence of false-positives, and indeed 13 false-positives were observed in the MRI exams, compared to only two false-positives using CESM. On the other hand, breast MRI proved to be more accurate in detecting ipsilateral additional foci of tumour cells: 88 % of additional foci were detected using breast MRI, against only 56 % using CESM. The number of multifocal breast cancer cases in our population was too small to study the reproducibility of these findings.
Recently, Fallenberg et al. confirmed the potential of CESM as a replacement or alternative for breast MRI in the preoperative assessment of disease extent [16]. In their study, tumour diameter as measured on mammography, CESM, MRI and histopathology were compared. The Pearson’s correlation coefficient (PCC) between CESM and MRI was highly significant: 0.943, p = 0.000. However, good correlation does not automatically imply good correlation between two measurements [17]. If there is a systematic over- or underestimation between measurements, PCC can be very high, but agreement is low. Therefore, analysis of agreement between two measurements, for example by using the Bland–Altman plots presented in this study, is recommended in the discussion on whether CESM can replace breast MRI for tumour size measurements.
As opposed to the studies by Jochelson et al. (who presented a descriptive analysis of their findings) and Fallenberg et al. (who studied only correlation between tumour size measurements), we studied maximum tumour diameter agreement measured using CESM and breast MRI. We found a good agreement between CESM and breast MRI-based measurements (Fig. 5).
In addition, we studied maximum tumour diameter agreement as measured using CESM and pathology. Although the agreement found was slightly less than that found for the CESM and breast MRI-based measurement, it was still considered good (Fig. 5). To the best of our knowledge, only three other studies evaluated these measurements. In the first, a pilot study, Dromain et al. evaluated 20 histologically proven breast cancers and concluded that correlation between tumour diameters assessed using contrast-enhanced mammography and using histopathological results was good: r 2 = 0.743 [18]. In the second study, Dromain et al. evaluated 142 breast lesions, assessing mean lesion size with mammography, ultrasound and a combination of mammography and CESM [19]. Mean differences between maximum tumour diameters found using these imaging techniques as compared to pathology were 1.3 mm, 5.3 mm and 0.7 mm, respectively. The third study by Fallenberg et al. consisted of 80 patients with newly diagnosed breast cancer. The PCC between CESM and histopathology was statistically significant: 0.733, p = 0.000 [16]. We observed a mean difference between CESM and pathology of only 1.4 mm.
Breast cancer screening programmes suffer from an important number of false-positive findings. The introduction of digital breast tomosynthesis might be able to increase cancer detection while decreasing the number of false-positive referrals, as was demonstrated recently by Skaane et al. [20].
Nonetheless, women that are still referred need a reliable diagnostic work-up to establish their final diagnosis. Zuley et al. showed that digital breast tomosynthesis might play an important role in this work-up, because they showed that it significantly improved the diagnostic accuracy of non-calcified lesions when compared with supplemental mammography views [21]. Michell et al. recently showed that in women referred from a breast cancer screening programme, digital breast tomosynthesis increased the diagnostic accuracy of the exam when compared to mammography [22]. In their study, AUC values were significantly higher with the addition of digital breast tomosynthesis combined with FFDM and film-screen mammography (AUC 0.967) when compared to FFDM plus film-screen mammography (AUC 0.895) and film-screen mammography alone (AUC 0.788). Although these numbers are in line with our current observations using CESM, we are of the opinion that CESM might be the more suitable technique (despite the use of intravenous contrast agent and a higher dose), because its accuracy reached 100 % for 113 cases and because it might be an alternative for the entire conventional work-up, in contrast to digital breast tomosynthesis, which is only an alternative for the mammographic work-up. For example, tomosynthesis would be highly suitable to rule out superposition densities in false-positive referrals, but many masses would still require further evaluation using ultrasound. As shown in our study, CESM has the potential to rule out superposition densities as well as tomosynthesis can, but it has the additional benefit that many masses caused by cysts can also be safely ruled out without the need for further imaging. In superposition densities, no enhancement whatsoever occurs in the region of interest, and in cysts a specific ‘void’ is seen, which strongly resembles a solar eclipse (Fig. 7). We hereby suggest the introduction of the term ‘eclipse’ for this medical sign of cysts.
