Abstract
To evaluate the feasibility of low-concentration contrast medium (CM) for vascular enhancement, image quality, and radiation dose on computed tomography aortography (CTA) using a combined low-tube-voltage and iterative reconstruction (IR) technique. Ninety subjects underwent dual-source CT (DSCT) operating in dual-source, high-pitch mode. DSCT scans were performed using both high-concentration CM (Group A, n = 50; Iomeprol 400) and low-concentration CM (Group B, n = 40; Iodixanol 270). Group A was scanned using a reference tube potential of 120 kVp and 120 reference mAs under automatic exposure control with IR. Group B was scanned using low-tube-voltage (80 or 100 kVp if body mass index ≥25 kg/m2) at a fixed current of 150 mAs, along with IR. Images of the two groups were compared regarding attenuation, image noise, signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR), iodine load, and radiation dose in various locations of the CTA. In comparison between Group A and Group B, the average mean attenuation (454.73 ± 86.66 vs. 515.96 ± 101.55 HU), SNR (25.28 ± 4.34 vs. 31.29 ± 4.58), and CNR (21.83 ± 4.20 vs. 27.55 ± 4.81) on CTA in Group B showed significantly greater values and significantly lower image noise values (18.76 ± 2.19 vs. 17.48 ± 3.34) than those in Group A (all Ps < 0.05). Homogeneous contrast enhancement from the ascending thoracic aorta to the infrarenal abdominal aorta was significantly superior in Group B (P < 0.05). Low-concentration CM and a low-tube-voltage combination technique using IR is a feasible method, showing sufficient contrast enhancement and image quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Computed tomography aortography (CTA) is a well-known minimally invasive method used to reveal aortic vascular anatomy and to detect pathologic conditions. CTA is frequently used to diagnose and follow-up various aortic diseases including acute aortic syndrome [1]. However, CTA is often done using a high-concentration iodinated contrast medium (CM) to acquire proper vascular attenuation throughout the aorta, as well as a wide scan range. This combination increases the total amount of iodine and radiation exposure [2, 3]. Reduction of the radiation dose and the incidence and severity of CM side effects, especially in contrast-induced nephropathy (CIN), have been addressed in many studies and the amount of iodine used is an important risk factor in CIN [4, 5]. A low-concentration iodinate CM may be helpful in reducing the iodine dose.
Several previous studies have shown that coronary CT angiography or CTA using low-tube-voltage kVp and a low-concentration of CM with iterative reconstruction (IR) maintains the CT attenuation of coronary arteries and image quality with the advantage of reducing the radiation dose when compared with conventional coronary CT angiography using high-tube-voltage and a high concentration of CM [6, 7]. Nowadays, automatic exposure control (AEC) is being used to reduce the radiation exposure. However, until now, there has been no study directly comparing CTA using a low-tube-voltage kVp and a low-concentration of CM with IR with conventional CTA using a high concentration of CM and AEC with IR.
The purpose of this study was to assess the feasibility of low-concentration CM for vascular enhancement, image quality, and radiation dose on CTA using a combined low-tube-voltage and IR technique performed using dual-source CT (DSCT).
Methods
Patient population
Between January 2013 and May 2014, 105 patients who were clinically referred for follow-up evaluation after surgery for aortic diseases, or who had abnormalities of the aorta, such as aortic aneurysm, acute aortic syndrome, Takayasu arteritis, Marfan syndrome, and renovascular hypertension, underwent DSCT scan. DSCT scans were performed using Iomeprol 400 (400 mg I/ml, Iomeron, Bracco, Milan, Italy) for patients from January to September 2013 (Group A). From October 2013 to May 2014, the DSCT scan protocol was changed to the use of Iodixanol 270 (270 mg I/ml, Visipaque, GE Healthcare, Little Chalfont, United Kingdom) (Group B). Group A patients were scanned with conventional CTA using AEC. Group B patients were scanned with the low dose CTA protocol and were further subdivided by tube voltage with respect to body mass index (BMI <25 or ≥25 kg/m2). Exclusion criteria included renal insufficiency (serum creatinine >1.5 mg/dl) and poor image quality due to severe motion artifact. Aortic dissection was another exclusion criterion, as it causes inhomogeneous enhancement of the aorta and separates the aortic lumen into true and false lumens. Fifteen patients were excluded and a total of 90 patients were enrolled. The 90 patients enrolled were presenting for CTA for a follow-up study after surgery (n = 25), or abnormalities of the aorta like aortic aneurysm (n = 15), intramural hematoma of the thoracic aorta (n = 20), a penetrating atherosclerotic aortic ulcer (n = 8), Takayasu arteritis (n = 3), Marfan syndrome (n = 1), and renovascular hypertension (n = 18). Sex, age, height, and body weight in each patient were recorded from the medical records. The Institutional Review Board approved the analyses of the clinical and imaging data. Written informed consent was not required as this investigation was retrospective.
