Abstract
Objectives
To test the feasibility of four-dimensional (4D) flow MRI to quantify the systolic wall shear stress (WSSsystole) and oscillatory shear index (OSI) in high-grade internal carotid artery (ICA) stenosis before and after endarterectomy (CEA).
Methods
Twenty patients with ≥60 % ICA stenosis were prospectively and consequently included. Four-dimensional flow MRI was used to measure individual time-resolved 3D blood flow velocities. Segmental WSSsystole and OSI were derived at eight wall segments in analysis planes positioned along the ICA, common (CCA) and external carotid artery (ECA).
Results
Regional WSSsystole of all patients decreased after CEA (P < 0.05). Changes were most prominent at the ICA bulb but remained unchanged in the CCA and ECA. OSI was significantly lower after CEA in the lateral vessel walls (P < 0.05). For analysis planes at the stenosis in- and outlet, a reduction of mean WSSsystole by 32 % and 52 % (P < 0.001) and OSI distal to the stenosis (40 %, P = 0.01) was found after CEA.
Conclusions
Our findings show the potential of in vivo 4D flow MRI to quantify haemodynamic changes in wall shear stress even in patients with complex flow conditions.
Key Points
• The 4D flow MRI allows in vivo measurement of individual 3D blood flow.
• Regional wall shear stress can be derived from such 3D flow data.
• Even complex flow in high-grade internal carotid artery stenosis can be analysed.
• This technique could be valuable for future studies of carotid atherosclerosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carotid endarterectomy (CEA) and carotid artery stenting are established treatments for high-grade internal carotid artery (ICA) stenosis, an important source of ipsilateral stroke [1]. The purpose of CEA is to restore normal carotid anatomy and blood flow by plaque removal. A number of studies have confirmed that recanalisation leads to a significant reduction of peak blood flow velocities and pressure gradients across the ICA [2–4]. However, the influence of CEA on wall shear stress (WSS), the friction force of blood that acts on the vessel wall and a contributing factor to altered endothelial cell function, is poorly understood [5].
Low WSS magnitude and high oscillatory shear index (OSI) have been linked to the development of local atherosclerosis in the carotid bifurcation and aorta in animal models [6, 7] and are considered atherogenic wall parameters. In this context, a computational fluid dynamic (CFD) model of the carotid bifurcation has shown that individual bifurcation geometry was associated with differences in the topographic distribution of critical wall parameters in healthy volunteers [8]. Findings of this CFD study have been confirmed by a 4D flow MRI study that quantified the in vivo 3D distribution of WSS and OSI in carotid bifurcations [9]. The results of this study demonstrated that critically low WSS and high OSI were concentrated at the dilated posterior wall of the ICA and thus at a typical site of carotid plaque development. In addition, the distribution of wall parameters was substantially altered in a small group of patients with moderate ICA stenosis.
To date, however, no studies have investigated the distribution of WSS and OSI in patients before and after CEA of high-grade ICA stenosis in vivo and the effect of CEA-induced changes in blood flow on regional WSS is unclear. It was therefore the aim to investigate the in vivo WSS and OSI distribution along the carotid bifurcation by pre- and post-interventional carotid 4D flow MRI in a cohort of 20 patients with ≥60 % ICA stenosis (following European Carotid Surgery Trial, ECST, criteria [10]) undergoing eversion CEA.
We hypothesised that plaque removal and consecutive reduction of high intrastenotic velocity gradients and poststenotic complex flow would result in measurable changes of WSS and OSI distribution.
