Abstract
Objective
To improve the detection of liver lesions in patients with hepatocellular carcinoma (HCC) via an iodine contrast enhancement tool.
Methods
Thirty-two patients with clinically proven HCCs underwent imaging with a three-phase protocol on a 256-slice MDCT. The contrast enhancement in the reconstructed slices was improved via a post-processing tool. Mean image noise was measured in four different regions: liver lesion, healthy liver, subcutaneous fat and bone. For each image set the image noise and contrast-to-noise ratio (CNR) were assessed. For subjective image assessment, four experienced radiologists evaluated the diagnostic quality.
Results
While employing the post-processing algorithm, CNR between the liver lesion and healthy liver tissue improves significantly by a factor of 1.78 (CNRwithout vC = 2.30 ± 1.92/CNRwith vC = 4.11 ± 3.05) (P* = 0.01). All results could be achieved without a strengthening of artefacts; mean HU values of subcutaneous fat and bone did not significantly change. Subjective image analysis illustrated a significant improvement when employing post-processing for clinically relevant criteria such as diagnostic confidence.
Conclusion
With post-processing we see a significantly improved detection of arterial uptake in hepatic lesions compared with non-processed data. The improvement in CNR was confirmed by subjective image assessment for small lesions and for lesions with limited uptake.
Key Points
• Enhancement with iodine-based contrast agents is an essential part of CT.
• A new post-processing tool significantly improves the diagnostics of hepatocellular carcinoma.
• It also improves detection of small lesions with limited iodine uptake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the third most common tumour and is ranked third for cancer mortality with more than 600,000 deaths worldwide per year [1, 2]. The yearly growing incidence of HCC is related to the increasing incidence of liver cirrhosis with different aetiologies such as alcoholism or the increasing number of hepatic viral infections such as hepatitis B and C [3, 4]. In the literature the development of HCC in a cirrhotic liver, except for the less common de novo carcinogenesis, is described as a multistep progression beginning from a low-grade dysplastic nodule with progression to a high-grade dysplastic nodule, to dysplastic nodules with microscopic lesions of HCC, to small HCC lesions and in the last step to manifest carcinoma [2]. In the early stages, there are almost no specific symptoms for the detection of HCCs. Nevertheless with regard to the prognosis of HCC patients it is crucial to detect HCC liver lesions at an early stage.
Nowadays, early diagnosis of HCC is possible using imaging techniques such as MRI and CT even without taking a biopsy from a suspect lesion [5]. Typically HCC lesions are hypervascular referring to the normal liver parenchyma [6]. This characteristic makes it necessary to perform three-phase dynamic CT in the form of native, arterial and portal venous phase after contrast media administration [7], with the arterial phase providing the most important information [6]. Such a multiphase protocol, as recommended by the Guidelines of the American Association for the Study of Liver Diseases (AASLD) and of the European Association for the study of the Liver (EASL) and the European Organisation for Research and Treatment of Cancer (EORTC) [8, 9], makes it possible to improve the differential diagnosis of liver lesions and the sensitivity for the detection of HCC.
Nevertheless, the diagnosis of HCC with CT sometimes remains difficult. Because of the changes in liver parenchyma due to liver cirrhosis, for example, small dysplastic liver nodules cannot be sufficiently distinguished from suspicious HCC lesions. With regard to factors such as tumour variability and vascularity or leakiness of the vascular endothelial cells [6, 10], there are HCC lesions with a limited uptake of contrast media [7, 11]. In this context some studies have shown that 27 to 34 % of the HCCs can be hypovascular [6, 12, 13]. Depending on the size of the lesions the detection can be challenging. Several studies reported a detection sensitivity of 29 % for HCC lesions with a mean diameter smaller than 10 mm and up to 94 % for lesions with a mean diameter larger than 20 mm [14–20]. On the contrary there is a high specificity of dynamic-enhanced CT for the detection of HCC of 75–96 % [6, 15, 16].
The possibility of enhancing the iodine contrast medium could improve the detection of small hypovascular HCC lesions. Besides its own vascularisation, peak arterial enhancement of a liver lesion depends on different factors such as the degree of liver cirrhosis affecting the liver haemodynamics or the injection rate, i.e. the total amount of contrast agent injected over a period of time [1, 21–24]. Improvement of the peak arterial enhancement can be achieved for example by a higher injection rate or a higher concentration, i.e., a higher dose of contrast agent [23, 25]. However, the increase in contrast agent brings along a number of risks such as contrast-medium-induced nephropathy, i.e. the aggravation of a pre-existing restricted renal function [26, 27]. Contrast-medium-induced nephropathy is one of the most common causes of acute renal failure in hospitalised patients [28].