CT scan protocol
All procedures were performed using a second-generation DSCT scanner (SOMATOM Definition Flash; Siemens Healthcare, Forchheim, Germany) operating in dual-source, high-pitch mode with a pitch of 2.0, collimation of 2 × 2 × 64 × 0.6 mm using a z-flying focal spot, rotation time of 0.28 s, reference tube potential of 120 kVp, and 120 reference mAs of AEC (CARE kV, CAREdose 4D, Siemens Healthcare) in Group A, and a tube potential of 80 or 100 kVp if BMI ≥25 kg/m2 and fixed 150 mAs in Group B. Scans in both groups were performed in a craniocaudal direction during deep inspiratory breath-hold of the entire chest, abdomen, and pelvis, including the supraclavicular fossa to symphysis pubis. Contrast administration was controlled with bolus tracking and the region of interest (ROI) was placed in the abdominal aorta at the level of the upper pole of the right kidney. The automatic trigger scan started 12 s after the attenuation threshold of 100 Hounsfield units (HU) in both groups. The total injected amount of CM was decided according to the patient’s weight (1.2 ml/kg) and each patient received CM at a flow rate of 4.5 ml/s followed by a 40 ml saline chaser through an 18-gauge needle in the antecubital vein using a dual-syringe power injector (Stellant D, Medrad, Indianola, PA, USA). The amount of total iodine used in each patient was calculated as: [iodine concentration (mg I/ml) × 1.2 ml/kg × body weight (kg)]/1000 (mg/g).
CT image reconstruction
Original scan data were transmitted to the Siemens workstation (SyngoMMWP VE40A, Siemens Medical Solutions). Transverse images were reconstructed at 0.75 mm slice thickness with a 0.5 mm increment using a sonogram-affirmed IR (SAFIRE, Siemens Healthcare, Forchheim, Germany) algorithm with a medium-sooth convolution kernel (I30f), medium strength of level 3, image matrix of 512 × 512 pixels, and a field of view of 200–330 mm. Additional multiplanar reconstruction (MPR) with a slice thickness of 3.0 mm and an increment of 2.0 mm using the same IR techniques was performed and transferred to a regular picture archiving and communication system (PACS) workstation (Maroview V5.4.10.42, INFINITT Healthcare, Seoul, Korea).
Image analysis
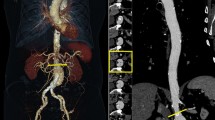
For quantitative evaluation of contrast enhancement, attenuation, and noise values in various locations of the ascending and descending thoracic aorta, and the suprarenal and infrarenal abdominal aorta were measured in consensus by two radiologists with 9 and 2 years of experience in interpreting CTA. To measure the values in axial images, a circular ROI cursor was drawn to encompass the vessel lumen with an effort to avoid calcification, thrombus, and motion artifacts. The locations for each measurement were the ascending and descending thoracic aorta, suprarenal and infrarenal abdominal aorta at the levels of the pulmonary trunk, left inferior pulmonary vein, orifice of the celiac axis, and the inferior mesenteric artery (Fig. 1). Attenuation and noise (standard deviation of the measured attenuation) values expressed in HU within each circular ROI were measured twice and the average value was calculated at each anatomic site. Based on these measurements, the signal-to-noise ratio (SNR) was calculated by the attenuation value/noise value of each anatomic site. Attenuation of the right psoas muscle at the level of the lower pole of the right kidney was also measured by placing an ROI of 200 mm2 [8]. Contrast-to-noise ratio (CNR) was calculated as follows: CNR = (mean attenuation of each site of aorta − attenuation of psoas muscle)/noise.
