Abstract
Objectives
To evaluate MRI using T1 and T2* mapping sequences in patients with suspected hepatic iron overload (HIO).
Methods
Twenty-five consecutive patients with clinically suspected HIO were retrospectively studied. All underwent MRI and liver biopsy. For the quantification of liver T2* values we used a fat-saturated multi-echo gradient echo sequence with 12 echoes (TR = 200 ms, TE = 0.99 ms + n × 1.41 ms, flip angle 20°). T1 values were obtained using a fast T1 mapping sequence based on an inversion recovery snapshot FLASH sequence. Parameter maps were analysed using regions of interest.
Results
ROC analysis calculated cut-off points at 10.07 ms and 15.47 ms for T2* in the determination of HIO with accuracy 88 %/88 %, sensitivity 84 %/89.5 % and specificity 100 %/83 %. MRI correctly classified 20 patients (80 %). All patients with HIO only had decreased T1 and T2* relaxation times. There was a significant difference in T1 between patients with HIO only and patients with HIO and steatohepatitis (P = 0.018).
Conclusions
MRI-based T2* relaxation diagnoses HIO very accurately, even at low iron concentrations. Important additional information may be obtained by the combination of T1 and T2* mapping. It is a rapid, non-invasive, accurate and reproducible technique for validating the evidence of even low hepatic iron concentrations.
Key Points
• Hepatic iron overload causes fibrosis, cirrhosis and increases hepatocellular carcinoma risk.
• MRI detects iron because of the field heterogeneity generated by haemosiderin.
• T2* relaxation is very accurate in diagnosing hepatic iron overload.
• Additional information may be obtained by T1 and T2* mapping.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hereditary haemochromatosis (HH) is a systemic iron overload syndrome that is typically associated with homozygosity for the C282Y polymorphism of the HFE gene that induces excessive intestinal absorption of iron from the diet. Excess iron accumulates mainly in the liver. Cardiac, pancreatic and pituitary iron overload are typical manifestations of early onset severe iron overload syndromes such as juvenile haemochromatosis, which is caused by defects of the haemojuvelin or hepcidin gene. In addition to these primary genetic disorders of iron metabolism, iron overload can also result from excessive iron absorption or repeated transfusion secondary to ineffective erythropoiesis and anaemia [1]. As humans have no route for the regulated excretion of iron, excess iron is mainly stored in the liver where iron-induced organ damage can ultimately result in cirrhosis and hepatocellular carcinoma. Depending on the underlying cause of iron overload, hepatic iron accumulates either in hepatocytes or in macrophages [2].
Haemochromatosis is a progressive disorder and clinical manifestations of the disease can be prevented by phlebotomy or iron chelation. Genetic testing for haemochromatosis allows the determination of individuals at risk of developing iron overload, but the penetrance of haemochromatosis is only 15 % in individuals homozygous for the C282Y polymorphism. This illustrates that methods that detect hepatic iron overload (HIO) early are required [3, 4].
Serum iron parameters have poor sensitivity and specificity for the assessment of body iron stores [5]. Therefore the most reliable method of assessing body iron stores is the quantification of iron in liver biopsies. Limitations of this method include sampling errors and its invasiveness.
Magnetic resonance imaging (MRI) is therefore an attractive non-invasive alternative for the quantification of hepatic iron [6–8]. Detection of iron by MRI is based on local field heterogeneities generated mostly by the paramagnetic effect of haemosiderin particles. However, only a few accepted and applicable techniques are available: calculation of the liver to muscle signal intensity (SI) ratio in gradient echo sequences described by Gandon et al., measurement of R2 and R2* which was rigorously validated by St Pierre et al. (R2 mapping) and Wood et al. (R2* mapping), calculation of relaxation time constants in T2* by Chandarana et al. or chemical shift imaging as published by Lim et al. [9–16].
The objective of this study was to investigate the impact of HIO on T1 and T2* relaxation times and to correlate changes in the relaxation times with the results obtained by liver biopsy. Our intention is to further improve and validate the MR protocol for the accurate assessment of hepatic iron content.