With respect to risks associated with iodine as a contrast material, the purpose of our study was to improve the detection of liver lesions in patients with HCC via an iodine contrast enhancement tool (vContrast, Philips Healthcare, Cleveland, OH, US) without applying a higher dose of contrast agent as commonly used.
Materials and methods
Patient population
Thirty-two patients (25 men and 7 women; mean age 61 years; age range 31–81 years) with proven HCC, who underwent a CT examination between 2009 and 2011, were enrolled in this study. All the liver lesions of the patients analysed in our study were HCC lesions proven by image appearance in correlation with serum levels of alpha-fetoprotein and in some uncertain cases proven by biopsy. The typical image appearance of HCC lesions is an early arterial uptake of contrast agent followed by a washout in venous phases. Twenty-five patients in this study were treated with trans-arterial chemoembolisation (TACE), three patients underwent radiofrequency thermotherapy (RFTT) and three patients had already undergone surgery with partial hepatectomy because of HCC lesions. One patient did not get any treatment before the study examination.
Exclusion criteria for this examination were pregnancy and lactation, any contraindication to iodinated contrast agent such as a severe anaphylactic reaction in the past, renal failure (serum creatinine level >1.3 mg/dl) or hyperthyroidism (serum TSH level <0.4 mIU/l). Because this was a retrospective study, we used the criteria that are standard in our department in the clinical day-to-day routine, although they may differ from other departments.
CT technique and image reconstruction
All patients were examined with a three-phase protocol of the liver (native, arterial and venous phase) using a 256-slice multidetector CT (Philips Brilliance iCT; Philips Healthcare, Cleveland, OH, USA) with a tube voltage of 120 kV and average tube current of 130 mAs depending on the body mass index. After performing an unenhanced CT acquisition, each patient received 1 ml per kg body weight of non-ionic contrast agent (Imeron 400, Bracco Imaging, Milan, Italy) intravenously. With the bolus trigger technique the arterial phase and venous phase CT data acquisitions started approximately at 15 s and 40 s retrospectively [29]. The contrast agent was administered using a mechanical dual-head power injector (Stellant, MEDRAD, Inc., Indianola, PA) at a flow of 3.5 ml/s.
Post-processing tool
Noel et al. reported on further details concerning the vContrast algorithm [30]. The algorithm has two main components:
-
1)
Low contrast clustering (LCC) estimate. By this component, the study images are approximated with a piece-wise smoothed (PWS) approximation. This component has high sensitivity to low-contrast structures, and it has the special capability of preserving the shape and intensity values of the low contrast structures very accurately.
-
2)
Structural enhancement. By this component, low-contrast structures are enhanced. As the LCC approximation is available, it enables the structures in the study to be enhanced without the introduction of new artefacts and without the amplification of study noise. Thus, it improves the study’s CNR. In this component, an enhancement map is derived by enhancement of the structures in the LCC approximation.
The final vContrast images are obtained by adding the enhancement map obtained in the structures enhancement component to the original study. To enhance the contrast, vContrast was applied to the axial CT data in the arterial phase to improve the contrast differences within a given density range (lower threshold 50/upper threshold 300; amplification 110 %).
Quantitative image analysis
The quantitative measurements were performed at a commercially available post-processing workstation. For each patient the two CT image sets with and without using the post-processing algorithm were displayed side by side. CT attenuation values (in Hounsfield units) were obtained for the liver lesions, the normal liver parenchyma, subcutaneous fat and bone by measuring the regions of interest at the same image position with and without enhancement with vContrast.
The size range of the focal liver lesions of the 32 patients was between 4 mm and up to 100 mm. A subgroup of 14 patients was diagnosed with lesions smaller than 10 mm. For the ROI in the normal liver parenchyma, large vessels or focal lesions were avoided. The attenuation of the bone was measured in the corticalis of the vertebral bodies and the attenuation of the subcutaneous fat was measured in the right flank. Mean image noise was measured as the standard deviation of the HU values in the four ROIs. For each image set the contrast-to-noise-ratio (CNR) was assessed for the liver lesions with healthy liver as the background. The CNR was calculated as follows:
where μ s is the mean HU value of the liver lesion, μ b is the mean HU value of the background, i.e. the normal liver parenchyma, and σ b is the standard deviation of the background respectively the normal liver parenchyma. For the liver lesions the mean value of the CNR with and without employing vContrast was assessed and statistically analysed using a paired t-test. Finally, the mean HU values for bone and subcutaneous fat were calculated and statistically analysed also using a paired t-test.
