Abstract
Background
Sarcopenia was previously linked to clinical outcomes for several cancer types, including esophageal cancer (EC), but most studies only measured the quantity of skeletal muscle mass. We aim to assess the clinical significance of evaluating the quantity and quality of skeletal muscle in patients with EC who underwent neoadjuvant chemotherapy (NAC) followed by esophagectomy.
Methods
We included 333 consecutive patients with EC who underwent NAC followed by esophagectomy. The psoas muscle index (PMI) and intracellular muscle adipose tissue content (IMAC) were measured by computed tomography. We defined low PMI combined with high IMAC as severe sarcopenia, and assessed its impact on clinical outcomes.
Results
Thirty-seven patients (11.1%) had severe sarcopenia. Compared with patients without severe sarcopenia, those with severe sarcopenia showed a significantly worse NAC response rate (54.1% vs 74.7%; P = 0.008), worse pathological response rate (24.3% vs 40.2%, P = 0.061), higher morbidity rate (67.6% vs 38.5%; P = 0.001), particularly for pneumonia (32.4% vs 14.9% P = 0.007) and expectoration disorder (37.8% vs 13.5% P < 0.001), and unfavorable survival (3-year overall survival rate: 54.1% vs 66.6% P = 0.027). Multivariable analysis of overall survival showed that severe sarcopenia (HR 1.68, P = 0.025) and cT (HR 1.52, P = 0.032) were independent prognostic factors of poor outcome.
Conclusions
PMI combined with IMAC represents a new criterion for sarcopenia that might be useful for predicting NAC response, postoperative complications, and long-term survival in patients with EC undergoing multidisciplinary treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Esophageal cancer (EC) is a highly aggressive malignant tumor with unfavorable prognosis1 As treatment, esophagectomy is quite invasive and was associated with a high rate of postoperative complications.2,3 EC closely correlates with poor nutritional status, because many patients manifest dysphagia and weight loss, which may lead to chemotherapy intolerance and poor postoperative outcomes.4 Furthermore, neoadjuvant chemotherapy (NAC), a standard treatment for resectable, locally advanced EC in Japan,5,6 could impair nutritional or performance status in patients who must undergo multimodal treatments. In this situation, predicting the risk of unfavorable clinical outcomes might contribute to optimizing the treatment strategy for multimodal therapy in patients with EC.
Sarcopenia is characterized by reduced skeletal muscle mass and function, related to aging or disease.7 Sarcopenia is associated with adverse clinical outcomes in several types of cancer, including colorectal8, pancreatic,9 and small cell lung cancers,10 and EC.11 Previous reports assessed the quantity of muscle using either the skeletal muscle mass index (SMI) or psoas muscle mass index (PMI). However, in diagnosing sarcopenia, these single parameters do not always provide consistent results.12 Thus, we need a more accurate method for assessing sarcopenia.
On the other hand, intracellular adipose tissue content (IMAC), a new parameter for describing sarcopenia, reflects the “quality” of muscle. IMAC was expected to be a potential predictor of clinical outcomes in cancer therapies.13,14,15,16 In fact, a previous study reported that IMAC identified a subgroup of patients with normal PMI who showed unfavorable prognosis in extrahepatic biliary cancer17 However, in EC, particularly among patients who receive multidisciplinary treatments, it remains unclear whether measuring IMAC, in addition to PMI, might provide better prediction of clinical outcome. The present retrospective study aimed to evaluate the clinical utility of a novel sarcopenia category, based on both PMI and IMAC measurements, in a large series of patients with EC who underwent NAC followed by surgery.
Patients and Methods
Patient Eligibility
The present study included 333 consecutive patients with EC who underwent NAC followed by surgical resection at the Department of Gastroenterological Surgery, Graduate School of Medicine, Osaka University from January 2010 to March 2017. Patients who received preoperative radiotherapy were excluded. We collected data related to the characteristics of the primary tumor and oncologic staging, other physical status, surgical and neoadjuvant treatment, NAC-related adverse events, response to NAC, postoperative complications, and prognostic factors. Clinicopathological factors were classified according to the Union for International Cancer Control (UICC) tumor–node–metastasis (TNM) classification of malignant tumors, 8th edition.18 According to the UICC classification, metastasis to supraclavicular lymph nodes, which was previously considered to be locoregional lymph node metastasis, was defined as “distant” lymph node metastasis. Therefore, by definition, patients with supraclavicular lymph node metastases (but no metastases to distant organs) were classified as stage IV in the present study.