Materials and methods
Patients
We retrospectively studied 25 patients (19 men and 6 women; mean age 43 years, range 28–69 years) who underwent liver MRI and who had concomitant liver biopsy between March 2003 and October 2010. All patients were referred to our department for evaluation of HIO on the basis of clinical criteria or because of increased serum ferritin (>300 μg/L in male patients and >200 μg/L in female patients) or transferrin saturation (>45 % in male patients and >50 % in female patients). Each patient in this study underwent both percutaneous needle biopsy of the liver and MR imaging before biopsy. No patient had a history of blood transfusion or was a thalassemia major carrier. The time interval between biopsy and MRI imaging was less than 1 month (mean 5 days, range 1–16 days). All patients were tested for the C282Y and H63D polymorphisms of the HFE gene. None of the patients had phlebotomy or abnormal bleeding between liver biopsy and MRI. Written informed consent was obtained from each patient before performing the imaging procedure and the liver biopsy. Institutional review board approval was granted by means of a general waiver for studies with retrospective data analysis (local research ethics committee, Medical University of Innsbruck; 20 February 2009).
Liver biopsy
Ultrasound-guided percutaneous liver biopsy was carried out using a 16-gauge Tru-cut biopsy needle. HIO was defined as a liver iron concentration (LIC) greater than 25 μmol Fe/g or hepatic iron index (HII) ≥ 1.9 [17, 18].
Hepatic steatosis was assessed by determining the percentage of fatty hepatocytes [19]. A fraction of greater then 5 % was defined as steatohepatitis (SH).
MR imaging techniques
Magnetic resonance imaging of the liver was performed on a clinical 1.5-T system equipped with at 45 mT/m gradient system (Avanto 76 × 32, Siemens Healthcare Sector, Erlangen, Germany). All subjects underwent our routine liver MRI protocol for iron evaluation including the following breath-hold sequences with a flexible six-element body matrix coil in transverse orientation: for the quantification of liver T1 values we used a fast T1 mapping sequence based on an inversion recovery snapshot fast-low angle shot (FLASH) sequence which allowed the acquisition of a single T1 map during one breath-hold with 5-mm slice thickness. This sequence was originally described by Haase et al. and details of sequence implementation and T1 calculation have been published elsewhere [20, 21]. T2* values were obtained using a fat-saturated multi-gradient echo sequence with 12 echoes (TR = 200 ms, TE = 0.99 ms + n × 1.41 ms, flip angle 20°). During one breath-hold a single slice with 10-mm slice thickness was acquired and the acquisition was repeated for five different slice positions. The matrix was held constant at 128 × 128 pixels with 360 × 360-mm field of view. Total facility time per patient was about 8 min.
MRI assessment and evaluation
Images were analysed off-line using ImageJ (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA) whereby T1 and T2* parameter maps were calculated from acquired images. The fitting algorithm for the T1 maps has been described before [20]. For the calculation of T2* maps a simple mono-exponential fitting routine was applied. Using these parameter maps the T1 and T2* times were measured in three regions of interest (ROIs) larger than 1 cm2 (range 1–3 cm2), whereby identical ROIs were used in T1 and T2* maps. Two ROIs were placed in the right lobe and one in the left lobe (Fig. 1). They were placed with care taken to avoid artefacts, major vascular structures and lesions (e.g. cysts, haemangioma etc.). The average of all measurements (mean T1 or T2*) was calculated for each sequence.
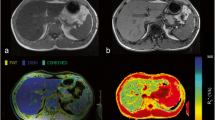
Example of a 31-year-old patient (male) with elevated serum ferritin and transferrin saturation. T1 (b) and T2* times (c) were measured in three ROIs. Two ROIs were placed in the right and one was placed in the left liver lobe with care taken to avoid artefacts, major vascular structures and lesions. The average of all measurements (mean T1 or T2*) was calculated with results of normal T1 and T2* values. Image a shows a T2 HASTE sequence for anatomical correlation
On the basis of previously published studies values in the range 530–610 ms were considered to be normal for T1 in the liver and 18–30 ms for T2* [22–25].