Qualitative image analysis
For each patient the arterial phase CT slices with and without employing vContrast were evaluated independently by four radiologists (an average of 4 years’ experience). The images were read on a commercially available diagnostic workstation and were presented in random order. Before the reading session, the following seven evaluation criteria, including individual scoring scales, were provided to the readers: (a) subjective image noise (1, minimal noise; 2, less than average noise; 3, average noise; 4, above average noise; 5, unacceptable noise), (b) artefacts (1, no artefacts; 2, minor artefacts not interfering with the diagnostic decision; 3, major artefacts affecting visualisation of major structures; 4, artefacts affecting diagnostic information), (c) diagnostic confidence (1, completely confident; 2, probably confident; 3, confident only for limited clinical situation; 4, poor confidence), (d) overall image quality (1, excellent; 2, good; 3, moderate; 4, low; 5, poor), (e) artificial image appearance (1, none; 2, weak; 3, moderate; 4, strong), (f) sharpness (1, excellent; 2, average; 3, low) and (g) detectability of the liver lesions (1, excellent; 2, good; 3, moderate; 4, low). For each assessment criterion, the mean values and standard deviation were calculated and the mean values were statistically analysed with a paired t-test.
Results
Regarding the results using vContrast, we found a significant improvement in the CNR between liver lesions and healthy liver (P* = 0.01) by a factor of 1.78 (CNR without vC = 2.30 ± 1.92/CNRwith vC = 4.11 ± 3.05) compared with the unprocessed data. Table 1 illustrates the mean Hounsfield units for the four evaluated ROIs.
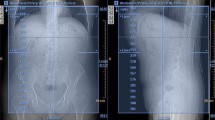
Figures 1 and 2 illustrate examples of HCC patients with and without using the post-processing vContrast algorithm. As one can observe in Fig. 1, a strength of vContrast is that it not only improves the assessment of large HCC tumours as in Fig. 1a and b, but also improves the detection of very small lesions with diameters of 4 or 5 mm (e.g. Fig. 1c and d). The real dimension of some lesions as shown in Fig. 2 could only be assessed when using vContrast.
a-d Axial contrast-enhanced multidetector CT images using a preset soft-tissue window (window width, 380 HU; window level, 50 HU) acquired during the arterial phase. A large hypervascular HCC lesion without (a) and with (b) using vContrast. A small hypervascular HCC lesion without (c) and with (d) employing vContrast
The effect of vContrast on an HCC case (a, left; b, right). Axial contrast-enhanced multidetector CT images using a preset soft-tissue window (window width, 380 HU; window level, 50 HU) acquired during the arterial phase. a The unprocessed image set shows a liver lesion with only slight contrast enhancement. b After using vContrast the hypervascular liver lesion shows an obvious enhancement and can be detected much more easily
Figure 3 illustrates mean CNR values in a boxplot. Only 3 of the 32 patients showed a higher CNR for the unprocessed data. The CNR for the rest of the examined patients showed an improvement compared with the unprocessed data. These three patients had small lesions with diameters smaller than 10 mm, making it difficult to place the ROIs exactly in the centre of the liver lesions. All three patients had already undergone TACE in which a mixture of epirubicin and lipiodol is applied; in these cases the contrast enhancement is sometimes difficult to distinguish on the periphery of a lipiodol-accumulating lesion. While employing vContrast the HU values of the corticalis of the bone and subcutaneous fat for each patient were slightly higher but in fact when comparing the average of the HU values of both regions the differences were not significant in a paired t-test.
The improvement in CNR could be confirmed by the subjective image analysis performed by four experienced radiologists for small lesions as well as for lesions with a limited uptake of contrast agent. For the images being processed with vContrast subjective image assessment overall showed a significant improvement for important clinical criteria such as diagnostic confidence (P* < 0.001), detectability of the liver lesions (P* < 0.001), sharpness (P* < 0.001) and overall image quality (P* < 0.001). Although the processed images seem to appear more artificial using vContrast, no significant strengthening of artefacts has been observed and no significant change in the subjective image noise was found. Our results were achieved without losing any diagnostically valuable information.
