Abstract
Objectives
To investigate effects of menopausal status, oral contraceptives (OC), and postmenopausal hormone therapy (HT) on normal breast parenchymal contrast enhancement (CE) and non-mass-like enhancing areas in magnetic resonance mammography (MRM).
Methods
A total of 459 female volunteers (mean age 49.1 ± 12.5 years) underwent T1-weighted 3D MRM 1–5 min after bolus injection of gadobutrol. Quantitative analysis was performed in normal breast parenchyma by manually tracing regions of interest and calculating percentage CE. Semiquantitative analysis was performed in non-mass-like enhancing areas, and signal intensity changes were characterised by five predefined kinetic curve types. The influence of OC (n = 69) and HT (n = 24) on CE was studied using random effects models.
Results
Breast parenchymal enhancement was significantly higher in premenopausal than in postmenopausal women (P < 0.001). CE decreased significantly with the use of OC (P = 0.01), while HT had negligible effects (P = 0.52). Prevalence of kinetic curve types of non-mass-like enhancement differed strongly between pre- and postmenopausal women (P < 0.0001), but was similar in OC users and non-OC users (P = 0.61) as well as HT users and non-HT users (P = 0.77).
Conclusions
Normal breast parenchymal enhancement and non-mass-like enhancing areas were strongly affected by menopausal status, while they were not affected by HT use and only moderately by OC use.
Key Points
• Breast parenchymal enhancement at MR mammography is stronger in premenopausal than postmenopausal women.
• The prevalence of strong enhancing non-mass-like areas is greater before menopause.
• Such enhancing non-mass-like areas may impair lesion detection in premenopausal women.
• Breast parenchymal enhancement is only marginally affected by hormone use.
• Discontinuation of hormone use before MR mammography may be unnecessary.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dynamic magnetic resonance mammography (MRM) is the most sensitive method for detecting early invasive breast cancer [1], combining morphological information with functional characterisation based on analysis of contrast enhancement kinetics of the breast. With the increasing use of MRM for screening of asymptomatic women at high risk for breast cancer, a thorough understanding of tissue morphology and contrast kinetics in normal breast tissue becomes more important.
Normal parenchyma typically shows non-mass-like enhancement [2]. The Breast Imaging-Reporting and Data System (BI-RADS) categorises this enhancement as minimal, mild, moderate, or marked, while patterns are described as diffuse, patchy, or nodular [3]. Benign and malignant enhancement profiles overlap [4, 5], and early and strong parenchymal enhancement, in particular, may complicate differential diagnosis and obscure malignant lesions [5–9]. For use of MRM in screening, it is especially desirable to have comprehensive qualitative and quantitative data on morphological and kinetic features of the healthy breast from a large, unselected population of women; however, such studies are scarce [10, 11].
Several studies show that endogenous hormonal variations, dependent on menopausal status and menstrual cycle phase, affect breast tissue composition [12–14], tissue relaxation times [15] and apparent diffusion coefficient [16], and contrast enhancement kinetics of normal breast parenchyma [17, 18] and of breast lesions [2, 19] in MRM.
Exogenous hormones, such as hormone therapy (HT) in postmenopausal women, are well known to increase breast density in X-ray mammography by 8.7–35.6% [20] and parenchymal tissue-to-fat ratio in MRM [21]. Although HT and oral contraceptives (OC) are widely used, only a few studies have evaluated effects of HT [10, 22], and no study has determined effects of OC on contrast enhancement of normal breast parenchyma in MRM.
Therefore, the purpose of the present study was first to investigate effects of menopausal status and second to evaluate effects of OC and HT on contrast enhancement kinetics of normal breast parenchyma and non-mass-like enhancing areas in MRM.