Materials and methods
Study population
Patients with ≥60 % (ECST criteria [10], i.e. ≥40 % NASCET criteria) asymptomatic or symptomatic ICA stenosis scheduled for CEA were prospectively and consequently included during a 12-month period between July 2008 und July 2009. All patients underwent eversion carotid endarterectomy and did not receive patch material to repair the ICA bulb during the CEA procedure because eversion CEA is the preferred surgical procedure for plaque removal at our institution. As a result, we assumed that the inter-individual variation of bifurcation geometry due to surgery was minimal. The degree of ICA stenosis was determined using extra- and intracranial 2D duplex ultrasound by two highly experienced examiners. During the study period 83 patients fulfilled the inclusion criteria. Fourteen patients refused to participate in the study or interrupted the initial MRI examination. Owing to the short interval between admission to the hospital and CEA (emergency CEA was performed in 2 patients) MRI was not available in 13 patients. Five patients had to be excluded because of ferromagnetic devices that were not compatible with 3-T MRI. One patient weighed over 135 kg and could not be examined in the MRI, and five patients with symptomatic ICA stenosis were too unstable to undergo MRI examination. Of the 43 remaining patients 29 completed both pre- and postoperative 4D flow MRI measurements and were considered for further evaluation. The study was approved by the local ethics committee and written informed consent was obtained from all participants.
MRI measurements
Magnetic resonance imaging measurements were performed on a 3-T MRI system (TIM-TRIO; Siemens; Erlangen, Germany) using a combined 12-element head and 6-element neck coil. For an anatomical overview, 3D time-of-flight MR angiography (MRA) in an axial slab covering the left and right carotid bifurcation was performed (flip angle = 25°; time to echo (TE)/time to repeat (TR) = 3.7 ms/20 ms; spatial resolution = 0.5 × 0.8 × 1.0 mm3; slab thickness = 52 mm).
In patients with a glomerular filtration rate >40 ml/min who agreed to receive contrast agent a time-resolved (TWIST) MRA and subsequent contrast-enhanced (CE) MRA were performed before CEA and reconstructed as maximum intensity projections (MIP) allowing for a 360° degree rotation for optimal visual assessment of stenosis location and length. MRA was executed after injection of 0.1 mmol/kg body weight gadolinium-based contrast agent (gadobenate dimeglumine, Multihance, Bracco, Italy) at 3.5 ml/s (voxel size = 1.2 × 1.5 × 4.0 mm3, field of view (FOV) = 250 × 380 mm2, TE/TR = 0.89 ms/2.3 ms, flip angle = 20°, bandwidth = 920 Hz/pixel).
Four-dimensional flow MRI consisted of a prospectively electrocardiogram gated radiofrequency-spoiled gradient echo sequence with interleaved three-directional velocity encoding [11]. The predominantly axial 3D imaging volume was angulated based on the TOF MRA data in order to include the distal 2 cm of the common carotid artery (CCA), the carotid bifurcation, and the proximal 5 cm of the ICA and external carotid artery. Imaging parameters were as follows: flip angle 15°, TE/TR = 3.1 ms/45.6 ms, velocity sensitivity = 200 cm/s, spatial resolution = 1.1 × 0.9 × 1.4 mm3, slab thickness = 50.4 mm, 36 slices/slab, and temporal resolution = 45.6 ms. For follow-up examination after eversion carotid endarterectomy the velocity sensitivity was set to 120 cm/s to account for the reduced flow velocities in the ICA after plaque removal.
Data processing and WSS quantification
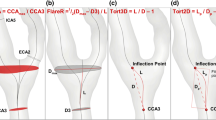
Data processing included noise filtering, correction for eddy currents, Maxwell terms, and velocity aliasing [12]. For each 4D flow MR data set a 3D phase-contrast MR angiogram (PC-MRA) was calculated and visualised as a 3D isosurface as shown in Fig. 1 (EnSight; CEI; Apex, NC, USA) [13]. Next, analysis planes were positioned in the carotid bifurcation according to anatomical landmarks (Fig. 1): analysis plane 2 was anchored at the flow bifurcation point (branching point between external carotid artery and ICA) and angulated normally to the ICA. All other analysis planes were generated by shifting the plane centre upstream (ICA, additional planes 3–6) or downstream (distal CCA = plane 1) in 4-mm intervals. The external carotid artery plane was positioned 4 mm above the flow diverter, i.e. at the site where the common carotid artery branches into the ICA and ECA. One reference analysis plane was positioned at the most distal point of the ICA in order to measure blood flow as far distally to the stenotic jet as possible. Each analysis plane was manually angulated normally to the arterial lumen [9].