Measurement of the attenuation and noise values at four different locations and visual homogeneity of luminal attenuation in the aorta (a, b ascending thoracic aorta, c, d descending thoracic aorta, e, f suprarenal abdominal aorta, g, h infrarenal abdominal aorta, i, j curved planar reformation). The mean attenuation and noise values are shown in the images. a, c, e, g, i A 41-year-old woman in whom a CT aortography was scanned using a tube potential of 120 kVp, 120 reference mAs and Iomeprol 400. b, d, f, h, j A 40-year-old women in whom a CT aortography was scanned with low dose CTA protocol and Iodixanol 270. These images of low-concentration contrast medium and a low-tube-voltage combination technique show sufficient contrast enhancement and image quality
Qualitative analyses were independently assigned in a blind fashion by the aforementioned two radiologists, and all images in the two groups were reviewed in random order. All images were evaluated primarily at a window level of 60 HU and a width of 1000 HU. Subjective image quality was graded according to the quality of the general image impression, enhancement, sharpness, and image noise on CTA using a 5-point rating scale (5 = excellent; 4 = good; 3 = moderate; 2 = fair; 1 = poor). Grades of 2, 3, 4, and 5 were assumed to be diagnosable.
Measurement of radiation dose
The CT dose index volume (CTDIvol) and dose-length product (DLP) were recorded during CT scans. The estimated effective dose (ED) was calculated by DLP × k (conversion coefficient) at the thorax, abdomen, or pelvis as:
Tissue weighting factors (TWFs) as DLP to effective dose conversion coefficients were 0.017 in the thorax (T), 0.015 in the abdomen (A), and 0.019 in the pelvis (P) [9].
Data and statistical analyses
Statistical analyses were performed using statistics software (SPSS, version 12.1.1, Chicago, IL, USA). Quantitative data were expressed as the mean ± standard deviation (SD) and analyzed using an independent t test in normally distributed data and a Mann–Whitney U test in non-normally distributed data. The independent t test was performed to analyze the differences between the two groups regarding attenuation value, image noise, SNR, CNR, and radiation dose. Repeated-measures ANOVA was performed to evaluate the difference in homogeneity of contrast enhancement between the two groups. The Chi square test was used to determine the difference in proportion to the subjective scoring for image quality between both groups. Cohen’s kappa statistics were used to assess inter-observer agreement for the subjective assessment of image quality. The kappa value was analyzed using the guideline of Landis and Koch (>0.81 almost perfect agreement; 0.61–0.80 substantial agreement; 0.41–0.60 moderate agreement; 0.20–0.40 fair agreement; <0.20 poor agreement) [10]. For all data analysis, a P value <0.05 was considered statistically significant.
Results
Patient demographics are shown in Table 1. CT scans were successful and diagnostic image quality was attained in all 90 patients (53 male, 37 female; mean age, 60.7 ± 17.3 years). There were no significant differences in age, height, body weight, BMI, or ejection fraction (EF) between the two groups (P > 0.05).
Quantitative evaluation of contrast enhancement
The average mean attenuation (454.73 ± 86.66 vs. 515.96 ± 101.55 HU), SNR (25.28 ± 4.34 vs. 31.29 ± 4.58), and CNR (21.83 ± 4.20 vs. 27.55 ± 4.81) on CTA images in Group B showed significantly greater values and significantly lower image noise values (18.76 ± 2.19 vs. 17.48 ± 3.34) than those in Group A (all Ps < 0.05) (Table 2). Except for attenuation in the ascending aorta, there were significant differences in attenuation, SNR, and CNR values at various locations of the ascending and descending thoracic aorta, and suprarenal and infrarenal abdominal aorta between Group A and Group B, with greater attenuation, SNR, and CNR in Group B (P < 0.05) (Fig. 1; Table 2). No significant differences were found between the two groups regarding the image noise at each of the four locations in the aorta, although there were slightly lower overall image noise values in Group B. Regarding the comparison of contrast enhancement homogeneity from the ascending thoracic aorta to the infrarenal abdominal aorta, Group B showed a significantly more homogeneous attenuation level than Group A (P = 0.007; Figs. 1, 2). Comparisons of attenuation, image noise, SNR, and CNR in each location on CTA in the subgroups of Group B according to BMI are shown in Table 3. There was a significant decrease in attenuation at each CTA location, except in the ascending aorta in subgroup B (BMI ≥25 kg/m2) (P < 0.05).