Materials and methods
Study population
Between June 2008 and September 2011, all women were enrolled from the Study of Health in Pomerania (SHIP), a prospective population-based cohort study in Northeast Germany [23] that includes a whole-body MRI examination. In addition to the whole-body MRI, women were offered participation in an optional MRM [24]. Women with known reactions to any kind of contrast agent or drugs, and pregnant or breastfeeding women were not offered MRM. Of 1,475 female study participants, aged 20–83 years, who were enrolled in the whole-body MRI examination, a total of 651 (44.1%) agreed to undergo additional MRM.
All women underwent a structured interview to obtain information on history of breast diseases, history of breast surgery including breast implants, menopausal status, day of the menstrual cycle, and medication history. All participants were asked to bring the packaging from medication they had taken within the last 14 days. For each medication the Anatomical Therapeutic Chemical (ATC) classification system code was recorded. Menopause was defined as cessation of menstrual bleeding for at least 12 months. Body height and weight were measured, and the body mass index (BMI) was calculated.
SHIP was approved by the institutional review board, and written informed consent was obtained from each participant before enrolment.
Dynamic contrast-enhanced MR mammography examination
MR imaging was performed using a whole-body 1.5 Tesla MR system (Magnetom Avanto; Siemens Medical Solutions, Erlangen, Germany). Intravenous access was established, and the woman was placed prone with the uncompressed breasts suspended in a commercial four-channel circularly polarised bilateral breast phased-array receiver coil. The protocol was identical for all participants and included axial dynamic, T1-weighted, time-resolved angiography with stochastic trajectories (TWIST) and three-dimensional imaging without fat suppression [8.86 ms/4.51 ms (repetition time/echo time); 25° flip angle; 340 mm field of view; 0.9 × 0.7 × 1.5 mm voxels]. Following acquisition of the first unenhanced sequence, an intravenous gadobutrol bolus (Gadovist, Bayer Healthcare, Leverkusen, Germany) was administered with a power injector at a dose of 0.1 mmol/kg body weight at a rate of 1.0 mL/s, followed by a saline flush (20 mL) injected at the same rate. The sequence was repeated five times without time gaps. Each sequence took 58.27 s.
Inclusion and exclusion criteria for data analysis
Inclusion criteria for analysis of normal breast parenchyma were female gender and age 20 years or older. Breast-related exclusion criteria were history of recent or previous breast disease or history of breast surgery including breast implants (n = 12), breasts with complete involution precluding measurement of representative parenchyma (n = 68), and breasts with mass lesions according to the BI-RADS MRI lexicon [3] (n = 97). Perimenopausal women with cessation of menstrual bleeding for less than 12 months (n = 15) were also excluded. A total of 192 (29.5%) subjects were excluded from analysis, resulting in a final study population of 459 women.
Quantitative analysis of contrast enhancement in normal breast parenchyma
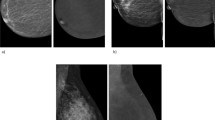
To limit possible bias in the reproducibility of measurement resulting from variable repartitioning of fibroglandular tissue throughout the breast, measurements were performed in two slices above and two slices below the nipple, where breast tissue is usually constant and more homogeneous [18, 22]. First, images were postprocessed for quantitative analysis using the Syngo 2008A MultiModality Workplace (Siemens Medical Solutions, Erlangen, Germany). Image subtraction was done to identify any non-mass-like enhancement that was excluded from the analysis of normal breast parenchyma. Non-mass-like enhancement was defined as early-phase contrast enhancement that was apparent in the first postcontrast image because this has been associated with malignant tumour growth [5, 18, 22, 25, 27, 28]. Second, a region of interest (ROI) was drawn manually to include all fibroglandular tissue of the breast in the four slices selected. Visible fat and cysts identified on the unenhanced image without subtraction or non-mass-like enhancement identified on the first postcontrast image with subtraction were excluded (Fig. 1). Third, a time-signal intensity curve was created automatically for each ROI on a pixel-by-pixel basis representing mean values of T1 signal intensity (SI) and standard deviations for all dynamic frames. Percentage contrast enhancement was calculated as [SI(t1–5) − SI(t0)]/SI(t0) × 100, where SI(t0) is the signal intensity before and SI(t1–5) after gadobutrol administration [17, 18, 22]. To exclude inter-reader variability only one radiologist performed all readings.