On the basis of a synthesis of clinical history with follow-up information, liver biopsy and genetic results, final clinical diagnosis of hepatic iron overload (HIO), steatohepatitis (SH) or a combination of both (HIO + SH) was made. Mean follow-up time was 22 months (range 12–32 months).
On the basis of these data, patients were divided into four groups: patients with HIO, patients with SH, patients with a combination of both (HIO + SH) or healthy patients without pathological liver iron or steatosis, referred to hereafter as group HIO, group SH, group HIO + SH and group H.
Statistical analysis
The R Project for Statistical Computing (R version 2.13.1) was used. The non-parametric Kruskal–Wallis test was performed for any significant differences between T1 and T2* relaxation times of the different groups (H/HIO/SH/HIO + SH). The Wilcoxon rank sum test including P value corrections for multiple testing (Holm method) was applied to compare the groups pairwise. P values less than 0.05 were considered to indicate a statistically significant result. Receiver operating characteristic (ROC) curves were drawn to assess cut-off levels for the MRI algorithm of T2* mapping to determine the diagnostic performance of MRI for the diagnosis of HIO. Maximum accuracy was taken as a criterion to define the optimal cut-off points. Applying the cut-off levels thus obtained to the present data, we used the Wilson method to estimate the 95 % confidence intervals (95 % CI) for the diagnostic parameters sensitivity, specificity, positive and negative predictive values (PPV, NPV) and accuracy.
Results
Histopathological findings with clinical correlation
Of 25 patients in the study group, 9 had evidence of iron overload only, 5 had steatohepatitis and 10 patients had both. One patient had a normal liver iron concentration and no evidence of fatty liver.
Histopathological analysis showed liver fibrosis grade 3 in 4 patients, 1 patient had grade 2 and 3 patients grade 1 fibrosis. Fibrosis was associated with HIO and/or steatosis in all patients.
The results of HFE gene testing are shown in Table 1; 4 patients had the haemochromatosis-associated genotype C282Y homozygosity and an additional 4 patients were compound heterozygous for C282Y and H63D.
MRI findings
All results are summarised in Table 1. Seventeen patients (68 %) had evidence of hepatic iron deposition and reduced T2* relaxation times (<18 ms). In this group 8 patients (47 %) had concomitant SH, on 4/8 (50 %) we found reduced T1 relaxation times (<530 ms) and the other 4 had normal T1 relaxation times. The remaining 9 patients (53 %) with hepatic iron deposition only revealed reduced T1 relaxation times (example is shown in Fig. 2).
In 3 patients (12 %) we found decreased T2* and slightly increased T1 relaxation times (>620 ms); all of them had liver fibrosis and SH (example is shown in Fig. 3). Two patients (8 %) with SH only had normal T2* and high T1 relaxation times. Two patients (8 %) with hepatic siderosis, fibrosis and SH had normal T2* and slightly increased T1 relaxation times. One patient (4 %) with no pathological result at liver biopsy had normal T1 and T2* relaxation times. Correlations between T1 and T2* are shown in Fig. 4. Figure 5 shows correlation of liver fibrosis with T1 and T2* relaxation times. In Tables 2 and 3 and Figs. 6 and 7 T1 and T2* relaxation times are summarised and correlated with the final diagnosis.
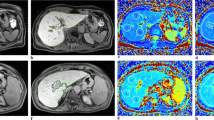
A 51-year-old patient (male) with suspected haemochromatosis based on elevated serum parameters undergoing MRI with T1 (b) and T2* mapping (c). Analysis showed high T1 values (644 ms) and decreased T2* values (13 ms). Histopathological workup did not show any evidence of hepatic iron but steatosis and fibrosis grade 1. Image a shows a T2 HASTE sequence for anatomical correlation
Correlation between T1 and T2* in all patients. Filled circles healthy patient without pathological liver iron or steatosis, filled triangles patients with hepatic iron overload, filled diamonds patients with steatohepatitis, open squares patients with a combination of hepatic iron overload and steatohepatitis
The optimal threshold for T2* relaxation time to determine patients with hepatic iron overload is 10.07 ms as ascertained by ROC curve analysis (Fig. 8 and Table 4). At this threshold the diagnostic accuracy is 88 % (CI 0.70–0.96), sensitivity is 84 % (CI 0.62–0.94) and specificity is 100 % (CI 0.61–1.0). The second cut-off was at 15.47 ms with an accuracy of 88 % (CI 0.70–0.96), sensitivity of 89.5 % (CI 0.69–0.97) and specificity of 83 % (CI 0.44–0.99). On the basis of these cut-off points, MRI correctly classified 20 patients (80 %).