Discussion
In our study we evaluated the post-processing algorithm vContrast to enhance the contrast in arterial phase liver CT acquisitions of patients with HCC. Based on our findings it has been demonstrated that this post-processing tool has the potential to improve the detection of arterial uptake in liver lesions. In particular the results show a significant improvement in CNR of the HCC lesions compared with the unenhanced data. The significant advancement of the CNR could be confirmed by subjective image assessment, which was performed by four experienced radiologists. With greater clinical importance this subjective evaluation revealed a significant improvement for criteria such as diagnostic confidence, overall image quality, sharpness and most importantly the detectability of liver lesions. On the other hand such post-processing tools as vContrast carry the potential risk of decreasing the diagnostic quality with respect to image artefacts. Indeed, the enhanced images can appear a little more artificial but without a subjective strengthening of artefacts and without losing any diagnostically valuable information. An important strength of vContrast, with regard to the HCC diagnostics, is the improved detectability of liver lesions and increased diagnostic confidence. In this context it is a great advantage to always have both the processed and the unprocessed image sets to compare the change in the enhancement of suspect liver lesions.
For dynamic CT the detection of small HCC liver lesions, especially for those with diameters of under 10 mm, sometimes seems to be a challenge [15, 19]. Subjective image analysis in our study shows an improvement in the detection of those small lesions with vContrast compared with the unenhanced data. This statement can also be translated to lesions with limited uptake of contrast media, which play a very important role in HCC diagnostics. Only 3 of the 32 patients who had small lesions with diameters less than 10 mm showed a higher CNR for the unprocessed data: 2 lesions measuring 7 mm and 1 lesion measuring 9 mm. One reason for the higher CNR in the unprocessed data of these three cases could be their treatment with TACE, i.e. selective arterial injection of lipiodol to mark HCC lesions, because in those lesions a differentiation between the accumulated hyperdense lipiodol and the enhancement of contrast agent can sometimes be difficult. But even for these lesions vContrast can help to depict a slight but significant enhancement of contrast agent in the marginal areas after treatment with TACE, helping to detect a relapse in an already treated lesion.
Considering the presented results one could come to the conclusion that it seems feasible to reduce the volume of contrast medium when using an iodine contrast enhancement tool such as vContrast. Such a reduction could be a great benefit especially for patients with a restricted renal function. For those patients it is clinical routine to reduce the total amount of iodine contrast medium to avoid aggravation of renal insufficiency, i.e. acute renal failure. Using vContrast the reduction of the administered dose of iodine contrast agent seems to be possible without losing diagnostic information.
In conclusion, we strongly believe that if an iodine contrast enhancement post-processing algorithm such as vContrast can be proven to have diagnostic merit, the radiological depiction of arterial uptake in liver nodules can be significantly improved.
References
Kudo M (2011) Diagnostic imaging of hepatocellular carcinoma: recent progress. Oncology 81:73–85
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Parkin DM, Bray FI, Devesa SS (2001) Cancer burden in the year 2000. The global picture. Eur J Cancer 37:4–66
Willatt JM, Hussain HK, Adusumilli S, Marrero JA (2008) MR imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology 247:311–330
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022
Bolog N, Andreisek G, Oancea I, Mangrau A (2011) CT and MR imaging of hepatocellular carcinoma. J Gastrointest Liver Dis 20:181–189
Murakami T, Hori M, Kim T, Kawata S, Abe H, Nakamura H (2004) Multidetector row CT and MR imaging in diagnosing hepatocellular carcinoma. Intervirology 47:209–226
Llovet JM, Ducreux M, Lencioni R, Di Bisceglie AM, Galle PR, Dufour JF, Greten TF, Raymond E, Roskams T, De Baere T, Ducreux M, Mazzaferro V, Bernardi M, Bruix J, Colombo M, Zhu A (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943
Bruix J, Sherman M (2005) Practice Guidelines Committee of the AASLD. Management of hepatocellular carcinoma. Hepatology 42:1208–1236
Heilmaier C, Lutz AM, Bolog N, Weishaupt D, Seifert B, Willmann JK (2009) Focal liver lesions: detection and characterisation at double-contrast liver MR imaging with ferocarbotran and gadobutrol versus single-contrast MR imaging. Radiology 253:724–733