Method of quantitative analysis of contrast enhancement in normal breast parenchyma. Figures show how the region of interest (ROI) was drawn to measure T1 signal intensity of normal breast parenchyma in two women aged 34 years (a and b) and 43 years (c and d). In case one, on the unenhanced image without subtraction (a) no cysts were seen, and on the first enhanced image with subtraction (b) no non-mass-like enhancement was identified. Therefore, the manually drawn ROI included all visible parenchyma (a). In case two, on the first postcontrast image with subtraction (d) non-mass-like contrast enhancement was identified at the lower boundary of the parenchyma. Therefore, the ROI was drawn on the unenhanced image without subtraction (c) exclusive of the enhancing area in d
Semiquantitative analysis of non-mass-like enhancement
Semiquantitative analyses were performed for all non-mass-like enhancing areas and foci visible on subtracted images. A ROI was placed in each area of non-mass-like enhancement and focus. Five kinetic curve types of contrast enhancement were defined according to Kaiser and Zeitler [25] (Fig. 2). Kinetic curve types I–III are typical for benign lesions, whereas kinetic curve type IV is suspicious, and kinetic curve type V is highly suspicious for malignancy in MRM [25]. When several non-mass-like enhancing areas or foci with different curve types were present within one breast, the curve type most suspicious for malignancy was chosen for evaluation.
Predefined curve types of contrast enhancement behaviour. Curve type I Minimal continuous contrast enhancement up to 25%; curve type II weak continuous contrast enhancement of 26–60%; curve type III strong continuous contrast enhancement of 61–80%; curve type IV strong continuous contrast enhancement of more than 80% during the first 2 min after contrast medium injection, followed by a plateau; curve type V strong continuous contrast enhancement of more than 80% during the first 2 min after contrast medium injection, followed by washout
Statistical analysis
Because the time courses of contrast enhancement were similar in both breasts, we modelled the mean contrast enhancement across both breasts in all further analyses. Two-level random effects models were applied to analyse contrast enhancement in both breasts, using the STATA xtmixed routine with six time points at level 1 and individuals at level 2. This approach takes the correlated longitudinal data structure into account [26]. All calculations were stratified by menopausal status, except for the test on CE differences between pre- and postmenopausal women. Regression model building was based on deviance tests using restricted maximum likelihood estimates to evaluate variance components of the model. A random intercept model strongly outperformed a model without random effects in premenopausal ( χ 2 = 2,208.56, df = 1, P < 0.001) and postmenopausal women ( χ 2 = 2,734.36, df = 1, P < 0.001), as did a linear time random slope model vs. the random intercept model (premenopausal: χ 2 = 968.81, df = 2, P < 0.001; postmenopausal: χ 2 = 382.15, df = 2, P < 0.001). An unstructured covariance matrix was chosen because it outperformed an independent matrix in the random slope model (premenopausal: χ 2 = 12.60, df = 1, P < 0.001; postmenopausal: χ 2 = 7.02, df = 1, P < 0.001). Full maximum likelihood estimation was used to evaluate fixed-effects terms in the models. All models included age and BMI. Prevalence differences in the five kinetic curve types between OC/HT users and non-OC/HT users were assessed using χ 2 test.
A P-value <0.05 was labelled statistically significant. Analysis was performed using STATA 12 (StataCorp, College Station, TX, USA) and SPSS 15.0.1 (SPSS Software, Munich, Germany).
Results
Subject characteristics and descriptive statistics are listed in Table 1. Out of 459 women, 234 (51.0%) were premenopausal and 225 (49.0%) were postmenopausal. Hormonal contraceptive use (ATC G03A) was reported by 69 premenopausal women. There were 24 women using postmenopausal hormone therapy: 12 women using estrogens only (ATC G03C) and 12 women progestogens and estrogens in combination (ATC G03F). No case of progestogens only use (ATC G03D) was recorded. No woman reported adverse events from gadobutrol administration.