Neoadjuvant Chemotherapy and Surgery
Patients underwent one of two NAC regimens: (1) two cycles of 70 mg/m2 docetaxel and 70 mg/m2 cisplatin delivered by rapid intravenous infusion on day 1, combined with 700 mg/m2 5-fluorouracil (5-FU), delivered by continuous intravenous infusion for 5 days (days 1–5), every 3 weeks19,20 (the DCF regimen); or (2) two cycles of 35 mg/m2 adriamycin and 70 mg/m2 cisplatin delivered by rapid intravenous infusion on day 1, along with 700 mg/m2 5-FU delivered as continuous intravenous infusion for 7 days (days 1–7), every 4 weeks (the ACF regimen)21,22,23,24,25,26
All patients underwent two cycles of NAC, and 3–4 weeks later, they received radical subtotal esophagectomy with either two- or three-field lymphadenectomy, followed by reconstruction with a gastric conduit or pediculate jejunum, as described previously.27,28 NAC-related adverse events were classified according to the Common Terminology Criteria for Adverse Events, version 4.0.29,30 Postoperative complications were classified using the Clavien–Dindo classification system.31 The Human Ethics Review Committee of Osaka University Graduate School of Medicine approved the protocol for this retrospective study, and each participant provided signed consent.
PMI and IMAC Measured with Computed Tomography
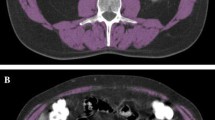
Whole-body computed tomography (CT) scans were performed before and after NAC, as routine care for all eligible patients.32,33 Psoas muscle mass was measured on CT scans with the Synapse Vincent system (Fuji Film Co. Ltd., Tokyo, Japan). Briefly, both sides of the psoas muscle region were selected automatically (Fig. 1a, b), and the cross-sectional psoas muscle area (cm2) was measured at the level of the third lumbar vertebra (L3). The psoas muscle index (PMI) was calculated by adjusting for patient height, as follows:
-
PMI (cm2/m2) = total psoas area at L3 (cm2)/height2 (m2)
At the same cross section, the average CT values (Hounsfield units) of the multifidus muscle and subcutaneous fat were measured (Fig. 1b). IMAC was calculated, as previously reported, with the following formula:
-
IMAC = CT value of multifidus muscle/CT value of subcutaneous fat
The cutoff values for PMI were set at 6.36 cm2/m2 for males and 3.92 cm2/m2 for females. The cutoff values for IMAC were set at – 0.375 for males and – 0.216 for females. These cutoff values were based on the average minus two standard deviations (SDs), observed in healthy Japanese individuals under the age of 50 years.34,35 In this study, patients with low PMI and high IMAC were allocated to the “severe sarcopenia” group. This group was compared with the other patients (nonsevere group) in terms of clinicopathological parameters. In the same manner, the visceral fat area (VFA) and subcutaneous fat area (SFA) were measured at the level of L3 (Supplementary Information).