Calculation and distribution of wall shear stress
For calculation of wall shear stress, all analysis planes were imported into a home-built analysis tool programmed in MatLab (The Mathworks, USA) [14]. For each analysis plane, the peak systolic WSS (WSSsystole) in N/m2 and the oscillatory shear index (OSI) in % were calculated using an eight-segment wall model (see Figs. 1, 2 and 3) [15]. WSSsystole was calculated as the average over four time frames centred at peak systole. In addition, global WSSsystole and OSI for the CCA, ICA, and ECA were calculated as the mean over all segments.
Distribution of average WSSsystole and OSI in each of the eight wall segments of the individually chosen analysis planes (depending on stenosis geometry) before and after carotid endarterectomy in the 20 patients. Significance level of *<0.05 and **<0.01 in wall segments that show a significant change in values between pre- and postoperative measurements
Determination of individual stenosis anatomy
Based on the standardised analysis planes, the planes closest to the in- and outlet of ICA stenosis were identified for each patient. Depending on stenosis morphology, i.e. length and extent of tapering, plane locations were variable between patients. As an example, Fig. 4 illustrates different distances of the stenosis maximum from the flow bifurcation (i.e. 15 mm and 3 mm, respectively) for two patients with ICA stenosis. Consequently, analysis planes at the in- and outlet of the stenotic plaque had to be defined and considered on an individual basis (Fig. 3). The distance of the stenosis maximum from the flow diverter and plaque length was measured in millimetres with electronic callipers using IMPAX software (version EE R20 VIII P1, Agfa, Vienna, Austria). Measurements were performed by one experienced reader based on the MIPs of the TWIST angiography or, if unavailable, based on the MIP of TOF angiography.
Contrast-enhanced MR angiography of two patients with high-grade internal carotid artery (ICA) stenosis before carotid endarterectomy. a and b: ICA stenosis with 75 % lumen narrowing: the physiologically dilated ICA bulb is preserved and most of the stenosis is located 15 mm distally to the flow diverter. c and d: ICA stenosis with 65 % lumen narrowing. Unlike the other patient, the ICA bulb is not preserved and the maximum of the stenosis is located only 3 mm distally to the flow diverter indicating that the stenosis begins in the distal common carotid artery
Statistical analysis
Continuous variables are reported as mean ± standard deviation. Differences in global WSS and OSI were evaluated by calculating pre- and post-interventional differences (in %). Statistical significance was assessed by the application of a paired two-sided t-test comparing pre- and post-intervention for the CCA, ECA, and ICA. For segmental WSS and OSI, pre- and post-interventional values were compared separately for each segment using a paired two-sided t-test. All tests used a significance level of P < 0.05. The given probability values represent values unadjusted for multiple testing.
Results
Study population
The median of the interval between CEA and postoperative MRI was 2 days. Analysis planes could not be positioned at the ICA bulb because of the presence of a flow void at the high-grade ICA stenosis in five patients. In addition, inadequate ECG triggering resulted in corrupt flow velocity curves in four patients. As a result, 9 of the 29 patients had to be excluded from the analysis. The average time required for ECG-gated 4D flow MRI depended on the individual heart rate and was 15-20 min. Post-processing of the acquired raw data including calculation of WSS and OSI was approximately 90 min per data set. Patients' characteristics and the degree of ICA stenosis in the remaining 20 patients are summarised in Table 1.
Distribution of WSSsystole and OSI within standard analysis planes
Figure 1 illustrates the pre- and post-interventional distribution of WSSsystole along eight wall segments in all analysis planes along the carotid bifurcation. The individual data points represent average WSSsystole over all 20 patients before (red squares) and after CEA (black circles) of the ICA stenosis. No significant changes in WSSsystole were observed as a result of CEA in the CCA and ECA. However, WSSsystole significantly decreased at the posterior wall of the ICA bulb, in more distal ICA locations, and in most of the lateral wall segments of the ICA. Consistent with these findings, pre- and post-interventional global WSSsystole was similar for the CCA (0.49 ± 0.20 N/m2 vs. 0.47 ± 0.32 N/m2, P = 0.84) and the ECA (0.87 ± 0.45 N/m2 vs. 0.87 ± 0.73 N/m2, P = 0.95) but significantly lower (37 % reduction) at the ICA after CEA (0.72 ± 0.30 N/m2 vs. 0.45 ± 0.21 N/m2, P = 0.0005).