Quantitative analysis of contrast enhancement in normal breast parenchyma
Contrast enhancement of normal breast parenchyma was approximately 30% higher in premenopausal than in postmenopausal women (P < 0.001). Mean enhancement was 6.07, 10.72, 14.88, 18.27, and 20.27% in postmenopausal women and 11.51, 19.83, 25.54, 30.13, and 33.74% in premenopausal women at 1, 2, 3, 4, and 5 min after contrast respectively. Note that age effects on contrast enhancement were negligible in both pre- (P = 0.89) and postmenopausal women (P = 0.73).
Hormone-related changes in absolute signal intensities stratified by menopausal status are displayed in Table 2. Before menopause, contrast enhancement was significantly lower in OC users compared to non-OC users (P = 0.01, Fig. 3). Differences in contrast enhancement between these groups were mainly due to a significantly lower increase from baseline and the first minute after contrast injection in OC users. After menopause, enhancement was similar in HT users and non-HT users (P = 0.52, Fig. 3). A subanalysis of HT compositions yielded similar results for estrogen-only users (P = 0.43) and for users of progestogens and estrogens in combination (P = 0.63).
Variation in contrast enhancement as a function of use of oral contraceptives (OC) or postmenopausal hormone therapy (HT). Results correspond to the calculations in Table 2. In non-OC users mean contrast enhancement was 12.65, 21.14, 27.44, 31.73, and 34.83% at 1, 2, 3, 4, and 5 min respectively. Contrast enhancement decreased significantly (P = 0.01) with the use of OC to 8.53, 16.69, 23.25, 27.61, and 30.19%. In non-HT users mean contrast enhancement was 5.83, 10.44, 14.58, 17.78, and 19.89% and was only slightly higher (P = 0.52) with the use of HT: 6.98, 12.20, 17.15, 20.78, and 22.74%
The menstrual cycle phase was distributed randomly in premenopausal women. The association with contrast enhancement was negligible in size (χ 2 = 7.7, df = 10, P = 0.66) even when taking account of potential nonlinear associations.
Semiquantitative analysis of non-mass-like enhancement
Non-mass-like enhancement and foci were observed in all examinations and were mostly symmetrical in both breasts. In 21 women non-mass-like enhancement showed suggestive morphology, meaning linear, ductal, or segmental distribution pattern with clumped or dendritic internal enhancement pattern according to BI-RADS [3]. These lesions were not excluded from the analysis because follow-up 3 months later with mammography and MRM revealed no malignant tumour growth. None of the non-mass-like enhancing areas or foci showed kinetic curves corresponding to a plateau phenomenon (type IV) or washout effect (type V). The distribution of kinetic curve types differed strongly between pre- and postmenopausal women (P < 0.0001, Fig. 4). In premenopausal women curve types II and III were more common. Subgroup analysis showed marginal differences in the distribution of curve types between OC users and non-OC users (P = 0.61) as well as between HT and non-HT users (P = 0.77, Fig. 5).