Method for measuring body composition parameters from cross-sectional CT images. Left: Representative images taken at the third lumbar level show a normal muscle content and b severe sarcopenia, based on PMI and IMAC values. Right: Colored images illustrate the methods for measuring PMI and IMAC. Blue: bilateral psoas muscle areas calculated by automatically tracing the appropriate areas with the Synapse Vincent program. Green: region of interest (ROI) in the multifidus muscle measured precisely by manual tracing. Red: bilateral small, square areas represent subcutaneous fat tissue, distant from major vessels. The IMAC was calculated as the mean CT value of the ROI in the multifidus muscles, divided by the area of subcutaneous fat. CT computed tomography, PMI psoas muscle index, IMAC intramuscular adipose tissue content
Inflammatory Markers
Nutrition and Inflammatory Parameters
Patient inflammatory status was evaluated as follows: patients with both C-reactive protein (CRP) > 0.5 mg/dl and serum albumin < 3.5 g/dl were assigned mGPS = 2, patients with one of these blood chemistry abnormalities were assigned mGPS = 1, and those with neither abnormality were assigned mGPS = 0;36 the Prognostic Nutritional Index (PNI) was defined as 10 × serum albumin [g/dl] + 0.005 × peripheral lymphocyte count [/mm3];37 and the neutrophil–lymphocyte ratio (NLR) was defined as neutrophils (mm3)/lymphocytes (mm3).38 The cutoff values for these parameters were set to ensure the largest difference between the two groups, based on previous reports.36,38
Evaluation of Tumor Response to NAC
All patients underwent restaging with CT, endoscopy, and positron emission tomography in tandem with CT to evaluate the clinical response at 2–3 weeks after NAC. Clinical tumor response was evaluated by esophagoscopy and CT after each cycle of chemotherapy,27,33 in accordance with criteria established by the Japanese Society for Esophageal Disease (JSED).6 The histopathological tumor response was evaluated according to JSED histological criteria.6,22
Statistical Analysis
Data were analyzed using JMP14 software (SAS Institute Inc., Cary, NC, USA). Continuous data are reported as mean ± standard deviation. Differences between groups were analyzed with the Pearson chi-squared (χ2) test for categorical variables. The Mann–Whitney U-test was used for group comparisons of continuous data at a single time point. Kaplan–Meier survival curves and log-rank analyses were performed to evaluate potential survival differences between groups. Because our main focus was to identify pretreatment prognostic factors, we only included pretreatment factors in the univariable analysis for OS. Items that showed significant associations on univariable analysis were entered into multivariable analyses with logistic regression models. Results are expressed as odds ratio (OR) or hazard ratio (HR) with 95% confidence interval (95% CI). P values < 0.05 are considered significant.
Results
PMI and IMAC Values and Their Correlation
The median (range) PMI was 7.11 (3.06–25.3) for men and 5.03 (3.43–7.45) for women. We created a low-PMI group that comprised 98 (33.3%) males and 5 (12.8%) females. On the other hand, the median (range) IMAC was – 0.424 (– 0.961 to – 0.028) for men and – 0.212 (– 0.767 to 0.065) for women. We created a high-IMAC group that comprised 90 (30.6%) males and 21 (53.8%) females. We found no significant relationship between PMI and IMAC values, regardless of gender (Fig. 2). Among all eligible patients, 37 (11.1%; 36 males and 1 female) had both low PMI and high IMAC, and these patients were categorized as “severe sarcopenia” in the present study (Fig. 2b, c). CT images of severe sarcopenia showed an extremely thin psoas muscle and a coarse multifidus muscle (Fig. 1b).
Correlations between PMI and IMAC values. Data show values for each patient in the indicated groups, and lines show correlations: a all patients (n = 334), b males (n = 295), and c females (n = 39). R2 values show that none of the correlations were significant. b, c Patients groups are indicated by dashed lines and a bold box. Normal values are shown in the top-right quadrant, moderate values in the top-left and bottom-right quadrants, and severe values (low PMI and high IMAC) inside the box. Severe sarcopenia was observed in 36 males and 1 female. PMI psoas muscle index, IMAC intramuscular adipose tissue content
Association between Severe Sarcopenia and Background Parameters
We compared clinicopathological characteristics between patients with severe sarcopenia and those without severe sarcopenia (nonsevere group; Table 1). The severe sarcopenia group had significantly greater median age (71 years) compared with the nonsevere group (67 years, P = 0.006). Notably, the severe sarcopenia group had significantly larger median VFA (90.4 cm2) than the nonsevere group (70.4 cm2, P = 0.010). However, we identified no significant differences between groups regarding other parameters, including sex, tumor location, cT, cN, cStage, pT, pN, pStage, comorbidity, American Society for Anesthesiologists physical status, NAC regimen, average relative dose intensity, CRP levels, prognostic nutritional index, NLR, or modified Glasgow Prognostic Scale. Moreover, the two groups were not significantly different in surgical factors, including operation time, blood loss, reconstruction route, reconstruction organ, surgical approach, or fields of lymphadenectomy.
