Abstract
Objective
To report short- and long-term effects of an audit process intended to optimise the radiation dose from multidetector row computed tomography (MDCT).
Methods
A survey of radiation dose from all eight MDCT departments in the state of Luxembourg performed in 2007 served as baseline, and involved the most frequently imaged regions (head, sinus, cervical spine, thorax, abdomen, and lumbar spine). CT dose index volume (CTDIvol), dose–length product per acquisition (DLP/acq), and DLP per examination (DLP/exa) were recorded, and their mean, median, 25th and 75th percentiles compared. In 2008, an audit conducted in each department helped to optimise doses. In 2009 and 2010, two further surveys evaluated the audit’s impact on the dose delivered.
Results
Between 2007 and 2009, DLP/exa significantly decreased by 32–69 % for all regions (P < 0.001) except the lumbar spine (5 %, P = 0.455). Between 2009 and 2010, DLP/exa significantly decreased by 13–18 % for sinus, cervical and lumbar spine (P ranging from 0.016 to less than 0.001). Between 2007 and 2010, DLP/exa significantly decreased for all regions (18–75 %, P < 0.001). Collective dose decreased by 30 % and the 75th percentile (diagnostic reference level, DRL) by 20–78 %.
Conclusions
The audit process resulted in long-lasting dose reduction, with DRLs reduced by 20–78 %, mean DLP/examination by 18–75 %, and collective dose by 30 %.
Key points
• External support through clinical audit may optimise default parameters of routine CT.
• Reduction of 75th percentiles used as reference diagnostic levels is 18–75 %.
• The effect of this audit is sustainable over time.
• Dose savings through optimisation can be added to those achievable through CT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The risk of radiation exposure from computed tomography (CT) is known to be high owing to the large number of examinations performed each year (67 million in the USA in 2008) and the high doses delivered per examination [1–4]. Moreover, a direct link has recently been established between radiation delivered by CT in childhood and cancer incidence in adults [5]. To address this risk, scientific societies and regulatory authorities have attempted to reduce the number of examinations by introducing stricter usage criteria, including reducing the dose per examination and per CT acquisition [6–8]. Despite these initiatives, the use of CT—and consequently radiation exposure—has been increasing worldwide [4].
In particular, one attempt to reduce dose is a directive produced by the European Union (EU) in 1997, which introduced the concept of surveying radiation dose from diagnostic examinations for both conventional and CT imaging procedures [8]. The EU defined the upper limit of the range of acceptable doses (diagnostic reference level, DRL) as the 75th percentile of the dose distribution [9, 10]. Surveys subsequent to this initiative revealed very wide ranges of doses delivered for identical examinations between radiology departments, suggesting a need to further improve processes in order to achieve a more consistent optimisation of dose [10]. According to the as low as reasonably achievable (ALARA) principle, dose optimisation aims to eliminate the amount of radiation not contributing to the image quality that still yields confident diagnoses [10]. By establishing these DRLs the expectation was a decrease in dose, but in actual fact an increase in dose was observed between the era of single detector row CT and that of multiple detector row CT (MDCT) [11, 12]. Given the supposedly increased efficiency of the newer generation CT machines, this observation was somewhat counterintuitive. Although some decrease has been reported on a local scale [13] from these machines, no decrease has been observed on a larger and nationwide scale [14–16]. In order to further reduce the dose across all departments, regardless of the machine used, the Ministry of Health in Luxembourg recently hypothesised that an audit process aimed at promoting dose reduction but which included for the first time a direct interaction with individual radiology departments tailored to the particular machine used in that department to ensure correct implementation of dose-optimised CT acquisition procedures might lead to a decreased dose delivered across all locations. This article describes this department-specific audit implementation process and reports its short- and long-terms effects.
Materials and methods
According to Luxembourg and EU legislation (i.e. Directive 95/46/EC regarding the protection of data of individuals) a purely observational study with complete anonymisation of the data at the source that removes any possibility of identifying the individual patients is not subject to mandatory ethical review [17].