Example of a simple cyst as a true negative finding in a 50-year-old patient referred because of a well-defined, dense mass in the right breast (a, arrow, upper row). No enhancement was observed (a, bottom row). More specifically, an enhancement void was observed (b, detail of white box), resembling a solar eclipse (c). Final diagnosis was confirmed by ultrasound (d) and cyst aspiration
CESM also suffers from some disadvantages, of which an increase in breast radiation dose is probably the most important one. Badr et al. recently showed that compared to FFDM, CESM dose increased by 54 % to 2.65 mGy per image [23]. This increase in dose should be taken into account when considering the benefits that CESM would have over conventional mammography, especially in young women. In addition, CESM will result in higher costs, because contrast is used and more time is needed to perform and read the exams. Future studies should also focus on the cost-effectiveness of this new technique.
This study has some limitations. First, our sample size was limited to 113 women in the final analysis and consisted of a population with a disease prevalence lower than published before. However, the population is preselected at the referral stage from the screening programme. Consequently, it is not possible to investigate how CESM would have performed in cases that were not included in the study population. Different screening radiologists might have resulted in different referrals, resulting in a different study population. The limited number of women might suggest that these study results are underpowered. Underpowered studies could have two important consequences: (1) undetected effects of practical importance, and (2) large variance of the parameter being measured. Even in this limited sample size, however, results were statistically significant to a high degree and therefore extremely unlikely to be based on chance. On the other hand, statistical significance does not contain information on the true differences between imaging modalities, and increasing the sample size would improve results with respect to approaching the ‘true’ effect of the parameters studied, and decrease variance. Furthermore, the number of breast cancer cases is small (particularly in situ cancers). Since sensitivity and specificity are correlated, this small number of abnormal cases might also influence specificity. For future studies, it would be recommendable to use a larger sample size, preferably evaluated by multiple readers.
A second potential limitation is that we were not able to assess breast density. As previously mentioned, breast density can influence mammography’s diagnostic accuracy. However, current commercially available (semi)automated breast density analysis software tools have not been validated for use in advanced mammography techniques, such as CESM. Visual inspection of breast density is inaccurate and observer-dependent [24]. Consequently, we could not perform a reliable analysis of breast density for this study.
A third potential study limitation is that no follow-up occurred in patients with abnormalities presumably caused by superposition of fibroglandular tissue. A follow-up period of at least 2 years would be preferable in order to rule out any malignancies occurring in the time frame between screening rounds. However, our standard reference procedure of diagnosing superposition densities is in line with the NHSBSP’s Clinical Guidelines for Breast Cancer Screening Assessment. Furthermore, a recently performed institutional quality control of this approach showed that this approach can be safely used to rule out breast cancer (Van Roozendaal et al., internal publication). In this institutional quality control, 582 referrals from breast cancer screening were analysed. Of these, 95 were diagnosed as ‘superposition cases’ with the before mentioned approach. Follow-up results were collected for these cases, divided into groups according to their follow-up policy: single breast MRI (37 cases), mammographic follow-up in 6 (41 cases) or in 12 months (4 cases), or no follow-up (13 cases). None of the superposition lesions proved to be malignant (median follow-up 26 months).
Conclusion
CESM is logistically feasible as a breast imaging tool in clinical practice. CESM significantly increases diagnostic accuracy in patients referred from breast cancer screening as compared to conventional mammography. Our study showed that diagnostic accuracy of CESM is high, even in populations with lower pretest breast cancer probability than previously published. The good agreement found between tumour diameters based on CESM, MRI and pathology supports CESM as a potential replacement for breast MRI in the preoperative evaluation of breast cancer patients.