Distribution of kinetic curve types of non-mass lesions in pre- and postmenopausal women. Of all premenopausal women, 101 (43.2%) showed curve type I, 101 (43.2%) were classified as curve type II, and 32 (13.6%) showed curve type III. Of all postmenopausal women, 148 (65.8%) showed curve type I, 70 (31.1%) were classified as curve type II, and 7 (3.1%) showed curve type III. Differences between the two groups were statistically highly significant (P < 0.0001)
Distribution of kinetic curve types of non-mass lesions according to the use of oral contraceptives (OC) and postmenopausal hormone therapy (HT). The distribution of curve types was not significantly different between non-OC users and OC users (P = 0.61): curve types I, II, and III were observed in 68 (41.2%), 76 (46.1%), and 21 (12.7%) non-OC users and in 33 (47.8%), 25 (36.2%), and 11 (16.0%) OC users. In postmenopausal women, the distribution of curve types was also not significantly different between non-HT users and HT users (P = 0.77): curve types I, II, and III were observed in 132 (65.7%), 62 (30.9%), and 7 (3.4%) non-HT users and in 15 (62.5%), 9 (37.5%), and 0 (0%) HT users
Discussion
Our analysis of 459 MRM datasets of women from a general population indicates that contrast enhancement of normal breast parenchyma and prevalence of non-mass-like enhancement are significantly affected by menopausal status. OC are associated with significantly lower parenchymal enhancement but had no effects on the prevalence of non-mass-like enhancement.
The significant variation in contrast enhancement of normal breast parenchyma with menopausal status and OC use underlines the presence of endogenous and, to a lesser extent, of exogenous hormonal effects on MRM. The mechanism of contrast enhancement in tumour and normal breast parenchyma is mainly related to microvessel density and permeability [27, 28].
In our study, contrast enhancement was significantly higher in premenopause than in postmenopause. Physiologically, the stronger enhancement may be explained by a histamine-like effect of estrogen, which induces vasodilatation and an increase in microvascular permeability [29], as well as by a mitogenic effect of progestin, which may lead to an increase in perfusion by promoting metabolic activity [30].
The significantly higher prevalence of curve types II and III non-mass-like enhancement in premenopausal women suggests that hormonal status also affects areas and foci of non-mass-like enhancement. This observation is consistent with recent investigations reporting a higher prevalence of background enhancement in premenopause compared to postmenopause [8, 9]. Early and strong parenchymal enhancement may complicate differential diagnosis and obscure malignant lesions, which may result in false-negative breast MRI findings [5–9]. Teifke et al. [31] found a small proportion of invasive breast carcinomas (7.3%; 3 of 41) to be masked by intensively enhancing glandular tissue. Uematsu et al. [9] reported the sensitivity and accuracy of MRM to decrease significantly in cases with moderate or marked background enhancement. In particular, contrast-enhancing areas that present with a similar non-mass-like morphology, such as preinvasive ductal carcinoma in situ, pose diagnostic problems [5, 6]. Ductal carcinoma in situ, especially when low-grade, may be obscured because it may present with patchy continuous enhancement [5, 32], resembling the non-mass-like enhancement of normal breast parenchyma we found to be more prevalent in premenopause. On the other hand, a suspicious finding in premenopausal women is more likely to be a false positive, possibly resulting in unnecessary biopsy or surgery [7].
In our study, HT had negligible effects on contrast enhancement of normal breast parenchyma, whereas Delille et al. [22] reported a higher extraction flow product (EFP) of non-diseased breast parenchyma in postmenopausal women receiving HT. The EFP represents the blood volume passing the parenchyma per unit of time and is based on a multi-compartment model established by Hulka et al. [27]. However, results are not directly comparable: methods of measuring contrast enhancement and EFP differ, and while our analysis was performed in a small number of women (n = 24) recruited from the general population, Delille et al. performed EFP measurements in the presumably healthy contralateral breasts of patients. Further studies must show whether EFP or contrast enhancement measurement is more adequate to determine HT effects on breast parenchyma.
We found a significantly lower contrast enhancement in OC users compared to non-OC users, which is consistent with a previous report by Marklund et al. [33]. They reported a tendency towards lower maximum signal intensity of normal breast parenchyma in a small group of OC users (n = 9) compared to non-OC users (n = 16). OC have a lower relative estrogen content than postmenopausal hormone preparations, whereas the relative progestin content is higher than in HT [34]. Therefore, breast tissue perfusion and permeability might be affected more strongly by progestin in OC users and estrogen in HT users. It is now generally accepted that the mechanism underlying MRI contrast enhancement is the extravasation of contrast media from microvessels into the extracellular matrix [27]. In vitro studies demonstrated that progesterone reversed the estradiol-induced lowering of the endothelial cell barrier function in human placenta [35] and reduced the permeability of the blood-brain barrier [36]. Therefore, the reverse effects of progestin on estrogen-induced microvessel permeability may explain the lower enhancement in OC users.