Correlation between Severe Sarcopenia and NAC-Related Adverse Events
Supplementary Table 1 presents the details of NAC-related adverse events in the two groups. The incidences of leukopenia (70.3% vs 53.7%, P = 0.056) and febrile neutropenia (62.2% vs 45.6%, P = 0.057) tended to be higher in the severe sarcopenia group compared with the nonsevere group. No significant difference between groups was observed in the overall frequency of adverse events (89.2% vs 84.8%, P = 0.477).
Impact of Severe Sarcopenia on Tumor Response to NAC
The two groups were compared regarding their tumor response to NAC (Table 2). The clinical response rate was significantly lower in the severe sarcopenia group compared with the nonsevere group (54.1% vs 74.7%, P = 0.008). Consistent with that finding, the pathological response, measured as the percentage of patients who showed moderate (grade 2) or marked (grade 3) NAC effectiveness, tended to be lower in the severe sarcopenia group (24.3%) than in the nonsevere group (40.2%, P = 0.061). Univariable and multivariable analyses of factors that might be associated with the clinical response to NAC showed that severe sarcopenia (OR 2.45, 95% CI 1.20–5.02; P = 0.014) and the ACF NAC regimen (OR 2.36, 95% CI 1.34–4.15; P = 0.003) were independent predictors of poor response (Table 2B).
Association between Severe Sarcopenia and Postoperative Complications
Table 3 presents a comparison of postoperative morbidity between the severe sarcopenia and the nonsevere group. The rates of overall complications (67.6% vs 38.5%, P = 0.001), particularly postoperative pneumonia (32.4% vs 14.9%, P = 0.007) and expectoration disorder (37.8% vs 13.5%, P < 0.001), were significantly higher in the severe sarcopenia group than in the nonsevere group (Table 3A). Univariable logistic regression analysis (Table 3B) revealed that age (P = 0.002), reconstruction with pediculate jejunum (P = 0.007), subcutaneous route (P = 0.002), blood loss (P = 0.011), and severe sarcopenia (P = 0.001) were significantly associated with overall complications. Multivariable analysis showed that, among these factors, severe sarcopenia (OR 2.69, 95% CI 1.27–5.68; P = 0.010) and age (OR 1.77, 95% CI 1.07–2.92; P = 0.026) were independent predictive factors of overall complications (Table 3B). We also compared postoperative complications between normal- and low-PMI groups, and between normal- and high-IMAC groups (Supplementary Table 2).
Influence of Pretherapeutic PMI and IMAC on Long-Term Survival
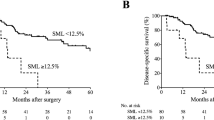
Supplementary Fig. 1 shows the overall survival (OS), recurrence-free survival (RFS), and cancer-specific survival (CSS) curves, according to the PMI or IMAC values. Neither PMI nor IMAC was identified as a significant prognostic factor on its own (Supplementary Fig. 1). On the other hand, when we combined the PMI and IMAC parameters, the 3-year OS rate was 54.1% for patients with low PMI/high IMAC, 67.5% for patients with low PMI/normal IMAC, 63.7% for patients with normal PMI/high IMAC, and 67.8% for patients with normal PMI/normal IMAC; moreover, the 3-year RFS rates were 40.5%, 55.0%, 52.3%, and 49.8%, respectively (Fig. 3). Similarly, the 3-year OS and RFS rates in the severe sarcopenia group were significantly worse than those in the nonsevere group (3-year OS: 54.1% vs 66.6%, P = 0.027; 3-year RFS: 40.5% vs 51.5%, P = 0.036). The severe sarcopenia group tended to have worse cancer-specific survival compared with the nonsevere group; however, the difference between groups was not statistically significant. Univariable logistic regression analysis revealed that cT (P = 0.036) and severe sarcopenia (P = 0.029) were significant factors of OS (Table 4). Multivariable analysis showed that both cT (HR 1.52, 95% CI 1.04–2.22; P = 0.032) and severe sarcopenia (HR 1.68; 95% CI 1.07–2.65, P = 0.025) were independent predictive factors of OS.