From February to June 2007, the Department of Radiation Safety in the Ministry of Health in the state of Luxembourg (500,000 inhabitants) conducted a nationwide survey on the dose of radiation delivered by CT and included all eight radiology departments equipped with MDCT in the country. According to EU guidelines, data of at least 20 consecutive patients who underwent standard examinations of each of the six most frequently imaged regions (head, sinus, cervical spine, thorax, abdomen including the pelvis, and lumbar spine) were collected [18, 19]. Using printed data sheets, the following information was collected: patient’s gender and age, acquisition parameters including mean tube current–time product, tube potential, pitch factor, and slice collimation, as well as dose descriptors expressed as both CT dose index volume (CTDIvol) and dose–length product per acquisition (DLP/acq). Sum of DLP/acq from consecutive acquisitions was also obtained and named DLP per examination (DLP/exa). CT parameters were computed using commercially available software (CT Expo V 1.6, University of Hannover, Germany) in order to cross verify the dose descriptors given by the CT machines [20]. For each of the eight MDCT departments, mean CTDIvol, mean DLP/acq, mean DLP/exa and mean E were calculated.
MDCT devices were equipped with 4–40 detector rows. Five devices were from Siemens (Siemens Healthcare, Forchheim, Germany) and three from General Electric (GE Healthcare, Milwaukee, WI, USA). As required by national and EU recommendations, all MDCT devices underwent yearly quality control processes by certified radiophysicists in order to ensure that they were working properly [9]. None of the devices were equipped with newly developed iterative reconstruction functionalities but all were equipped with automatic exposure control systems (AEC). The 75th, 50th and 25th percentiles of each type of examination and dose descriptor (CTDIvol, DLP/acq and DLP/exa) were calculated. Data of this first survey, summarised in Table 1, were analysed by two independent experts (a physicist and a radiologist) who both considered the 2007 dosimetric results to be high, some of them being even higher than the very first EU DRLs published in 1999 [12, 19]. After the review of the first survey, these experts discussed optimisation strategies with the heads of all radiology departments during a meeting held in November 2007. They recommended first optimising CTDIvol values and second considering reducing z-coverage, and in particular the number of acquisitions. On the basis of their observations and the potential reduction in radiation dose, the Ministry of Health initiated an audit process and funded the support of one of the experts—the radiologist who had clinical experience in the field—with the aim of helping radiology departments achieve MDCT dose optimisation. The audit process was in agreement with the Quality Assurance Audit for Diagnostic Radiology Improvement and Learning (QUAADRIL) recommendation from the International Atomic Energy Agency (IAEA) [21]. The main objective of the audit was to check all acquisition parameters, activate AEC systems if disabled, propose reduced CTDIvol, by adjusting tube potential and tube current values, and to invite local radiologists to consider z-coverage reduction as a complementary means of reducing radiation dose. The methods used for dose optimisation and the possible achievable objectives have been previously described in a book chapter [22].
All radiology departments accepted this audit process, which was then conducted between April 2008 and January 2009 (audit coverage being 100 % of MDCT devices in Luxembourg). During the audit, the expert radiologist checked all CT parameters, proposed reduced dose acquisition protocols named optimised protocols, and tested them together with the on-site radiologists during a 1-day visit. If approved, optimised protocols were archived in each MDCT unit with a specific optimised dose (OD) label. On-site radiologists collaborated with the expert in setting up optimised parameters, which they used thereafter rather than the default parameters. Mean CTDIvol values applied to a standard size patient weighing 75 kg, observed before and after optimised protocols, as stored in each unit, are listed in Table 2. An open line of communication with the auditing expert was offered to all departments enabling any radiologist to ask for further technical support after optimisation.
One year later, a second survey was conducted between April and June 2009. None of the MDCT devices had been replaced or upgraded. The only variable that had changed was the form of data collection, whereby paper sheets had been replaced by electronic extraction of CT parameters from DICOM headers of anonymised CT series stored in the picture archive communication system (PACS) [23]. Between 20 and 40 examinations were considered for each region. CTDIvol, DLP/acq and DLP/exa were calculated as in the 2007 survey.