Attenuation at different anatomic locations in the aorta (Asd TA ascending thoracic aorta, Desd TA descending thoracic aorta, Supra RA suprarenal abdominal aorta, Infra RA infrarenal abdominal aorta). In comparing Group A (Iomeprol 400) and Group B (Iodixanol 270), the average mean attenuation at the different locations in the aorta in Group B show greater values than those in Group A, as well as a superior homogeneous attenuation level of contrast enhancement from the ascending thoracic aorta to the infrarenal abdominal aorta
Subjective image quality
Qualitative image analysis regarding the general image impression, enhancement, sharpness, and image noise revealed mean scores of image quality that were all greater than 4. Except for sharpness, the proportion of subjective scoring for image quality regarding general image impression, enhancement, and image noise were not significantly different between the two groups (Table 4). The inter-observer agreements of linear weighted kappa values ranged from 0.69 to 0.81 (mean 0.78). There was substantial agreement between the two readers in subjective ratings of image quality.
Iodine weight and radiation dose estimation
The administered iodine weight was significantly lower in Group B (20.14 ± 3.58 g) than in Group A (31.02 ± 5.48 g) (P < 0.05; Table 4). A 35 % reduction in iodine weight was noted in Group B. Mean tube potential, tube current, CTDIvol, DLP, and estimated effective radiation dose was significantly lower in Group B than in Group A (P < 0.01; Table 5). In comparison to Group A, the average reduction in the mean estimated effective dose in Group B was 33.8 %.
Discussion
In the present study, attenuation, image noise, SNR, CNR, as well as subjective image quality of CTA images scanned with an intentionally reduced tube voltage using low concentration CM, were compatible with those obtained by conventional CTA, even with the use of recently updated AEC (care kV, care dose 4D) in DSCT in an effort to decrease radiation exposure. The findings indicate that low concentration CM and a low-tube-voltage combination technique using IR is a feasible method for reducing radiation dosages and iodine load.
In routine clinical practice, proper and homogeneous vascular attenuation across the wide scan range of the entire aorta is a primary goal in CTA. High concentration CM in conventional CTA protocols is usually preferred. However, a lower tube voltage helps to improve vascular attenuation, especially when using low concentration CM without any change in the injection rate or total amount of administered CM [11]. In fact, the reduced tube voltage could make the mean photon energy in the X-ray spectrum closer to the K-edge of iodine (33.2 keV), so reducing the tube voltage leads to a higher mean CT attenuation value of iodine [11–13]. However, an increase in the CT attenuation of iodine achieved by reducing the tube voltage will inevitably be accompanied by an increase in image noise resulting in a lower SNR, CNR, and degraded subjective image quality when compared to those obtained with a higher tube voltage [14–16]. In a previous study investigating renal vascular enhancement between 80 kVp-moderate concentration CM (300 mg I/ml) and 120 kVp-high concentration CM (370 mg I/ml), both renal vascular enhancement and subjective image quality were better in the 80 kVp-moderate concentration CM group [17]. However, in their study the authors used a sufficiently increased effective tube current time product (585 mAseff) in their low tube voltage (80 kVp-moderate concentration CM) CTA protocol. To overcome the image noise, the present study introduced an IR technique using a low tube voltage (80 kVp) and a low concentration CM (270 mg I/ml) with a low fixed current (150 mAs) protocol and found that the average of the mean attenuation and subjective image quality, as well as the average image noise, SNR, and CNR were significantly superior to those in the high concentration CM group using the AEC of the low dose CTA protocol. In fact, a considerable limitation of low tube voltage CTA is that the greater the patient’s BMI, the greater the background image noise. This limitation can be overcome using an IR technique [14, 18]. Several studies have also suggested that the reduced tube voltage with IR may increase image quality in comparison with reconstruction of filtered back projections under the same conditions of tube voltage and tube current output [19–22]. These prior findings are similar to the present results, where the IR technique was also applied in both groups using a different tube voltage and tube current. Presently, the average of mean image noise in all four locations in the aorta in Group B in this study was significantly lower than in Group A. However, the data does not seem to suggest that the IR technique in lower dose CTA is more efficient than high-dose CTA, because the mean image noise values at each of the four locations in Group B were not significantly different from those in Group A.