Murakami T, Kim T, Takamura M, Hori M, Takahashi S, Federle MP, Tsuda K, Osuga K, Kawata S, Nakamura H, Kudo M (2001) Hypervascular hepatocellular carcinoma: detection with double arterial phase multidetector row helical CT. Radiology 218:763–767
Kudo M (1999) Imaging diagnosis of hepatocellular carcinoma and premalignant/borderline lesions. Semin Liver Dis 19:297–309
Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, Venturi AM, Piscaglia F (2005) Characterisation of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology 42:27–34
Peterson MS, Baron RL, Marsh JW Jr, Oliver JH 3rd, Confer SR, Hunt LE (2000) Pretransplantation surveillance for possible hepatocellular carcinoma in patients with cirrhosis: epidemiology and CT-based tumour detection rate in 430 cases with surgical pathologic correlation. Radiology 217:743–749
Kim SH, Choi D, Kim SH, Lim JH, Lee WJ, Kim MJ, Lim HK, Lee SJ (2005) Ferucarbotran-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol 184:1069–1076
Ronzoni A, Artioli D, Scardina R, Battistig L, Minola E, Sironi S, Vanzulli A (2007) Role of MDCT in the diagnosis of hepatocellular carcinoma in patients with cirrhosis undergoing orthotopic liver transplantation. AJR Am J Roentgenol 189:792–798
Valls C, Cos M, Figueras J, Andía E, Ramos E, Sánchez A, Serrano T, Torras J (2004) Pretransplantation diagnosis and staging of hepatocellular carcinoma in patients with cirrhosis: value of dual-phase helical CT. AJR Am J Roentgenol 182:1011–1017
Lim JH, Kim CK, Lee WJ, Park CK, Koh KC, Paik SW, Joh JW (2000) Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic livers: accuracy of helical CT in transplant patients. AJR Am J Roentgenol 175:693–698
Kim YK, Kim CS, Chung GH, Han YM, Lee SY, Chon SB, Lee JM (2006) Comparison of gadobenate dimeglumine-enhanced dynamic MRI and 16-MDCT for the detection of hepatocellular carcinoma. AJR Am J Roentgenol 186:149–157
Marin D, Di Martino M, Guerrisi A, De Filippis G, Rossi M, Ginanni Corradini S, Masciangelo R, Catalano C, Passariello R (2009) Hepatocellular carcinoma in patients with cirrhosis: qualitative comparison of gadobenate dimeglumine-enhanced MR imaging and multiphasic 64-section CT. Radiology 251:85–95
Kim T, Murakami T, Takahashi S, Tsuda K, Tomoda K, Narumi Y, Oi H, Nakamura H (1998) Effects of injection rates of contrast material on arterial phase hepatic CT imaging. AJR Am J Roentgenol 171:429–432
Bea KT, Heiken JP, Brink JA (1998) Aortic and hepatic peak enhancement at CT: effect of contrast medium injection rate – pharmacokinetic analysis and experimental porcine model. Radiology 206:455–464
Awai K, Takeda K, Onishi H, Hori (2002) Aortic and hepatic enhancement and tumor-to-liver contrast: analysis of the effect of different concentrations of contrast material at multi-detector row-helical CT. Radiology 224:757–763
Tanikake M, Shimizu T, Narabayashi I, Matsuki M, Masuda K, Yamamoto K, Uesugi Y, Yoshikawa S (2003) Three-dimensional CT angiography of the hepatic artery: use of multi-detector row helical CT and a contrast agent. Radiology 227:883–889
Oliver JH, Baron RL (1998) High flow injection rates versus low flow injection rates: does increasing the injection rate result in greater detection of enhancement of hepatocellular carcinoma during hepatic arterial phase CT? Radiology 209:215
Wong GT, Irwin MG (2007) Contrast-induced nephropathy. Br J Anaesth 99:474–483
Wong PCY, Li Z, Guo J, Zhang A (2012) Pathophysiology of contrast-induced nephropathy. Int j cardiol 158:186–192
Nash K, Hafeez A, Hou S (2002) Hospital-acquired renal insufficiency. Am J Kidney Dis 39:930–936
Rengo M, Bellini D, De Cecco CN, Osimani M, Vecchietti F, Caruso D, Maceroni MM, Lucchesi P, Iafrate F, Palombo E, Paolantonio P, Ferrari R, Laghi A (2011) The optimal contrast media policy in CT of the liver. Part II: clinical protocols. Acta Radiol 52:473–480
Noël PB, Bendik E, Münzel D, Schneider A, Goshen L, Gringauz A, Lamash Y, Vlassenbroek A, Fingerle AA, Rummeny EJ, Dobritz M (2013) A method for improving iodine contrast enhancement in abdominal computed tomography: experimental study in a pig model. Eur Radiol 4:985–990
Acknowledgments
AV is an employee of Philips HealthCare. The other authors have no financial disclosures and had complete, unrestricted access to the study data at all stages of the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bendik, E., Noël, P.B., Münzel, D. et al. Evaluation of a method for improving the detection of hepatocellular carcinoma. Eur Radiol 24, 250–255 (2014). https://doi.org/10.1007/s00330-013-3006-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-3006-8