The number of CT examinations performed during the 2009 survey was obtained from radiology information systems (RIS) in each department. Collective dose savings were calculated for each type of examination in each department by multiplying the difference in mean DLP/exa values between 2007 and 2009 by the corresponding conversion factors and by the number of examinations performed in 2009 [24].
Delegates from the Ministry of Health made an annual visit to all radiology departments in 2009 and 2010 in order to encourage radiologists and radiographers to use the optimised dose protocols rather than the previous standard ones, which were still available, and to consider reductions in z-coverage for further optimisation.
In 2010, a third survey was conducted using the same methods as in 2009.
Statistical analysis
Data were summarized as mean and standard error on the mean (SEM), as well as 75th, 50th and 25th percentiles. ANOVA tests at two levels of between-group factors (hospital and year) were performed for mean CTDIvol, mean DLP/acq, mean DLP/exa and for each of the eight MDCT departments. The number of levels of hospitals and year factors are respectively eight and three. Statistical significance for all tests was set at P < 0.05. The statistical software used was IBM-SPSS V20.0.
Results
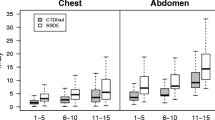
In 2008, according to the data provided by the RIS of each department, 95,376 examinations were performed in Luxembourg: 19,192 (20.1 %) on the head, 7,311 (7.7 %) on the sinus, 6,818 (7.1 %) on the cervical spine, 14,838 (15.6 %) on the thorax, 24,956 (26.2 %) on the abdomen and 15,093 (15.8 %) on the lumbar spine. The six selected examinations represented 92.5 % of all CT examinations performed in the country. The results of three surveys are summarised in Table 3 and illustrated in Figs. 1, 2, 3, 4, 5 and 6. Depending on the region, reduction in CTDIvol ranged from 11 to 71 % between 2007 and 2009 and was statistically significant (P ranging from less than 0.001 to 0.013). Expressed in DLP/exa, dose reduction was also statistically significant in all anatomical regions (P < 0.001) except the lumbar spine (P = 0.917). At follow-up between 2009 and 2010, CTDIvol significantly increased in the head and abdomen (P ranging from less than 0.001 to 0.046), significantly decreased in sinus, cervical and lumbar spine (P ranging from less than 0.001 to 0.016), but did not significantly change in the thorax (P = 0.693). Expressed in DLP/exa, radiation dose did not significantly change in the head and abdomen (P = 0.836 and 0.259, respectively).
75th percentile, median and 25th percentile of DLP/exa of MDCT of the head. Dose–length products (DLPs) are expressed in mGy cm. CT dose index (CTDI) phantom for the head was 16 cm in diameter. 75th percentile is used as a diagnostic reference level (DRL) whereas the 25th percentile is indicative of appropriate optimisation [14, 16]
Independent of statistically significant differences between mean values collected in 2007 and 2010, reductions in the 75th percentile of radiation doses (i.e. DRL) ranged from 20 to 78 %. On the basis of the assumption that the number of examinations performed each year in the country remained constant, the collective dose was reduced by 30 % between 2007 and 2009.
Discussion
This study has three findings:
-
1.
A single external audit that installs optimised dose protocols in each CT unit, specific to the machine of that unit, can substantially reduce the radiation dose delivered by CT.
-
2.
The dose reduction achieved is sustainable over time.
-
3.
Once personally involved in a dose reduction process, radiology departments tend to maintain the lower dose and to attempt further decreases through reductions in z-coverage. But most noteworthy is the fact that this study shows that, after implementing the whole optimisation process, radiation doses are among the lowest ever reported for MDCT [14–16].