In a retrospective analysis of 215 postmenopausal patients, Pfleiderer et al. [10] investigated effects of HT on contrast-enhancing areas. HT users showed highly significant differences in the distribution of curve types compared to non-HT users but showed no evidence of plateau or washout kinetic. We also saw no plateau or washout kinetics in any of the non-mass-like enhancing areas in our study. Therefore, it seems unlikely that HT or OC would mimic MR findings typical for breast cancer. In our study, the distribution of curve types I to III was similar in HT users and non-HT users. In contrast, Pfleiderer et al. [10] found more curve type III enhancing areas in HT users (23.3%) than in non-HT users (3.8%). First, this could be explained by the different study populations: Pfleiderer investigated a patient population including two women with ductal carcinoma in situ and invasive lobular cancer, whereas we excluded women with breast lesions. Second, the pharmaceutical form of hormone medication (combination therapy versus monotherapy, oral versus transdermal administration) was different in the study populations.
As neither OC nor HT use resulted in MR findings mimicking breast cancer, increased the prevalence of strongly enhancing non-mass-like lesions, or led to stronger enhancement of normal breast parenchyma, discontinuation of HT or OC prior to MRM does not seem to be necessary. However, further research is definitely needed to confirm these observations and to support our conclusion.
Our study has some limitations. Since analyses were performed in a general population our groups of OC users and HT users represent the prevalence of hormone medication use in this region. But both groups were probably too small to provide enough statistical power to reliably detect subtle differences in curve type distributions. Because HT effects on EFP were found to be different for monotherapy and combined therapy [22], the small group size of monotherapy and combined therapy users in our study could have contributed to the contrary results. Further studies are therefore needed to determine enhancement differences of normal breast parenchyma related to use of different HT and OC types. Finally, for quantitative analysis of breast parenchyma ROIs were traced manually. Manual segmentation of the breast parenchyma is a laborious and extremely time-consuming process, which is observer-dependent and therefore hardly reproducible and prone to errors. Thus, development of a computerised, reliable, and fast automatic approach for breast tissue analysis is needed. However such methods are still under investigation [12].
In conclusion, contrast enhancement of normal breast parenchyma and prevalence of strongly enhancing non-mass-like areas are significantly higher in premenopause, indicating that non-mass lesions may be obscured, impairing lesion detection. Contrast enhancement of normal breast parenchyma was not affected by HT and only moderately by OC. Prevalence of kinetic curve types of non-mass-like enhancement was not affected by OC or HT. Therefore, discontinuation of HT or OC use prior to MRM does not seem to be necessary. Further research is required to confirm OC- and HT-related findings and to determine the influence of different hormone compositions.