The change in the oscillatory shear index (OSI) before and after CEA is shown in Fig. 2. Variability between individual patients was high as indicated by the large standard deviations. Nevertheless, there was a significant decrease in OSI after CEA in lateral wall segments of most ICA locations close to the posterior wall of the proximal ICA and in the CCA and distal ICA. Pre- and post-interventional global OSIs were reduced in the CCA (14.8 ± 9.1 % vs. 10.8 ± 6.8 %, P = 0.03), similar in the ECA (10.4 ± 7.4 % vs. 7.7 ± 7.1 %, P = 0.19) and significantly lower (25 % reduction) for the ICA (12.6 ± 5.7 % vs. 9.4 ± 4.8 %, P = 0.03).
Distribution of WSSsystole and OSI considering individual plaque anatomy
A comparison of the distribution of WSSsystole and OSI in locations directly proximal and distal to the ICA stenosis is shown in Fig. 3. CEA resulted in a significant decrease in WSSsystole in all eight segments distal to the former ICA stenosis. In addition, significant post-treatment WSSsystole reductions were found in 5/8 segments along the posterior ICA wall proximal to the stenosis. Segmental OSI decreased at the lateral wall proximal and distal to the former ICA plaque but not at the posterior wall.
The segmental differences led to a significant reduction of mean post-interventional WSSsystole by 32 % in locations proximal (0.37 ± 0.13 N/m2 vs. 0.25 ± 0.14 N/m2, P = 0.005) and by 52 % in locations distal to the ICA stenosis (0.75 ± 0.46 N/m2 vs. 0.36 ± 0.19 N/m2, P = 0.0005). The relative reductions in the OSI were 24 % and 40 % compared with preoperative values for locations proximal (14.8 ± 7.5 % vs. 11.2 ± 5.2 %, P = 0.05) and distal (11.8 ± 8.7 % vs. 7.1 ± 3.6 %, P = 0.01) to the stenosis, respectively.
Discussion
The findings of our study demonstrate that 4D flow MRI was able to quantify wall parameters in 20 patients with ≥60 % ICA stenosis before and after recanalisation. Post-CEA we observed significant reductions in WSSsystole and OSI in the recanalised ICA, at both the site of the plaque and up- and downstream of the vessel. The enlargement of the ICA after plaque removal led to a consecutive deceleration of local blood flow velocities. This occurred in particular at the posterior wall of the ICA due to the reconstruction of the physiological dilatation of the ICA bulb by eversion CEA. Accordingly, the gradient of adjacent velocities at the wall decreased and led to a measurement of decreased WSSsystole [11]. CEA also changed post-stenotic complex flow towards a more laminar blood flow profile. Thus, lower oscillations of blood flow in the distal ICA resulted in a decrease of OSI at this site postoperatively. In addition, we speculate that the reconstruction of normal ICA geometry led to a less pulsatile flow waveform and thus generally reduced OSI.
In vivo measurements of fluid dynamics in post-endarterectomy carotid vessels are a prerequisite to understanding the interaction of fluid dynamic parameters with the carotid wall and the initiation and progression of atheroma. The ability to quantify such changes in vivo in ICA stenosis has important implications: identification of patients at increased risk of stroke and optimal timing of recanalisation procedures before brain infarction are highly challenging. Moreover, some patients suffer periprocedural stroke with devastating consequences and 3 %/year develop restenosis after CEA or CAS [16]. Risk factors for stroke and re-stenosis, however, are largely unknown and constitute an important research goal in order to improve individual treatment decisions and surveillance. Regional changes in WSS and OSI may help to identify patients who are at risk of plaque progression or rupture because of flow-mediated endothelial cell dysfunction. While our study did not directly investigate the link between altered wall parameters and risk of plaque progression or stroke, it is an important prerequisite that demonstrates the potential to detect significant changes in WSS and OSI in high-risk patients. Further longitudinal studies are now warranted to investigate the diagnostic value of 4D flow-derived WSS and OSI to identify patients at higher risk of plaque progression, re-stenosis, or stroke.