We found a significant association between reduced T2* relaxation times and hepatic siderosis. T1 relaxation times were increased in patients with SH only but decreased in all patients with hepatic iron deposition only (<530 ms). T2* relaxation times did not show a significant difference between patients with HIO and patients with a combination of HIO and SH (P = 0.11). In relation to T1 relaxation times we found a significant difference between patients with HIO and patients with HIO + SH (P = 0.018).
Discussion
Genetic haemochromatosis, thalassaemia and transfusion-dependent anaemia are the main causes of systemic iron overload. The liver is the primary iron storage site in humans. Fibrosis, cirrhosis and hepatocellular carcinoma are frequent iron-induced complications [4].
Magnetic resonance imaging has been investigated as a non-invasive method for determining liver iron concentrations by detecting the paramagnetic effect produced by the presence of stored iron, inducing local irregularities in the magnetic field, which cause water protons around these deposits to lose phase coherence. Two different methods are in use: calculation of relaxation time constants and the calculation of the signal intensity (SI) ratio between liver and skeletal muscle [6, 7, 11, 15]. To our knowledge this is the first study to evaluate an MRI sequence protocol including T2* and T1 maps in patients with suspected HIO.
Our purpose was to evaluate the combined use of T2* and T1 mapping in patients with suspected HIO. By defining cut-off values for the T2* relaxation time for determining hepatic siderosis we were able to classify 20 patients (80 %) correctly. Results of our T2* calculation are in accordance with previously published values [22]. In all patients with HIO only we found reduced T1 and T2* relaxation times. The significant difference in T1 relaxation time between patients with HIO only and patients with concomitant HIO and SH proved that when it comes to a more complex diffuse liver disease T1 relaxation time shows a tendency towards rather normal or increased values. By adding the information on T1 we were not able to determine HIO more precisely, but we received additional information on possible SH or on SH concomitant with iron overload.
Most of the previously described and validated methods for measuring SI ratio and relaxometry in SE and GRE sequences require elaborate post-processing and calibration curves or have long acquisition times [6, 7, 26–28]. This limits their use in clinical practice. GRE sequences are more sensitive than spin echo (SE) sequences for mild degrees of iron overload; the sensitivity to iron also varies significantly with the TR, TE and flip angle applied [6]. Many studies also describe measurements only in the right liver lobe providing no benefit over the sampling limitations of liver biopsy [6, 10]. We decided to use GRE sequences whereby relaxometry data could be acquired during a single breath-hold, thus enabling very short data acquisition times [29]. ROIs were placed in both liver lobes to minimise sampling errors. An additional benefit of the shorter acquisition times of GRE images is the reduction of motion artefacts. In all 25 MRI examinations artefacts were of sufficiently low influence; average facility time per patient was about 8 min whereas post-processing with imageJ took about an additional 5 min.
Gandon et al. suggested a breath-hold T2*-weighted sequence to theoretically be the most optimal sequence. Only a few studies evaluate this sequence for liver iron determination and data on reference ranges for hepatic T2* relaxation times are scarce [6, 11, 22, 25]. According to Pepe et al. T2* relaxation times of 25.6 ± 3.4 ms with a lower limit of 18.8 ms are the physiological T2* range [25]. A study by Storey et al. delineates 25.5 ms as normal T2* relaxometry and Anderson et al. determined 33 ±7 ms as normal [22, 30]. The only comparable study concerning T2* relaxation time in patients with liver disease is by Chandarana et al. For the prediction of iron grades 1 or greater and 3 or greater they found a sensitivity and specificity of 90.5 % and 100 % and 100 % and 97 % at T2* cut-offs of less than 24 ms and less than 14 ms, respectively [11]. In our study, we had a sensitivity of 84 % and a specificity of 100 % with a cut-off at 10.07 ms and 89.5 %/83 % at 15.47 ms, respectively.