a Overall, b recurrence-free, and c cancer-specific survival among four groups, classified according to PMI and IMAC values. a, b Patients had normal values (red), low PMI values (yellow), high IMAC values (green), or low PMI and high IMAC values (blue). Comparison of patients with normal values (red) compared with patients with severe sarcopenia (blue) in d overall survival, e recurrence-free survival, and f cancer-specific survival. PMI psoas muscle index, IMAC intramuscular adipose tissue content
Discussion
The present results suggest that PMI combined with IMAC measurements could serve as a novel categorization of sarcopenia. Our measurements on CT scans performed before NAC revealed that, among patients with EC, low PMI combined with high IMAC values were significantly associated with worse response to chemotherapy, high morbidity rate, particularly pneumonia, and worse long-term survival. Thus, the new sarcopenia criterion, based on PMI and IMAC values, was useful in predicting clinical outcomes of patients with EC who underwent multidisciplinary treatments.
Several previous studies have described CT-based diagnosis for sarcopenia in patients with EC. Indeed, sarcopenia was associated with adverse events due to NAC, tumor response to NAC, postoperative complications, and prognosis.39,40 However, most of those studies only utilized the PMI to identify patients with sarcopenia. Moreover, no consistent evidence has shown the benefit of PMI measurement in patients with EC. In the present study, we also identified a significant association between the PMI, on its own, and postoperative complications (particularly pneumonia and expectoration disorder; Supplementary Table 2). However, the PMI was not associated with adverse events due to NAC, response to NAC, or long-term survival (Supplementary Fig. 1). This lack of association was presumably because PMI primarily reflects the physical or mechanical aspects of skeletal muscles. Therefore, the increased incidence of postoperative pneumonia and expectoration disorder among patients with low PMI were probably due to deterioration of strength in the respiratory41 and swallowing42 muscles.
Evaluating the “quality” of skeletal muscle with IMAC has recently attracted attention.13,14,15,16 The utility of IMAC for predicting clinical outcomes was reported previously in various cancers14,16,43 However, to the best of the authors’ knowledge, the present study is the first to use IMAC to evaluate muscle quality in patients with EC. We found a significant association between IMAC, on its own, and adverse events related to NAC, clinical response to NAC, and development of postoperative morbidity (Supplementary Table 2). The relationship between intramuscular fat accumulation and cancer treatment outcomes has been reported in Japan and Western countries. However, most reports that evaluated intramuscular fat accumulation with the IMAC were studies from Japan. Therefore, a consensus on the standardization or optimization of the method for evaluating intramuscular fat accumulation remains a future challenge.
The detailed mechanisms that link high IMAC to the tumor response remain unclear. Zoico et al. suspected that adipose tissue infiltration into skeletal muscle, which was observed on histological examination, might be associated with high mRNA expression of pro-inflammatory factors, including interleukin (IL)-6 and suppressor of cytokine signaling 3 (SOCS-3).44 These mediators might disturb the immune system and tumor microenvironment, which could lead to a poor response to chemotherapy and unfavorable survival. Accordingly, in contrast to the PMI, adipose tissue infiltration into muscle might reflect the inflammatory aspects of skeletal muscle. Thus, the novel sarcopenia criterion, based on both the PMI and IMAC, might reflect both the mechanical and inflammatory aspects of skeletal muscle. For this reason, this combination might be a better predictor of clinical outcomes than either factor alone, in patients with EC. In this study, severe sarcopenia was associated with OS and RFS, but not with CSS. This finding might be explained by the fact that the severe sarcopenia group had a higher proportion of death from other diseases compared with the nonsevere group (31.8% vs 10.8%, P = 0.180). Furthermore, we examined the recurrence sites and found significantly more cases of distant metastatic recurrence in the severe sarcopenia group than in the nonsevere group (severe vs nonsevere: 82.3% vs 53.1%, P = 0.022). In cases of severe sarcopenia, chronic inflammation may cause immune escape of tumor cells, by mechanisms such as T-cell exhaustion. Thus, our finding might be due to immune escape, which can affect systemic recurrence more than locoregional recurrence.