All carotid plaques were removed by eversion carotid endarterectomy because this is the preferred CEA technique at our institution. Thus, apart from a general enlargement of the diseased vessels because of atherosclerosis with outward remodelling, an almost physiological carotid bifurcation including an ICA bulb was restored by surgery. In addition, performance of the same surgical technique in all patients probably limited inter-individual variations of postoperative geometry. In contrast, CEA with patch repair may enlarge the internal carotid artery diameter compared to eversion CEA, which would result in different redistribution of local wall shear stress. The accuracy of our methodical approach has been previously validated for the carotid artery bifurcation in ten healthy volunteers [17]. The current study demonstrates that the proposed in vivo WSS analysis can be performed even under complex blood flow conditions such as in high-grade ICA stenosis causing haemodynamically relevant flow obstruction. Moreover, it shows that plaque removal leads to a significant reduction of both WSSsystole and OSI along the ICA. These parameters, however, did not change at the proximal CCA and ECA, which are typically not affected by plaque removal at the ICA. These findings underline the ability of our approach to selectively quantify regional 3D haemodynamics.
Comparable in vivo studies of WSS and OSI at the carotid bifurcation in humans are sparse. To our knowledge, this is the first study evaluating changes in WSS and OSI in high-grade ICA stenosis due to CEA in vivo. In a previous 4D flow MRI study of 64 carotid bifurcations of healthy young volunteers, critical wall parameters (i.e. low absolute WSS and high OSI) were concentrated at the posterior wall of the ICA bulb, a known atheroprone region. Moreover, this study showed that the distribution of absolute WSS and OSI was altered in six patients when moderate (i.e. 45–55 %) ICA stenosis was present. In 11 patients who had undergone CEA of 70–95 % ICA stenosis, the topology of WSS and OSI distribution of the carotid bifurcation was similar to that of volunteers. However, a systematic comparison of local haemodynamics before and after CEA was not performed and ICA stenoses were only moderate [9]. A recent case series of seven patients with <50 % and one patient with 50–70 % ICA stenosis calculated WSS using Doppler ultrasound and computational fluid dynamics (CFD). Patients were treated with statins over 6 months and the change in plaque size and composition was analysed by multi-contrast MRI at 3 T [18]. In agreement with the previous 4D flow MRI study [9], the incidence of low WSS at baseline (but not OSI) was highest at the posterior wall of the ICA bulb, correlating with plaque thickness and other indicators of plaque vulnerability. The effect that recanalisation had on WSS and OSI was not evaluated. Another study investigated local carotid haemodynamics in two patients who underwent CEA and carotid artery stenting of ICA stenosis using 3D CT angiography, Doppler ultrasound, and CFD [19]. Data on haemodynamic changes before CEA and carotid artery stenting were not acquired. Recanalisation induced slow helical flow and regionally low WSS in the ICA bulb after patch CEA, comparable to findings of previous studies [9, 11, 20, 21].
Limitations associated with the 4D flow MRI imaging protocol are the relatively low spatial and temporal resolution, which leads to an underestimation of absolute values of WSS and OSI. An increase in these MRI parameters and acceleration of data acquisition by parallel imaging may have the potential to improve the interpretation of intrastenotic maximum blood flow velocities. However, the available resolution in our study did not affect relative pre- and post-interventional changes of WSS or OSI. Moreover, previous studies have demonstrated good reproducibility and inter- and intra-observer reliability of WSS and OSI [17] and a recent study found good agreement of 4D flow MRI and high-resolution 2D duplex ultrasound [2] emphasising the accuracy of 4D flow MRI. Owing to metal artefacts at the vessel wall, even in patients undergoing CAS using nitinol stents, accurate follow-up measurements of WSS are currently not possible. In addition, the long process time of approximately 60 min for one single data set restricts the clinical use of this technique. However, promising new software prototypes are currently being evaluated in our institution and allow a significantly faster analysis of WSS, which is a prerequisite for application in clinical routine.