Good correlation was also reported in patients with concomitant SH—we could not find any confounding effect of steatosis on T2* results. Lim et al. evaluated the diagnostic performance of chemical shift imaging for hepatic iron quantification and compared this technique with T2* imaging. Contrary to our study, they observed that concomitant steatosis lowers the diagnostic accuracy of both sequences [9]. In our patients with SH only, T1 values were increased whereas most of the patients with hepatic iron deposition only or with concomitant SH had decreased T1. This is a paradoxical result because theoretically fatty liver tissue is expected to have a shorter T1 relaxation time than normal liver tissue. It has already been shown in in vivo MRI studies that fatty livers can show a thoroughly chameleonic presentation of MR tissue parameters [31].
Liver biopsy with determination of LIC and HII is currently considered the reference standard for the diagnosis of liver iron overload, whereas elevated serum iron, transferrin saturation and ferritin are unreliable indicators and can only suggest hepatic iron deposition. However, results can also indicate other forms of hepatocyte injury such as alcoholic or viral hepatitis, or other inflammatory disorders involving the liver [18]. Nevertheless, as an invasive procedure with bleeding risk liver biopsy is unsuitable for repeated analysis or in patients with impaired coagulation such as in liver cirrhosis. Contamination, inadequate specimen sizes, sampling errors, laboratory errors or an increased variability in cirrhotic livers impose further limitations on liver biopsy [8, 32, 33]. In our series 8 patients had fibrosis of the liver ranging from grade 1 to 3. In 3/8 patients histopathological analysis also revealed steatosis and MRI showed decreased T2* and increased T1 time. Five of 8 patients with liver fibrosis had concomitant hepatic siderosis and steatosis, two of whom showed normal T2* and increased T1 relaxation times. Overall, the incidence of liver fibrosis affected MRI results, especially the T2* time, in 5 patients. The study by Anderson et al. illustrated the significance of fibrosis for the precision of hepatic iron estimates [22]. Fibrosis may also represent an unsolved problem when estimating liver iron with MRI. As shown in our study, T2* relaxation time may be altered owing to fibrosis, whereas patients with concomitant steatosis may present increased T1 values. This is a fact that has not been previously specified. In recent times, it has become apparent that subjects may develop significant fibrosis even at low HIC [34]. In these cases, rather than quantifying the exact amount of hepatic iron, it should be of greater importance to simply stratify patients as having HIO or not. Therefore, in clinical practice, the main aim should be to detect even a slight increase in liver iron.
In general, studies calculating the relaxation time have always been performed in patients with secondary iron overload and with severely increased HIC [1, 22, 26]. Compared with T2* values obtained by Anderson et al., those in our study were often very close to previously established thresholds as suggested by borderline HIC and T2* relaxation times in many patients included in our study [22].
HFE genotyping is required to confirm the diagnosis of HH and in C282Y homozygotes with elevated transferrin saturation and hyperferritinaemia the diagnosis can be made without determination of the HIC. Quantification of HIC is required in those 5–15 % of patients in whom phenotypic HH is not associated with HFE gene mutations. In such patients, evaluation with MRI or biopsy of the liver with iron quantification is indicated [35]. In our study 8 patients could be classified as having HFE-associated HH, all of whom had HIO and showed decreased T2* values.
The penetrance of HFE haemochromatosis is 15 % in patients homozygous for C282Y, which further illustrates the need for validated and non-invasive methods for the repeated assessment of HIC. In addition to genetic haemochromatosis, hyperferritinaemia is also prevalent in patients with metabolic syndrome. This disorder is referred to as metabolic hyperferritinaemia or dysmetabolic iron overload [36]. MRI could be a crucial method for further diagnostic and therapeutic workup in patients suspected of having this syndrome. Although MRI has been recommended for the evaluation of such patients by the European Association for the Study of the Liver (EASL), the diagnostic accuracy of MRI in patients with dysmetabolic iron overload is unknown. Here we demonstrate the high accuracy for determining HIO in such a cohort.