References
Pisano ED, Gatsonis C, Hendrick E et al (2005) Diagnostic performance of digital versus film mammography for breast cancer screening. N Engl J Med 353:1773–1783
Carney PA, Miglioretti DL, Yankaskas BC et al (2003) Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med 138:168–175
Kuhl C (2007) The current status of breast MR imaging part I: Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 244:356–378
Lobbes MBI, Smidt ML, Houwers J, Tjan-Heijnen VC, Wildberger JE (2013) Contrast-enhanced mammography: techniques, current results and potential indications. Clin Radiol 68:935–944
Timmers JMH, Van Doorne-Nagtegaal HJ, Zonderland HM et al (2012) The Breast Imaging Reporting and Data System (BI-RADS) in the Dutch breast cancer screening programme: its role as an assessment and stratification tool. Eur Radiol 22:1717–1723
Stacul F, Van der Molen AJ, Reimer P et al (2011) Contrast-induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 21:2527–2541
Nationaal Borstkanker Overleg Nederland (NABON) National guideline breast cancer 2012, Amsterdam, NABON, 2012
Obuchowksi NA (2003) Receiver operating characteristic curves and their use in radiology. Radiology 229:3–8
Mann RM, Kuhl CK, Kinkel K, Boetes C (2008) Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol 18:1307–1318
Liston J, Wilson R (2010) NHSBSP clinical guidelines for breast cancer screening assessment, 3rd edn. NHS Cancer Screening Programmes, Sheffield
Dromain C, Balleyguier C, Adler G, Garbay JR, Delaloge S (2009) Contrast-enhanced digital mammography. Eur J Radiol 69:34–42
American College of Radiology (2003) Breast Imaging Reporting and Data System (BI-RADS), vol 4. American College of Radiology, Reston
Hanley JA, McNeil BJ (1983) A method of comparing the area under receiver operating characteristic curves derived from the same cases. Radiology 148:839–843
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Jochelson MS, Dershaw DD, Sung JS et al (2013) Bilateral contrast-enhanced dual energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 266:743–751
Fallenberg EM, Dromain C, Diekmann F et al (2014) Contrast-enhanced spectral mammography versus MRI: initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol 24:256–264
Lobbes MB, Nelemans PJ (2013) Good correlation does not automatically imply good agreement: the trouble with comparing tumour size by breast MRI versus histopathology. Eur J Radiol 82:e906–e907
Dromain C, Balleyguier C, Muller S et al (2006) Evaluation of tumour angiogenesis of breast carcinoma using contrast-enhanced digital mammography. Am J Roentgenol 187:528–537
Dromain C, Thibault F, Muller S et al (2011) Dual-energy contrast-enhanced digital mammography: initial clinical results. Eur Radiol 21:565–574
Skaane P, Bandos AI, Gullien R et al (2013) Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 267:47–56
Zuley ML, Bandos AI, Ganott MA et al (2013) Digital breast tomosynthesis versus supplemental diagnostic mammographic views for evaluation of noncalcified breast lesions. Radiology 266:89–95
Michell M, Iqbal A, Wasan RK et al (2012) A comparison of the accuracy of film-screen mammography, full-field digital mammography, and digital breast tomosynthesis. Clin Radiol 67:976–981
Badr S, Laurent N, Régis C et al (2014) Dual-energy contrast-enhanced digital mammography in routine clinical practice in 2013. Diagn Interv Imaging 95:245–258
Lobbes MB, Cleutjens JP, Lima Passos V et al (2012) Density is in the eye of the beholder: visual versus semi-automated assessment of breast density on standard mammograms. Insights Imaging 3:91–99
Acknowledgments
The scientific guarantor of this publication is Dr. M. Lobbes. The authors of this manuscript declare relationships with the following companies: M. Lobbes has received a speaking fee from GE Healthcare for two presentations on CESM. However, GE Healthcare did not provide any funding for this study. The authors had full control of data collection, data analysis and manuscript preparation at all times. The authors state that this work has not received any funding. One of the authors (P. Nelemans) has significant statistical expertise. Institutional review board approval was not required because in the Netherlands, research covered by the Medical Research Involving Human Subjects Act must be submitted to an accredited medical ethics committee for approval. Our medical ethics committee concluded that the research proposal of the current study does not, under Dutch law, require medical ethics approval because no extra burden is placed on research subjects. Written informed consent was waived by the institutional review board. Methodology: retrospective, diagnostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lobbes, M.B.I., Lalji, U., Houwers, J. et al. Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur Radiol 24, 1668–1676 (2014). https://doi.org/10.1007/s00330-014-3154-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3154-5