Notably, there was an unexpected finding regarding the comparison of contrast enhancement homogeneity from the ascending thoracic aorta to the infrarenal abdominal aorta, with Group B showing a significantly more homogeneous attenuation level than Group A. The homogeneity of contrast enhancement is mainly dependent on bolus geometry as well as many factors including cardiac output, body weight, and blood volume [23]. Furthermore, a higher heart rate, various characteristics of aortic pathology, as well as the variable voltage and currency of AEC, also influence the image quality of contrast enhancement. In the present study, however, the amount of CM in the scan protocol, EF, and body weight were not significantly different and the injection rate was also the same across both groups. Differences in concentration and iodine delivery rates of CM (1.8 g I/s in Group A and 1.2 g I/s in Group B) may not explain this homogeneity. These findings could be attributed to the osmolarity of CM [24]. Iodixanol (270 mg I/ml) is a dimeric iodinated CM and isoosmolar to plasma (290 mOsm/kg), whereas Iomeprol (400 mg I/ml) is hyperosmolar to blood (726 ± 34 mOsm/kg). Hyperosmolarity leads to the absorption of water from the extracellular space to the intravascular space and a diluted iodine concentration, whereas this effect does not occur with isoosmolar CM [25–27]. In particular, a dimeric iodinated CM has a larger molecular size, which results in slower efflux of CM from the vascular to the extravascular space [28]. This homogeneity may be effective in evaluating for distal lower abdominal aortic aneurysms showing slow and turbulent blood flow.
In the present study, the reduction in the estimated effective dose and iodine load in Group B was as much as 33.8 and 35 %, respectively. Advantages of a reduction in radiation exposure and reduced iodine administration are essential to patients who require re-examination for follow-up CTA and who are at risk for CM induced nephropathy [29–31].
The present study has some limitations. First, although most of the enrolled patients in Group B had a normal weight (BMI 18.5–24.9 kg/m2) or were overweight (30 %, BMI 25–29.9 kg/m2), and just one patient was obese (BMI ≥30 kg/m2), the intentionally reduced tube voltage of CTA protocol using low concentration CM may not be applicable to obese patients. Second, the diagnostic performance of the reduced tube voltage for the CTA protocol using low concentration CM was not evaluated. Only the performance of a reduced tube voltage and low concentration of CM regarding image quality rather than the diagnostic accuracy according to various aortic diseases was evaluated. Third, the drawing of the circular ROIs at the different anatomic levels was not exactly equal for each anatomic level. They were adjusted to the maximal vessel lumen with an effort to avoid calcification, thrombus, and motion artifacts. Fourth, branches of the aorta, such as the celiac trunk and superior mesenteric artery, were not evaluated, although those are also important in detecting the involvement of various aortic diseases. However, sometimes these branches are too small to assess the attenuation without increasing measurement error, and sufficient contrast enhancement and image quality of the aorta suggests there would also be sufficient quality in the major branches of the aorta. Fifth, the attenuation measurements were made in consensus rather than independently.
Despite these limitations, this study was able to demonstrate a substantial and sustained image quality as well as a reduction in the radiation dose and iodine load using a CTA protocol with a low tube voltage and a low concentration of CM.
In conclusion, low concentration CM and low-tube-voltage CTA with DSCT using IR is a feasible method for showing sufficient contrast enhancement and image quality, and can be done using a significantly lower radiation dose and iodine load in non-obese patients.