After a single on-site auditing visit, we did indeed observe a reduction in both the radiation doses delivered and the amplitude of their differences between departments. To our knowledge, such decreases in dose and variation of two consecutive nationwide surveys applied to MDCT have never been reported. Although we did not have a control arm to capture the theoretical possibility of dose reduction happening in the absence of an audit, our result suggests that an audit, which includes an interactive, hands-on phase to help implement optimisation protocols, might be the key process for obtaining important dose reductions that are consistently maintained over time. Our observation supports the recommendation proposed by Samara et al. to conduct audits that support local departments in their dose management [14]. Their recommendation was based on the lack of decrease in dose levels recorded between two consecutive surveys conducted without any external support to radiology departments [14, 15].
As this process resulted in an efficient radiation dose reduction, it merits some additional comments. Firstly, we considered the 25th percentile of surveys and not DRLs as reasonably achievable objectives, as the 75th percentile represents the upper limit of acceptable practice in Europe [25]. The 25th percentile used as an objective for optimisation was within the reported ranges of optimised doses for CT [25–28]. Secondly, in order to ensure consistent and accurate implementation of the protocols, as well as the acceptance of the image quality in their clinical practice, we spent all the time necessary with on-site radiologists to achieve each step of the optimisation process. It is important to say that both standard and optimised protocols were available throughout the audit, which meant that there was no guarantee that optimised protocols would be used. Nevertheless, analysis of DICOM headers in the second and third surveys showed that optimised protocols were indeed preferred to standard ones, suggesting that they provided sufficient diagnostic confidence. Thirdly, in order to ensure that we were as close as possible to clinical conditions, we introduced optimised protocols during clinical practice, as opposed to ‘phantom’ experiments. Fourthly, whenever possible, we used smoother reconstruction algorithms among those provided by the manufacturers to reduce the noise associated with the dose reduction. Fifthly, we considered that, among all the CT units involved, we would deliver a comparable dose regardless of manufacturer, number of detector rows and type of AEC system [29].
One could speculate that the 30 % collective dose reduction observed after the single audit visit could be explained by the absence of any previous dose management in the radiology departments involved in this trial. Indeed, all of these departments were still working with original protocols that had been installed and proposed by the manufacturers. However, we observed that once involved in a dose reduction process, radiology departments tended to decrease the dose further over time. This further dose reduction may also be related to the radiologist’s engagement in managing the dose through z-coverage and number of multiphasic examinations, or to the repeated dose monitoring itself. Unfortunately, this was accompanied by slightly increased CTDIvol values for the head and abdomen. Despite these encouraging results in DLP/exa values, we do believe that audits need to be repeated in order to maintain the individual efforts in achieving the lowest possible dose levels. In the near future, continuous dose monitoring with specific software will probably be useful in tracking radiation dose, detecting and preventing abnormal events, identifying inadequate protocol choice by CT radiographers, and in producing more reliable data for dose monitoring and surveys [30].
This study has several limitations. Firstly, the sample size in the state of Luxembourg is rather small and represents approximately the size of a European city, such as Gothenburg, Sweden. Extrapolating our results to larger countries would require a much large number of experts involved in such an audit process. However, as MDCT devices are similar all around the world, there is no reason to believe that the process could not be successful everywhere.
Secondly, we had no control group with which to compare the specific impact of our audit and were only able to compare our data with those reported in other European countries, such as Switzerland [14–16]. However, it would have been unethical and indeed unfeasible to randomly offer the audit support to only one-half of the departments involved.
Thirdly, the size of an individual patient was not taken into account. Recommended survey methods make the assumption that on a sufficiently large sample of consecutive patients, the average body weight would be similar to that of a so-called standard patient weighing 70–75 kg [26]. Consequently, small differences in CT dose between surveys may be due to differences in body size. This factor may have influenced the data for body regions where AEC systems were switched on (i.e. some head, all spine, chest and abdomen examinations), but not those where AEC systems were switched off (i.e. some head and all sinus examinations).