There was a dropout of patients who were screened and finally included in the study. Most of our patients suffered from acute stroke and it was challenging to perform an MRI examination shortly before carotid surgery. In addition, MRI examination required cooperation of patients in order to minimize motion artefacts and a robust ECG trigger for accurate data acquisition. These two factors currently limit the application in clinical routine. Further methodical improvements are therefore desirable that will reduce total MRI examination times to less than 30 min to gain higher patient acceptance and comfort and that will improve the reliability of the ECG-triggering for optimal timing of flow measurement. However, as dropout was not due to the degree or morphology of ICA stenosis we do not expect a significant bias on our results. In some patients we observed insufficient data quality. An optimised carotid coil that can be directly attached to the surface of the neck (in combination with a higher number of coils) [22] could further improve the signal-to-noise ratio and overall image quality.
Another drawback of our study is related to the lack of a control group of age-matched patients without ICA stenosis. Similarly, cutoff and normal values of WSSsystole and OSI along the carotid bifurcation have not been established for different age groups, limiting the predictive value of the WSSsystole and OSI derived in this study. Currently, the optimal wall parameters have yet to be established. In our analysis we chose WSSsystole over time-averaged WSS as an exploratory metric. Previous intergroup comparisons of WSS have shown that time-averaged WSS often does not exhibit significant intergroup differences because of averaging during the haemodynamically inactive diastolic period [23]. Future follow-up studies are thus needed to identify which of the WSS parameters (time averaged, peak systolic or oscillatory) optimally and independently predict development, progression, and occurrence of vulnerable plaques and thus allow for risk stratification in future patients.
Plaques of the ICA are typically eccentric. Accordingly, WSSsystole or OSI may be different within one analysis plane depending on whether that segment resides at the site of the plaque/wall thickening or at an unaffected wall segment opposite to the plaque. In addition, individual plaque composition such as predominant calcification versus a predominant large lipid-rich/necrotic core could have an influence on local haemodynamics due to the difference of elasticity of the plaque. Thus, co-registration of local WSS/OSI with local plaque distribution using source data of three-dimensional MR angiography would be of great interest to optimally study the potential role of WSS/OSI on ICA plaques. Unfortunately, multi-contrast plaque MRI, which would be needed for co-registration of plaque composition, was not part of the imaging protocol in this study. The three-dimensional and high-resolution MR-based assessment of wall thickening and plaque characteristics and its association with critical WSS/OSI should thus be further investigated in longitudinal patient studies.
In conclusion, 4D flow MRI was feasible for assessing the in vivo distribution of WSSsystole and OSI in patients with high-grade ICA stenosis. Plaque removal and thus reduced blood flow velocity gradients at the wall led to a selective and significant reduction of both average WSSsystole and OSI at the ICA. Four-dimensional flow MRI can be easily combined with other MR imaging techniques such as MR angiography or multi-contrast MRI for plaque imaging during the same session, allowing for co-registration of 3D flow and structural data. It therefore constitutes a highly promising tool for future large-scale longitudinal studies in patients evaluating the fluid-structure interactions at the carotid bifurcation.