Abbreviations
- ATC:
-
Anatomical Therapeutic Chemical classification system
- BI-RADS:
-
Breast Imaging-Reporting and Data System
- BMI:
-
Body mass index
- CE:
-
Contrast enhancement
- HT:
-
Postmenopausal hormone therapy
- MRM:
-
Magnetic resonance mammography
- OC:
-
Oral contraceptives
- ROI:
-
Region of interest
- TWIST:
-
Time-resolved angiography with stochastic trajectories
References
Hauth EAM, Jaeger H, Maderwald S et al (2006) Evaluation of quantitative parametric analysis for characterization of breast lesions in contrast-enhanced MR mammography. Eur Radiol 16:2834–2841
Kuhl CK, Bieling HB, Gieseke J et al (1997) Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology 203:137–144
American College of Radiology (2003) ACR Breast Imaging-Reporting and Data System (BI-RADS): breast MRI atlas, 4th ed. American College of Radiology, Reston, VA
Sardanelli F, Podo F (2007) Breast MR imaging in women at high-risk of breast cancer. Is something changing in early breast cancer detection? Eur Radiol 17:873–887
Jansen SA, Newstead GM, Abe H, Shimauchi A, Schmidt RA, Karczmar GS (2007) Pure ductal carcinoma in situ: kinetic and morphologic MR characteristics compared with mammographic appearance and nuclear grade. Radiology 245:684–691
Teifke A, Lehr H, Vomweg T, Hlawatsch A, Thelen M (2003) Outcome analysis and rational management of enhancing lesions incidentally detected on contrast-enhanced MRI of the breast. AJR Am J Roentgenol 181:655–662
Hambly NM, Liberman L, Dershaw DD, Brennan S, Morris EA (2011) Background parenchymal enhancement on baseline screening breast MRI: impact on biopsy rate and short-interval follow-up. AJR Am J Roentgenol 196:218–224
Baltzer PA, Dietzel M, Vag T et al (2011) Clinical MR mammography: impact of hormonal status on background enhancement and diagnostic accuracy. Rofo 183:441–447
Uematsu T, Kasami M, Watanabe J (2011) Does the degree of background enhancement in breast MRI affect the detection and staging of breast cancer? Eur Radiol 21:2261–2267
Pfleiderer SOR, Sachse S, Sauner D (2004) Changes in magnetic resonance mammography due to hormone replacement therapy. Breast Cancer Res 6:R232–238
Jansen SA, Lin VC, Giger ML, Li H, Karczmar GS, Newstead GM (2011) Normal parenchymal enhancement patterns in women undergoing MR screening of the breast. Eur Radiol 21:1374–1382
Chan S, Su M-YL, Lei F-J et al (2011) Menstrual cycle-related fluctuations in breast density measured by using three-dimensional MR imaging. Radiology 261:744–751
Hussain Z, Roberts N, Whitehouse GH, García-Fiñana M, Percy D (1999) Estimation of breast volume and its variation during the menstrual cycle using MRI and stereology. Br J Radiol 72:236–245
Graham SJ, Stanchev PL, Lloyd-Smith JO, Bronskill MJ, Plewes DB (1995) Changes in fibroglandular volume and water content of breast tissue during the menstrual cycle observed by MR imaging at 1.5 T. J Magn Reson Imaging 5:695–701
Dean KI, Majurin ML, Komu M (1994) Relaxation times of normal breast tissues. Changes with age and variations during the menstrual cycle. Acta Radiol 35:258–261
O’Flynn EAM, Morgan VA, Giles SL, Desouza NM (2012) Diffusion weighted imaging of the normal breast: reproducibility of apparent diffusion coefficient measurements and variation with menstrual cycle and menopausal status. Eur Radiol 22(7):1512–1518
Müller-Schimpfle M, Ohmenhaüser K, Stoll P, Dietz K, Claussen CD (1997) Menstrual cycle and age: influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology 203:145–149
Delille J-P, Slanetz PJ, Yeh ED, Kopans DB, Garrido L (2005) Physiologic changes in breast magnetic resonance imaging during the menstrual cycle: perfusion imaging, signal enhancement, and influence of the T1 relaxation time of breast tissue. Breast J 11:236–241
Rieber A, Nüssle K, Merkle E, Kreienberg R, Tomczak R, Brambs HJ (1999) MR mammography: influence of menstrual cycle on the dynamic contrast enhancement of fibrocystic disease. Eur Radiol 9:1107–1112