Abbreviations
- CTA:
-
Computed tomography aortography
- CM:
-
Contrast medium
- CNR:
-
Contrast-to-noise ratio
- DSCT:
-
Dual-source computed tomography
- IR:
-
Iterative reconstruction
- SNR:
-
Signal-to-noise ratio
References
Johnson PT, Heath DG, Kuszyk BS, Fishman EK (1996) CT angiography with volume rendering: advantages and applications in splanchnic vascular imaging. Radiology 200(2):564–568
Rau MM, Setty BN, Blake MA, Ouellette-Piazzo K, Hahn PF, Sahani DV (2007) Evaluation of renal transplant donors with 16-section multidetector CT angiography: comparison of contrast media with low and high iodine concentrations. J Vasc Interv Radiol 18(5):603–609
Lell MM, Anders K, Uder M, Klotz E, Ditt H, Vega-Higuera F, Boskamp T, Bautz WA, Tomandl BF (2006) New techniques in CT angiography. Radiographics 26(Suppl 1):S45–S62
Katzberg RW, Barrett BJ (2007) Risk of iodinated contrast material-induced nephropathy with intravenous administration. Radiology 243(3):622–628
Nyman U, Almen T, Aspelin P, Hellstrom M, Kristiansson M, Sterner G (2005) Contrast-medium-induced nephropathy correlated to the ratio between dose in gram iodine and estimated GFR in ml/min. Acta Radiol 46(8):830–842
Zheng M, Liu Y, Wei M, Wu Y, Zhao H, Li J (2014) Low concentration contrast medium for dual-source computed tomography coronary angiography by a combination of iterative reconstruction and low-tube-voltage technique: feasibility study. Eur J Radiol 83(2):e92–e99
Kanematsu M, Goshima S, Miyoshi T, Kondo H, Watanabe H, Noda Y, Bae KT (2014) Whole-body CT angiography with low tube voltage and low-concentration contrast material to reduce radiation dose and iodine load. AJR Am J Roentgenol 202(1):W106–W116
Nakayama Y, Awai K, Funama Y, Liu D, Nakaura T, Tamura Y, Yamashita Y (2006) Lower tube voltage reduces contrast material and radiation doses on 16-MDCT aortography. AJR Am J Roentgenol 187(5):W490–W497
European Commission Study Group (2000) European guidelines on quality criteria for computed tomography. Publication no. Report EUR 16262 EN. Office for Official Publications of European Communities, Brussels, Belgium
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Brooks RA (1977) A quantitative theory of the Hounsfield unit and its application to dual energy scanning. J Comput Assist Tomogr 1(4):487–493
Nakazato R, Dey D, Gutstein A, Le Meunier L, Cheng VY, Pimentel R, Paz W, Hayes SW, Thomson LE, Friedman JD, Berman DS (2009) Coronary artery calcium scoring using a reduced tube voltage and radiation dose protocol with dual-source computed tomography. J Cardiovasc Comput Tomogr 3(6):394–400
Bahner ML, Bengel A, Brix G, Zuna I, Kauczor HU, Delorme S (2005) Improved vascular opacification in cerebral computed tomography angiography with 80 kVp. Invest Radiol 40(4):229–234
Nakayama Y, Awai K, Funama Y, Hatemura M, Imuta M, Nakaura T, Ryu D, Morishita S, Sultana S, Sato N, Yamashita Y (2005) Abdominal CT with low tube voltage: preliminary observations about radiation dose, contrast enhancement, image quality, and noise. Radiology 237(3):945–951
Sahani DV, Kalva SP, Hahn PF, Saini S (2007) 16-MDCT angiography in living kidney donors at various tube potentials: impact on image quality and radiation dose. AJR Am J Roentgenol 188(1):115–120
Waaijer A, Prokop M, Velthuis BK, Bakker CJ, de Kort GA, van Leeuwen MS (2007) Circle of Willis at CT angiography: dose reduction and image quality—reducing tube voltage and increasing tube current settings. Radiology 242(3):832–839
Cho ES, Yu JS, Ahn JH, Kim JH, Chung JJ, Lee HK, Lee KH (2012) CT angiography of the renal arteries: comparison of lower-tube-voltage CTA with moderate-concentration iodinated contrast material and conventional CTA. AJR Am J Roentgenol 199(1):96–102
Sigal-Cinqualbre AB, Hennequin R, Abada HT, Chen X, Paul JF (2004) Low-kilovoltage multi-detector row chest CT in adults: feasibility and effect on image quality and iodine dose. Radiology 231(1):169–174
Marin D, Nelson RC, Schindera ST, Richard S, Youngblood RS, Yoshizumi TT, Samei E (2010) Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm—initial clinical experience. Radiology 254(1):145–153