References
Brott TG, Hobson RW 2nd, Howard G et al (2010) Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 363:11–23
Harloff A, Zech T, Wegent F, Strecker C, Weiller C, Markl M (2013) Comparison of blood flow velocity quantification by 4D flow MR imaging with ultrasound at the carotid bifurcation. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A3419
Sachar R, Yadav JS, Roffi M et al (2004) Severe bilateral carotid stenosis: the impact of ipsilateral stenting on Doppler-defined contralateral stenosis. J Am Coll Cardiol 43:1358–1362
Aleksic M, Matoussevitch V, Heckenkamp J, Brunkwall J (2006) Changes in internal carotid blood flow after CEA evaluated by transit-time flowmeter. Eur J Vasc Endovasc Surg 31:14–17
Malek AM, Alper SL, Izumo S (1999) Hemodynamic shear stress and its role in atherosclerosis. JAMA 282:2035–2042
Cheng C, Tempel D, van Haperen R et al (2006) Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113:2744–2753
Tomita H, Hagaman J, Friedman MH, Maeda N (2012) Relationship between hemodynamics and atherosclerosis in aortic arches of apolipoprotein E-null mice on 129S6/SvEvTac and C57BL/6J genetic backgrounds. Atherosclerosis 220:78–85
Lee SW, Antiga L, Spence JD, Steinman DA (2008) Geometry of the carotid bifurcation predicts its exposure to disturbed flow. Stroke 39:2341–2347
Markl M, Wegent F, Zech T et al (2010) In vivo wall shear stress distribution in the carotid artery: effect of bifurcation geometry, internal carotid artery stenosis, and recanalization therapy. Circ Cardiovasc Imaging 3:647–655
European Carotid Surgery Trialists’ Collaborative Group (1991) MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet 337:1235–1243
Harloff A, Albrecht F, Spreer J et al (2009) 3D blood flow characteristics in the carotid artery bifurcation assessed by flow-sensitive 4D MRI at 3T. Magn Reson Med 61:65–74
Bock J, Kreher BW, Hennig J, Markl M (2007) Optimized pre-processing of time-resolved 2D and 3D phase contrast MRI data. Abstract. ISMRM May; Berlin, Germany. p 3138
Bock J, Frydrychowicz A, Stalder AF et al (2010) 3D phase contrast MRA and flow visualization in the thoracic aorta at 3T: feasibility and effect of standard and blood-pool contrast agents. Magn Reson Med 63:330–338
Stalder AF, Russe MF, Frydrychowicz A, Bock J, Hennig J, Markl M (2008) Quantitative 2D and 3D phase contrast MRI: optimized analysis of blood flow and vessel wall parameters. Magn Reson Med 60:1218–1231
Barker AJ, Markl M, Burk J et al (2012) Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging 5:457–466
Lal BK, Beach KW, Roubin GS et al (2012) Restenosis after carotid artery stenting and endarterectomy: a secondary analysis of CREST, a randomised controlled trial. Lancet Neurol 11:755–763
Markl M, Wallis W, Harloff A (2011) Reproducibility of flow and wall shear stress analysis using flow-sensitive four-dimensional MRI. J Magn Reson Imaging 33:988–994
LaDisa JF Jr, Bowers M, Harmann L et al (2010) Time-efficient patient-specific quantification of regional carotid artery fluid dynamics and spatial correlation with plaque burden. Med Phys 37:784–792
Hayase H, Tokunaga K, Nakayama T et al (2011) Computational fluid dynamics of carotid arteries after carotid endarterectomy or carotid artery stenting based on postoperative patient-specific computed tomography angiography and ultrasound flow data. Neurosurgery 68:1096–1101
Harloff A, Markl M, Frydrychowicz A, Hennig J, Weiller C (2009) Diagnosing stroke aetiologies. Morphologic and functional analysis of the aorta and carotid arteries by MRI. Nervenarzt 80:929–940
Gallo D, Steinman DA, Bijari PB, Morbiducci U (2012) Helical flow in carotid bifurcation as surrogate marker of exposure to disturbed shear. J Biomech 45:2398–2404
Tate Q, Kim SE, Treiman G, Parker DL, Hadley JR (2012) Increased vessel depiction of the carotid bifurcation with a specialized 16-channel phased array coil at 3T. Magn Reson Med 69:1486–1493
Barker AJ, Lanning C, Shandas R (2010) Quantification of hemodynamic wall shear stress in patients with bicuspid aortic valve using phase-contrast MRI. Ann Biomed Eng 38:788–800
Acknowledgements
We thank Hansjörg Mast for performing all MRI measurements.
Andreas Harloff is supported by Deutsche Forschungsgemeinschaft grant no. HA 5399/3-1; Michael Markl is supported by NIH NHLBI grant R01HL115828; NUCATS Institute NIH grant UL1RR025741, and the Northwestern Memorial Foundation Dixon Translational Research Grants Initiative.
Disclosures
None of the authors has a conflict of interest related to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harloff, A., Berg, S., Barker, A.J. et al. Wall shear stress distribution at the carotid bifurcation: influence of eversion carotid endarterectomy. Eur Radiol 23, 3361–3369 (2013). https://doi.org/10.1007/s00330-013-2953-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-2953-4