Fourthly, the observed dose reduction is expressed as a percentage of the initial 2007 value. It was indeed not possible to make completely sure that the initial protocol corresponded to default parameters proposed by the manufacturer, or had been adapted by the manufacturer at the time of installation. Therefore, the percentage of dose reduction might not be consistently achievable with any machine in any country, and could be lower or even higher.
Lastly, image quality and CT diagnosis performance were not investigated. That said, it is probably quite fair to say that an optimised protocol providing insufficient image quality would not have been used given the choice over a ‘standard’ protocol that did provide sufficient image quality. As radiology departments had this choice and consistently chose to use the optimised protocol, we feel safe in assuming that the image quality was indeed sufficient.
In conclusion, a clinical audit on radiation dose, which includes an interactive, hands-on phase to help implement optimisation protocols, can result in enduring reduction of diagnostic reference levels by 20 to 78 %, mean DLP/examination by 18 to 75 % and the collective dose delivered by MDCT by 30 %.
References
Mettler FA, Bhargavan M, Faulkner K et al (2009) Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources: 1950–2007. Radiology 253:520–553
Brenner DJ, Hricak H (2010) Radiation exposure from medical imaging. JAMA 304:208–209
Brenner DJ, Hall EJ (2007) Computed tomography—an increasing source of radiation exposure. N Engl J Med 357:2277–2284
Hricak H, Brenner DJ, Adelstein SJ, Frush DP, Hall EJ, Howell RW, McCollough CH, Mettler FA, Pearce MS, Suleiman OH, Thrall JH, Wagner LK (2011) Managing radiation use in medical imaging: a multifaceted challenge. Radiology 258:889–905
Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Craft AW, Parker L, Berrington de González A (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380:499–505
American College of Radiology (2013) Appropriateness criteria—diagnostic imaging. http://www.acr.org/Quality-Safety/Appropriateness-Criteria/Diagnostic. Accessed 2 Apr 2013
The Royal College of Radiology (2013) RCR referral guidelines. http://www.rcr.ac.uk/content.aspx?PageID=995. Accessed 2 Apr 2013
The Council of the European Union (1997) Council Directive 97/43 of 30 June 1997 on health protection of individuals against the dangers of ionizing radiation in relation to medical exposure, and repealing directive 84/466/Euratom. http://eur-lex.europa.eu/Result.do?T1=V3&T2=1997&T3=0043&RechType=RECH_naturel&Submit=Search. Accessed 2 Apr 2013
Commission E (2000) European guidelines on quality criteria for computed tomography, EUR 16262EN. Office for Official Publications of the European. Communities, Luxembourg
Golding SJ (2010) Radiation exposure in CT: what is the professionally responsible approach? Radiology 255:683–686
Shrimpton PC, Jessen KA, Geleijns J, Panzer W, Tosi G (1998) Reference doses in computed tomography. Radiat Prot Dosim 80:55–59
Shrimpton PC, Hillier MC, Lewis MA, Dunn M (2006) National survey of doses from CT in the UK: 2003. Br J Radiol 79:968–980
Lo GG, Chan JKF, Lau CW, Geng H, Yu SK (2010) Radiation dose survey: comparison between new-generation computed tomography and 64-slice multidetector computed tomography. J HK Coll Radiol 12:107–112
Samara ET, Aroua A, Bochud FO, Ott B, Theiler T, Treier R, Trueb PR, Vader JP, Verdun FR (2012) Exposure of the Swiss population by medical x-rays: 2008 review. Health Phys 102:263–270
Treier R, Aroua A, Verdun FR, Samara E, Stuessi A, Trueb PR (2010) Patient doses in CT examinations in Switzerland: implementation of national diagnostic reference levels. Radiat Prot Dosimetry 142:244–254