One major limitation of our study is its retrospective character. Therefore, the exact quantitative HIC (i.e. Perls’ Prussian Blue, mg/g dry-weight) was not available for all patients, rather we had HII or a simple yes/no result. Correlation between T1 and T2* values with the exact amount of HIC was not possible. Nevertheless definition of HIO or not was feasible in all patients [35]. ROC analysis for T2* yielded two cut-offs because of the small number of patients. It has to be noted that relaxation times strongly vary with field strength; hence, the used method holds for 1.5 T and the given measurement protocol.
We found that a decrease in T2* and T1 relaxation times (T2* < 15.47 ms and T1 < 530 ms) is a good predictor of HIO, especially in patients with hepatic siderosis only whereby 9/9 had reduced T1 and T2* relaxation times. In patients with negative HFE gene test, MRI can give important information for further diagnostic and therapeutic procedures. Liver biopsy still retains its importance in the assessment of hepatic fibrosis or inflammation, particularly because the incidence of liver fibrosis may change the results of T2* and T1 relaxation time measurements and lead to misdiagnosis. However, prospective studies are needed to further confirm our results.
In conclusion, the present study demonstrates that T2* relaxation times determined by MRI have excellent sensitivity and specificity in the diagnosis of the presence of HIO in patients with suspected HIO and that important additional information may be obtained by the combination of T1 and T2* mapping. It is a rapid, non-invasive, accurate and reproducible technique for validating the evidence of even low hepatic iron concentrations.
Abbreviations
- HH:
-
hereditary haemochromatosis
- HII:
-
hepatic iron index
- HIC:
-
hepatic iron concentration
- HIO:
-
hepatic iron overload
- LIC:
-
liver iron concentration
- MRI:
-
magnetic resonance imaging
- SH:
-
steatohepatitis
References
Alustiza JM, Castiella A, De Juan MD, Emparanza JI, Artetxe J, Uranga M (2007) Iron overload in the liver diagnostic and quantification. Eur J Radiol 61:499–506
Siegelman ES, Mitchell DG, Semelka RC (1996) Abdominal iron deposition: metabolism, MR findings, and clinical importance. Radiology 199:13–22
Stevens RG, Jones DY, Micozzi MS, Taylor PR (1988) Body iron stores and the risk of cancer. N Engl J Med 319:1047–1052
Niederau C, Fischer R, Sonnenberg A, Stremmel W, Trampisch HJ, Strohmeyer G (1985) Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med 313:1256–1262
Adams P, Brissot P, Powell LW (2000) EASL international consensus conference on haemochromatosis. J Hepatol 33:485–504
Gandon Y, Olivie D, Guyader D et al (2004) Non-invasive assessment of hepatic iron stores by MRI. Lancet 363:357–362
Bonkovsky HL, Rubin RB, Cable EE, Davidoff A, Rijcken TH, Stark DD (1999) Hepatic iron concentration: noninvasive estimation by means of MR imaging techniques. Radiology 212:227–234
Villeneuve JP, Bilodeau M, Lepage R, Cote J, Lefebvre M (1996) Variability in hepatic iron concentration measurement from needle-biopsy specimens. J Hepatol 25:172–177
Lim RP, Tuvia K, Hajdu CH et al (2010) Quantification of hepatic iron deposition in patients with liver disease: comparison of chemical shift imaging with single-echo T2*-weighted imaging. AJR Am J Roentgenol 194:1288–1295
Gandon Y, Guyader D, Heautot JF et al (1994) Hemochromatosis: diagnosis and quantification of liver iron with gradient-echo MR imaging. Radiology 193:533–538
Chandarana H, Lim RP, Jensen JH et al (2009) Hepatic iron deposition in patients with liver disease: preliminary experience with breath-hold multiecho T2*-weighted sequence. AJR Am J Roentgenol 193:1261–1267
St Pierre TG, Clark PR, Chua-Anusorn W (2005) Measurement and mapping of liver iron concentrations using magnetic resonance imaging. Ann N Y Acad Sci 1054:379–385