Boyd NF, Melnichouk O, Martin LJ et al (2011) Mammographic density, response to hormones, and breast cancer risk. J Clin Oncol 29:2985–2992
Reichenbach JR, Przetak C, Klinger G, Kaiser WA (1999) Assessment of breast tissue changes on hormonal replacement therapy using MRI: a pilot study. J Comput Assist Tomogr 23:407–413
Delille JP, Slanetz PJ, Yeh ED, Kopans DB, Halpern EF, Garrido L (2005) Hormone replacement therapy in postmenopausal women: breast tissue perfusion determined with MR imaging—initial observations. Radiology 235:36–41
Völzke H, Alte D, Schmidt CO et al (2011) Cohort profile: the study of health in Pomerania. Int J Epidemiol 40:294–307
Hegenscheid K, Kühn JP, Volzke H, Biffar R, Hosten N, Puls R (2009) Whole-body magnetic resonance imaging of healthy volunteers: pilot study results from the population-based SHIP study. Rofo 181:748–759
Kaiser WA, Zeitler E (1989) MR imaging of the breast: fast imaging sequences with and without Gd-DTPA. Preliminary observations. Radiology 170:681–686
Snijders T, Bosker R (1999) Multilevel analysis: an introduction to basic and advances multilevelmodeling. Sage Publications, London
Hulka CA, Smith BL, Sgroi DC et al (1995) Benign and malignant breast lesions: differentiation with echo-planar MR imaging. Radiology 197:33–38
Hulka CA, Edmister WB, Smith BL (1997) Dynamic echo-planar imaging of the breast: experience in diagnosing breast carcinoma and correlation with tumor angiogenesis. Radiology 205:837–842
Zeppa R (1969) Vascular response of the breast to estrogen. J Clin Endocrinol Metab 29:695–700
Potten CS, Watson RJ, Williams GT et al (1988) The effect of age and menstrual cycle upon proliferative activity of the normal human breast. Br J Cancer 58:163–170
Teifke A, Hlawatsch A, Beier T et al (2002) Undetected malignancies of the breast: dynamic contrast-enhanced MR imaging at 1.0 T. Radiology 224:881–888
Neubauer H, Li M, Kuehne-Heid R, Schneider A, Kaiser WA (2003) High grade and non-high grade ductal carcinoma in situ on dynamic MR mammography: characteristic findings for signal increase and morphological pattern of enhancement. Br J Radiol 76:3–12
Marklund M, Christensen R, Torp-Pedersen S, Thomsen C, Nolsøe CP (2009) Signal intensity of normal breast tissue at MR mammography on midfield: applying a random coefficient model evaluating the effect of doubling the contrast dose. Eur J Radiol 69:93–101
Kulier R, Helmerhorst FM, Maitra N, Gülmezoglu AM (2004) Effectiveness and acceptability of progestogens in combined oral contraceptives—a systematic review. Reprod Health 1:1
Fujimoto J, Sakaguchi H, Hirose R, Tamaya T (1998) Sex steroidal regulation of vessel permeability associated with vessel endothelial cadherin (V-cadherin). J Steroid Biochem Mol Biol 67:25–32
Wang J, Jiang C, Li X, Liu C, Cheng N, Hao Y (2009) The protective mechanism of progesterone on blood-brain barrier in cerebral ischemia in rats. Brain Res Bull 79:426–430
Acknowledgements
SHIP is part of the Community Medicine Research Net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (grant No. 03ZIK012), the Ministry of Cultural Affairs as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania. Whole-body MR imaging was supported by a joint grant from Siemens Healthcare, Erlangen, Germany, and the Federal State of Mecklenburg-West Pomerania. The University of Greifswald is a member of the ‘Center of Knowledge Interchange’ program of Siemens AG. Dynamic contrast-enhanced MR mammography research is part of the entire whole-body MRI study and was supported by Bayer Healthcare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hegenscheid, K., Schmidt, C.O., Seipel, R. et al. Contrast enhancement kinetics of normal breast parenchyma in dynamic MR mammography: effects of menopausal status, oral contraceptives, and postmenopausal hormone therapy. Eur Radiol 22, 2633–2640 (2012). https://doi.org/10.1007/s00330-012-2544-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2544-9