This study had several limitations. First, it was a retrospective cohort study conducted at a single institution, which could have introduced a potential selection bias. Second, the present study did not examine whether interventions, such as rehabilitation or nutritional support, during preoperative treatment contribute to the maintenance and improvement of muscle mass and muscle quality; in fact, active exercise and nutritional management were reported to improve muscle strength effectively in both healthy individual45 and patients with cancer.46 A future prospective study should be performed to investigate the effectiveness of nutritional and exercise interventions during NAC in EC patients. Third, severe sarcopenia was diagnosed by using cutoff values for PMI and IMAC based on data from healthy subjects; however, these cutoff values were not optimized for diagnosing sarcopenia. In fact, more than half of the male patients in this study were assigned to the low-PMI group and also to the low-IMAC group. Thus, assessments performed with these cutoff values might not have detected patients with “true” sarcopenia. Moreover, optimal cutoff values might vary according to ethnicity, nationality, lifestyle, and clinical setting; consequently, the values used here might not be universally applicable. Optimizing the cutoff values for PMI and IMAC is the greatest challenge in CT-based sarcopenia studies. Finally, we diagnosed sarcopenia using only CT-based parameters, similar to previous studies. The European Working Group on Sarcopenia in Older People (EWGSOP) recommends a set of approaches for diagnosing sarcopenia, including muscle mass, handgrip strength, and gait speed. Further study is necessary to evaluate associations between these CT-based parameters and the actual strength and functional capacity of skeletal muscle.
In conclusion, we found that severe sarcopenia, a novel category characterized by low PMI and high IMAC, was associated with the clinical response to chemotherapy, postoperative complications, and long-term survival, in a large series of patients with EC who underwent NAC followed by surgery. In the future, a prospective study with a large number of patients would be necessary to validate our findings. Nevertheless, the present study provides important information that might ultimately lead to improved clinical outcomes in patients with EC who must undergo multimodal treatments.
Data Availability
The data that support the findings of this study are available on request from the corresponding author, Tomoki Makino. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
References
Gertler R, Stein HJ, Langer R, et al. Long-term outcome of 2920 patients with cancers of the esophagus and esophagogastric junction: evaluation of the New Union Internationale Contre le Cancer/American Joint Cancer Committee staging system. Ann Surg. 2011;253(4):689–98.
Yamashita K, Makino T, Miyata H, et al. Postoperative infectious complications are associated with adverse oncologic outcomes in esophageal cancer patients undergoing preoperative chemotherapy. Ann Surg Oncol. 2016;23(6):2106–14.
Wu PC, Posner MC. The role of surgery in the management of oesophageal cancer. Lancet Oncol. 2003;4(8):481–8.
Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg. 2000;190(5):562–72.
Japanese Classification of Esophageal Cancer. 11th Edition: part I. Esophagus. 2017;14(1):1–36.
Japanese Classification of Esophageal Cancer. 11th Edition: part II and III. Esophagus. 2017;14(1):37–65.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23.
Miyamoto Y, Baba Y, Sakamoto Y, et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol. 2015;22(8):2663–8.
Onesti JK, Wright GP, Kenning SE, et al. Sarcopenia and survival in patients undergoing pancreatic resection. Pancreatology. 2016;16(2):284–9.
Go SI, Park MJ, Song HN, et al. Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Supportive Care Cancer. 2016;24(5):2075–84.
Ishida T, Makino T, Yamasaki M, et al. Impact of measurement of skeletal muscle mass on clinical outcomes in patients with esophageal cancer undergoing esophagectomy after neoadjuvant chemotherapy. Surgery. 2019;166(6):1041–7.
Deng HY, Zha P, Peng L, Hou L, Huang KL, Li XY. Preoperative sarcopenia is a predictor of poor prognosis of esophageal cancer after esophagectomy: a comprehensive systematic review and meta-analysis. Dis Esophagus. 2019;32(3).