Moscariello A, Takx RA, Schoepf UJ, Renker M, Zwerner PL, O’Brien TX, Allmendinger T, Vogt S, Schmidt B, Savino G, Fink C, Bonomo L, Henzler T (2011) Coronary CT angiography: image quality, diagnostic accuracy, and potential for radiation dose reduction using a novel iterative image reconstruction technique-comparison with traditional filtered back projection. Eur Radiol 21(10):2130–2138
Renker M, Nance JW Jr, Schoepf UJ, O’Brien TX, Zwerner PL, Meyer M, Kerl JM, Bauer RW, Fink C, Vogl TJ, Henzler T (2011) Evaluation of heavily calcified vessels with coronary CT angiography: comparison of iterative and filtered back projection image reconstruction. Radiology 260(2):390–399
Ebersberger U, Tricarico F, Schoepf UJ, Blanke P, Spears JR, Rowe GW, Halligan WT, Henzler T, Bamberg F, Leber AW, Hoffmann E, Apfaltrer P (2013) CT evaluation of coronary artery stents with iterative image reconstruction: improvements in image quality and potential for radiation dose reduction. Eur Radiol 23(1):125–132
Bae KT, Heiken JP, Brink JA (1998) Aortic and hepatic contrast medium enhancement at CT. Part II. Effect of reduced cardiac output in a porcine model. Radiology 207(3):657–662
Faggioni L, Neri E, Sbragia P, Pascale R, D’Errico L, Caramella D, Bartolozzi C (2012) 80-kV pulmonary CT angiography with 40 mL of iodinated contrast material in lean patients: comparison of vascular enhancement with iodixanol (320 mg I/mL) and iomeprol (400 mg I/mL). AJR Am J Roentgenol 199(6):1220–1225
Cademartiri F, Mollet NR, van der Lugt A, McFadden EP, Stijnen T, de Feyter PJ, Krestin GP (2005) Intravenous contrast material administration at helical 16-detector row CT coronary angiography: effect of iodine concentration on vascular attenuation. Radiology 236(2):661–665
Rienmuller R, Brekke O, Kampenes VB, Reiter U (2001) Dimeric versus monomeric nonionic contrast agents in visualization of coronary arteries. Eur J Radiol 38(3):173–178
Pannu HK, Thompson RE, Phelps J, Magee CA, Fishman EK (2005) Optimal contrast agents for vascular imaging on computed tomography: iodixanol versus iohexol. Acad Radiol 12(5):576–584
Blomley MJ, Dawson P (1997) Bolus dynamics: theoretical and experimental aspects. Br J Radiol 70(832):351–359
Seeliger E, Flemming B, Wronski T, Ladwig M, Arakelyan K, Godes M, Mockel M, Persson PB (2007) Viscosity of contrast media perturbs renal hemodynamics. J Am Soc Nephrol 18(11):2912–2920
Seeliger JE (1978) Rontgenological diagnosis. J Dent Assoc S Afr 33(9):491–492
Liss P, Persson PB, Hansell P, Lagerqvist B (2006) Renal failure in 57 925 patients undergoing coronary procedures using iso-osmolar or low-osmolar contrast media. Kidney Int 70(10):1811–1817
Acknowledgments
We gratefully acknowledge Cheol-oh Baek, Jung-Hee Jung, radiologic technicians working with Chungnam National University Hospital, Daejeon, Korea, for their contributions in performing DSCT scans.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Hee Jeong Shin declares that she has no conflict of interest. Author Song Soo Kim declares that he has no conflict of interest. Author Jae-Hwan Lee declares that he has no conflict of interest. Author Jae-Hyeong Park declares that he has no conflict of interest. Author Jin-Ok Jeong declares that she has no conflict of interest. Author Seon Ah Jin declares that she has no conflict of interest. Author Byung Seok Shin declares that he has no conflict of interest. Author Kyung-Sook Shin declares that she has no conflict of interest. Author Moonsang Ahn declares that he has no conflict of interest.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Shin, H.J., Kim, S.S., Lee, JH. et al. Feasibility of low-concentration iodinated contrast medium with lower-tube-voltage dual-source CT aortography using iterative reconstruction: comparison with automatic exposure control CT aortography. Int J Cardiovasc Imaging 32 (Suppl 1), 53–61 (2016). https://doi.org/10.1007/s10554-015-0816-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-015-0816-6