Stamm G (2012) Collective radiation dose from MDCT: critical review of surveys studies. In: Tack D, Kalra MK, Gevenois PA (eds) Radiation dose from multidetector CT. Springer, Heidelberg, pp 209–229
European Commission (1995) Directive 95/46/EC of the European Parliament and of the Council of 24 October 1995 on the protection of individuals with regard to the processing of personal data and on the free movement of such data. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:31995L0046:en:HTML. Accessed 21 Jan 2013
Brix G, Nagel HD, Stamm G, Veit R, Lechel U, Griebel J, Galanski M (2003) Radiation exposure in multi-slice versus single-slice spiral CT: results of a nationwide survey. Eur Radiol 13:1979–1991
European Commission. Appendix MSCT dosimetry, guidelines on radiation dose to the patient. http://www.msct.eu/PDF_FILES/Appendix%20MSCT%20Dosimetry.pdf . Accessed 2 Apr 2013
Stamm G, Nagel HD (2002) CT-expo—a novel program for dose evaluation in CT. RöFo 174:1570–1576
International Atomic Energy Agency (AIEA) (2010) Comprehensive clinical audits of diagnostic radiology practices: a tool for quality improvement quality assurance audit for diagnostic radiology improvement and learning (QUAADRIL). http://www-pub.iaea.org/mtcd/publications/PubDetails.asp?pubId=8187. Accessed 20 Apr 2013
Tack D (2007) Methods and strategies for radiation dose optimization and reduction in MDCT with special focus on the image quality. In: Tack D, Gevenois PA (eds) Radiation dose from adult and pediatric multidetector computed tomography. Springer, Berlin, pp 99–116
Jahnen A, Kohler S, Hermen J, Tack D, Back C (2011) Automatic computed tomography patient dose calculation using DICOM header metadata. Radiat Prot Dosimetry 147:317–320
Deak PD, Smal Y, Kalender WA (2010) Multisection CT protocols: sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology 257:158–166
European Commission (1999) Radiation protection 109: guidance on diagnostic reference levels for medical exposures. EC1999. http://www.google.be/url?sa=t&rct=j&q=radiation%20protection%20109&source=web&cd=1&ved=0CC4QFjAA&url=http%3A%2F%2Fec.europa.eu%2Fenergy%2Fnuclear%2Fradiation_protection%2Fdoc%2Fpublication%2F109_en.pdf&ei=0SoFUYqVJarV0QWR-4CgDg&usg=AFQjCNGlP4AT3FWU7nfk8AT2D3Tiy3j4cA&bvm=bv.41524429,d.d2k&cad=rja Accessed 26 Jan 2013
Tsapaki V, Aldrich JE, Sharma R, Staniszewska MA, Krisanachinda A, Rehani M, Hufton A, Triantopoulou C, Maniatis PN, Papailiou J, Prokop M (2006) Dose reduction in CT while maintaining diagnostic confidence: diagnostic reference levels at routine head, chest, and abdominal CT–IAEA-coordinated research project. Radiology 240:828–834
Kambadakone AR, Prakash P, Hahn PF, Sahani DV (2010) Low-dose CT examinations in Crohn’s disease: Impact on image quality, diagnostic performance, and radiation dose. AJR Am J Roentgenol 195:78–88
Lee SJ, Park SH, Kim AY, Yang SK, Yun SC, Lee SS, Jung GS, Ha HK (2010) Effect of altering automatic exposure control settings and quality reference mAs on radiation dose, image quality and diagnostic efficacy in MDCT enterography of active inflammatory Crohn’s disease. AJR Am J Roentgenol 195:89–100
Brink JA, Morin RL (2012) Size-specific dose estimation for CT: how should it be used and what does it mean? Radiology 265:666–668
Sodickson A, Warden GI, Farkas CE, Ikuta I, Prevedello LM, Andriole KP, Khorasani R (2012) Exposing exposure: automated anatomy-specific CT radiation exposure extraction for quality assurance and radiation monitoring. Radiology 264:397–405
Acknowledgments
The Ministry of Health in Luxembourg funded the audit process.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tack, D., Jahnen, A., Kohler, S. et al. Multidetector CT radiation dose optimisation in adults: short- and long-term effects of a clinical audit. Eur Radiol 24, 169–175 (2014). https://doi.org/10.1007/s00330-013-2994-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-2994-8