Fukuda Y, Asaoka T, Eguchi H, et al. Clinical Impact of Preoperative sarcopenia on the postoperative outcomes after pancreas transplantation. World J Surg. 2018;42(10):3364–71.
Ojima Y, Harano M, Sumitani D, Okajima M. Impact of preoperative skeletal muscle mass and quality on the survival of elderly patients after curative resection of colorectal cancer. J Anus Rectum Colon. 2019;3(4):143–51.
Okumura S, Kaido T, Hamaguchi Y, et al. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015;157(6):1088–98.
Waki Y, Irino T, Makuuchi R, et al. Impact of preoperative skeletal muscle quality measurement on long-term survival after curative gastrectomy for locally advanced gastric cancer. World J Surg. 2019;43(12):3083–93.
Okumura S, Kaido T, Hamaguchi Y, et al. Impact of the preoperative quantity and quality of skeletal muscle on outcomes after resection of extrahepatic biliary malignancies. Surgery. 2016;159(3):821–33.
James D. Brierley (Editor) MKGE, Christian Wittekind (Editor). TNM Classification of Malignant Tumours, 8th Edition. Oxford: Wiley-Blackwell; 2017.
Hagi T, Makino T, Yamasaki M, et al. Pathological regression of lymph nodes better predicts long-term survival in esophageal cancer patients undergoing neoadjuvant chemotherapy followed by surgery. Ann Surg. 2020. https://doi.org/10.1097/SLA.0000000000004238.
Makino T, Yamasaki M, Miyazaki Y, et al. Utility of initial induction chemotherapy with 5-fluorouracil, cisplatin, and docetaxel (DCF) for T4 esophageal cancer: a propensity score-matched analysis. Dis Esophagus. 2018;31(4).
Makino T, Yamasaki M, Takemasa I, et al. Dickkopf-1 expression as a marker for predicting clinical outcome in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16(7):2058–64.
Makino T, Yamasaki M, Takeno A, et al. Cytokeratins 18 and 8 are poor prognostic markers in patients with squamous cell carcinoma of the oesophagus. Br J Cancer. 2009;101(8):1298–306.
Hashimoto T, Makino T, Yamasaki M, et al. The pattern of residual tumor after neoadjuvant chemotherapy for locally advanced esophageal cancer and its clinical significance. Ann Surg. 2020;271(5):875–84.
Makino T, Yamasaki M, Tanaka K, et al. Metabolic tumor volume change predicts long-term survival and histological response to preoperative chemotherapy in locally advanced esophageal cancer. Ann Surg. 2019;270(6):1090–5.
Urakawa S, Makino T, Yamasaki M, et al. Lymph node response to neoadjuvant chemotherapy as an independent prognostic factor in metastatic esophageal cancer. Ann Surg. 2019. https://doi.org/10.1097/SLA.0000000000003445.
Yamasaki M, Yasuda T, Yano M, et al. Multicenter randomized phase II study of cisplatin and fluorouracil plus docetaxel (DCF) compared with cisplatin and fluorouracil plus adriamycin (ACF) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (OGSG1003). Ann Oncol. 2017;28(1):116–20.
Makino T, Yamasaki M, Miyata H, et al. Solitary lymph node recurrence of esophageal squamous cell carcinoma: surgical failure or systemic disease? Ann Surg Oncol. 2016;23(6):2087–93.
Makino T, Yamasaki M, Tanaka K, et al. Importance of positron emission tomography for assessing the response of primary and metastatic lesions to induction treatments in T4 esophageal cancer. Surgery. 2017;162(4):836–45.
Hagi T, Makino T, Yamasaki M, et al. Dysphagia score as a predictor of adverse events due to triplet chemotherapy and oncological outcomes in 434 consecutive patients with esophageal cancer. Ann Surg Oncol. 2019;26(13):4754–64.