St Pierre TG, Clark PR, Chua-anusorn W et al (2005) Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 105:855–861
Wood JC, Enriquez C, Ghugre N et al (2005) MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 106:1460–1465
Reeder SB, Sirlin CB (2010) Quantification of liver fat with magnetic resonance imaging. Magn Reson Imag Clin N Am 18:337–357, ix
Castiella A, Alustiza JM, Emparanza JI, Zapata EM, Costero B, Diez MI (2011) Liver iron concentration quantification by MRI: are recommended protocols accurate enough for clinical practice? Eur Radiol 21:137–141
Guyader D, Gandon Y (2000) Quantification of iron overload. Bull Acad Natl Med 184:337–347, discussion 347–338
Tavill AS (2001) Diagnosis and management of hemochromatosis. Hepatology 33:1321–1328
Chevallier P (2005) What can we expect from non invasive imaging methods to detect and grade liver fatty infiltration? Gastroenterol Clin Biol 29:1133–1135
Kremser C, Trieb TR, Judmaier W, DeVries AF (2006) Assessing tumor perfusion and treatment response in rectal cancer. Radiology 238:756–757, author reply 757–758
Haase A, Matthaei D, Bartkowski R, Duhmke E, Leibfritz D (1989) Inversion recovery snapshot FLASH MR imaging. J Comput Assist Tomogr 13:1036–1040
Anderson LJ, Holden S, Davis B et al (2001) Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 22:2171–2179
Bluml S, Schad LR, Stepanow B, Lorenz WJ (1993) Spin-lattice relaxation time measurement by means of a TurboFLASH technique. Magn Reson Med 30:289–295
rde Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC (2004) MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology 230:652–659
Pepe A, Lombardi M, Positano V et al (2006) Evaluation of the efficacy of oral deferiprone in beta-thalassemia major by multislice multiecho T2*. Eur J Haematol 76:183–192
St Pierre TG, Clark PR, Chua-Anusorn W (2004) Single spin-echo proton transverse relaxometry of iron-loaded liver. NMR Biomed 17:446–458
Kreeftenberg HG Jr, Mooyaart EL, Huizenga JR, Sluiter WJ (2000) Quantification of liver iron concentration with magnetic resonance imaging by combining T1-, T2-weighted spin echo sequences and a gradient echo sequence. Neth J Med 56:133–137
Alustiza JM, Artetxe J, Castiella A et al (2004) MR quantification of hepatic iron concentration. Radiology 230:479–484
Westwood M, Anderson LJ, Firmin DN et al (2003) A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J Magn Reson Imaging 18:33–39
Storey P, Thompson AA, Carqueville CL, Wood JC, de Freitas RA, Rigsby CK (2007) R2* imaging of transfusional iron burden at 3 T and comparison with 1.5 T. J Magn Reson Imaging 25:540–547
Chai JW, Lin YC, Chen JH et al (2001) In vivo magnetic resonance (MR) study of fatty liver: importance of intracellular ultrastructural alteration for MR tissue parameters change. J Magn Reson Imaging 14:35–41
Ludwig J, Hashimoto E, Porayko MK, Moyer TP, Baldus WP (1997) Hemosiderosis in cirrhosis: a study of 447 native livers. Gastroenterology 112:882–888
Emond MJ, Bronner MP, Carlson TH, Lin M, Labbe RF, Kowdley KV (1999) Quantitative study of the variability of hepatic iron concentrations. Clin Chem 45:340–346
Adams PC (2001) Is there a threshold of hepatic iron concentration that leads to cirrhosis in C282Y hemochromatosis? Am J Gastroenterol 96:567–569
Moyer TP, Highsmith WE, Smyrk TC, Gross JB Jr (2011) Hereditary hemochromatosis: laboratory evaluation. Clin Chim Acta 412:1485–1492
Moirand R, Mortaji AM, Loreal O, Paillard F, Brissot P, Deugnier Y (1997) A new syndrome of liver iron overload with normal transferrin saturation. Lancet 349:95–97
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Henninger, B., Kremser, C., Rauch, S. et al. Evaluation of MR imaging with T1 and T2* mapping for the determination of hepatic iron overload. Eur Radiol 22, 2478–2486 (2012). https://doi.org/10.1007/s00330-012-2506-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2506-2