Nc. I. Common Terminology Criteria for Adverse Events v4.0. NIH-publication. 2009;09-7473.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Makino T, Doki Y, Miyata H, et al. Use of (18)F-fluorodeoxyglucose-positron emission tomography to evaluate responses to neo-adjuvant chemotherapy for primary tumor and lymph node metastasis in esophageal squamous cell carcinoma. Surgery. 2008;144(5):793–802.
Makino T, Miyata H, Yamasaki M, et al. Utility of response evaluation to neo-adjuvant chemotherapy by (18)F-fluorodeoxyglucose-positron emission tomography in locally advanced esophageal squamous cell carcinoma. Surgery. 2010;148(5):908–18.
Hamaguchi Y, Kaido T, Okumura S, et al. Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation. 2017;101(3):565–74.
Hamaguchi Y, Kaido T, Okumura S, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition. 2016;32(11–12):1200–5.
Toiyama Y, Miki C, Inoue Y, Tanaka K, Mohri Y, Kusunoki M. Evaluation of an inflammation-based prognostic score for the identification of patients requiring postoperative adjuvant chemotherapy for stage II colorectal cancer. Exp Ther Med. 2011;2(1):95–101.
Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–5.
Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–30.
Nishigori T, Okabe H, Tanaka E, Tsunoda S, Hisamori S, Sakai Y. Sarcopenia as a predictor of pulmonary complications after esophagectomy for thoracic esophageal cancer. J Surg Oncol. 2016;113(6):678–84.
Murimwa GZ, Venkat PS, Jin W, et al. Impact of sarcopenia on outcomes of locally advanced esophageal cancer patients treated with neoadjuvant chemoradiation followed by surgery. J Gastrointest Oncol. 2017;8(5):808–15.
Bahat G, Tufan A, Ozkaya H, et al. Relation between hand grip strength, respiratory muscle strength and spirometric measures in male nursing home residents. Aging Male. 2014;17(3):136–40.
Wakabayashi H, Sakuma K. Rehabilitation nutrition for sarcopenia with disability: a combination of both rehabilitation and nutrition care management. J Cachexia Sarcopenia Muscle. 2014;5(4):269–77.
Shirai H, Kaido T, Hamaguchi Y, et al. Preoperative low muscle mass and low muscle quality negatively impact on pulmonary function in patients undergoing hepatectomy for hepatocellular carcinoma. Liver Cancer. 2018;7(1):76–89.
Zoico E, Corzato F, Bambace C, et al. Myosteatosis and myofibrosis: relationship with aging, inflammation and insulin resistance. Arch Gerontol Geriatr. 2013;57(3):411–6.
Wu PY, Huang KS, Chen KM, Chou CP, Tu YK. Exercise, nutrition, and combined exercise and nutrition in older adults with sarcopenia: a systematic review and network meta-analysis. Maturitas. 2021;145:38–48.
Zimmer P, Trebing S, Timmers-Trebing U, et al. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial. Supportive Care Cancer. 2018;26(2):615–24.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Ethics Approval and Consent to Participate
The Human Ethics Review Committee of Osaka University Graduate School of Medicine approved the protocol for this retrospective study, and each participant provided signed consent. All procedures were in accordance with the Declaration of Helsinki.
Consent for Publication
Consent for publication has been obtained from individuals whose data are included in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10434_2021_10025_MOESM3_ESM.tiff
Supplementary file3. Figure S1:Overall and recurrence free survival in patients treated foresophageal carcinoma, classified by either the PMI or the IMAC value. Red lines indicatepatients with normal values. Blue lines indicate patients with low PMI or high IMAC values.(A, D O verall survival , B, E ) recurrence free survival , and (C,F) cancer specific survivalPMI: psoas muscle index; IMAC: intramuscular adipose tissue content (TIFF 32221 kb)
Rights and permissions
About this article
Cite this article
Ishida, T., Makino, T., Yamasaki, M. et al. Quantity and Quality of Skeletal Muscle as an Important Predictor of Clinical Outcomes in Patients with Esophageal Cancer Undergoing Esophagectomy after Neoadjuvant Chemotherapy. Ann Surg Oncol 28, 7185–7195 (2021). https://doi.org/10.1245/s10434-021-10025-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10025-x