Abstract
Objectives
To review the role of imaging in the diagnosis of recurrent disease in previously treated non-small cell lung cancer (NSCLC) and discuss the imaging pitfalls.
Methods
A comprehensive review of published literature on CT and PET imaging of NSCLC recurrence was performed. Diagnostic and prognostic values are discussed. Representative imaging examples are illustrated.
Results
Up to 30% of NSCLC recurrences present as loco-regional, involving treated hemithorax and ipsilateral lymph nodes, while 70% present as metachronous distant metastases. CT and PET-CT play an important role in the early detection of recurrence; indications for imaging vary depending on pathological features.
Conclusion
Imaging plays a central role in the identification of recurrence and may predict prognosis.
Key Points
-
Lung cancer recurs after surgery in 30% to 75% of patients.
-
CT and PET-CT are crucial in identification of loco-regional recurrence.
-
Knowledge of potential pitfalls is essential, especially for parenchymal or nodal recurrence.
-
CT can diagnose metastases but further examinations (PET-CT, MRI) are often needed.
-
Morphological and functional imaging criteria may help in predicting recurrence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer death in men and the fourth in women, accounting worldwide for 1.6 million cases and 1.4 million deaths in 2008 [1, 2]; it had been estimated that in 2010, 116,750 men and 105,770 women would have been diagnosed with lung cancer, and that 157,300 men and women would have died of it [3]. Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancers and includes several subtypes, of which squamous cell carcinoma and adenocarcinoma account for 42% and 39% of the cases in smokers, respectively [4, 5]. When performed in early stages of NSCLC (stage I and II), surgery is associated with a favourable prognosis [6]. Patients with advanced lung cancer (stage IIIB) are treated with non-surgical multiple technique therapy and patients with distant metastases at diagnosis (stage IV) often receive palliative chemotherapy to improve the quality of life [7]. It has also been reported that selected patients with locally advanced NSCLC (ipsilateral mediastinal nodal involvement) in whom neoadjuvant therapy resulted in down staging may also benefit from surgery, with a significantly improved 5-year-survival rate [7, 8]. For patients with potentially resectable tumours who have significant comorbidities or do not consent to curative surgery, other therapeutic approaches include stereotactic body radiotherapy (SBRT) and radiofrequency ablation (RFA) [9–12].
Even when treated with curative intent, NSCLC is associated with high recurrence ranging from 30% to 75%, depending on the pathological stage and the treatment provided. More than 80% of recurrences [11] occur within the first 2 years of presentation with an additional annual recurrence rate of 3% to 6% per year thereafter [11–15]. There is no consensus as to whether early treatment of NSCLC recurrence results in a better outcome [16].
Imaging plays a central role in the detection of lung cancer recurrence. The aims of this review were to evaluate CT and PET-CT features of NSCLC recurrences and discuss the potential pitfalls of each imaging technique. Imaging findings that might predict recurrence are discussed.
Recurrence
Recurrence of NSCLC may be classified as loco-regional recurrence or distant metastases [17]. Loco-regional recurrence is located within the treated hemithorax and usually presents with nodules involving the resection staple line or the area that was treated with radiotherapy or RFA, as well as the bronchial stump, pleura, chest wall and lymph nodes. In distant metastases, multiple organ metastases are frequently detected, including metachronous pulmonary nodules [11, 17].
In addition to recurrences, new primary lung cancer is also reported in 1 to 2% of NSCLC patients per year following initial radical therapy [10, 18, 19]. Actually, there is no simple method for distinguishing recurrence from new primary or metachronous lung cancer. A recent revision by Rubins et al. [10] of criteria by Martini and Melamed [20] claimed that a new lesion is a metachronous tumour when a different histology is found, or when the same histology is found after a disease-free interval of 4 years or more, or when the new cancer originates from a carcinoma in situ and no extrapulmonary metastases were present at the time of diagnosis. Maeda et al. [11] however stated that selected populations of lung cancer patients are at significant risk of late recurrence after 5 years. In fact, a confident differentiation of a new primary from a metastasis is often impossible [18]. Pulmonary metastases often appear on CT as round and smooth nodules located peripherally, while primary lung cancers usually have ill-defined margins, but cavitating and ill-defined lung metastases have also been described [21]. It has been stated that the primary goal must be to determine whether the new tumour is treatable with curative intent, whether it should be a new primary lung cancer or a recurrence [20].
Loco-regional recurrence
About 30% of NSCLC recurrences are reported to be loco-regional [22]. Adenocarcinoma and squamous cell carcinoma both tend to recur loco-regionally, but there is no concordance among studies about which of these histological subtypes recurs more frequently [17]. Local recurrence after radical surgical resection (R0) [23] is reported in 20% of patients with stage I NSCLC, and in up to 50% patients with stage III NSCLC [24]. Conversely, bronchioloalveolar carcinoma (BAC) does not seem to cause loco-regional recurrences and instead, ipsilateral metachronous pulmonary nodules not involving the staple line are reported to be the most common pattern of recurrence [17, 25, 26]. BAC pulmonary recurrence is also difficult to assess, in particular because it is often not fluoro-deoxiglucose (FDG) avid with false–negative PET findings in 40% of cases [27]. BAC should therefore be followed by serial CT and is probably best regarded as a separate entity to squamous and adenocarcinomas.
Parenchymal and bronchial recurrence
Parenchymal recurrences following surgical approaches can be identified by CT or PET-CT. Differentiation of recurrence on contrast-enhanced CT from post-surgical changes after segmentectomy may be challenging. A soft-tissue attenuation nodule near the surgical clips showing contrast enhancement in early post-surgical evaluations (up to 3 months after surgery) may represent hypervascularisation coexisting with inflammatory reaction. Follow-up with serial CT may be helpful to differentiate this from recurrence; interval growth of an enhancing solid lesion on the resection staple line will be suggestive of the latter [28, 29]. This may also be confirmed by PET-CT when increased metabolic activity is found (Fig. 1). A review of 8 studies of 571 patients with NSCLC treated with curative intent demonstrated pooled PET and PET-CT sensitivity of 82–100%, specificity of 62–100%, positive predictive value (PPV) of 50–100%, and negative predictive value (NPV) of 80–100%. Hellwig et al. [30] investigated the prognostic value of PET-CT in NSCLC recurrence in 62 patients and stated that standardised uptake value (SUV) in NSCLC recurrence was significantly higher than in post-therapeutic changes (10.6 ± 5.1 vs. 2.1 ± 0.6). Moreover, lower FDG uptake in recurrent tissues predicted longer median survival, up to 46 months when SUV was <11, and up to 3 months when SUV was >11 [30].
Recurrence after segmentectomy. A 61-year-old patient who had already undergone left upper lobe (LUL) resection for non-small cell lung carcinoma (NSCLC), underwent lower left lobe (LLL) segmentectomy for a parenchymal recurrence. 5-month-follow-up CT A demonstrated solid tissue on the resection staple line (arrow), near the surgical clip. PET-CT performed after 3 months B demonstrated further growth of solid tissue on the resection staple line (arrowhead) and intense metabolic activity indicating parenchymal recurrence
Bronchial recurrence following pneumonectomy, lobectomy or sleeve lobectomy is a potential pitfall for CT and PET-CT, because the finding of an obstructing soft-tissue mass at the bronchial stump on post-surgical CT may be interpreted as residual scar tissue, while narrowing of the bronchus could be related to extrinsic compression from an adjacent inflammatory node [31, 32]. Again, interval growth of enhancing tissue consecutive CT may be indicative of recurrence, although alternative problems such as inflammation cannot be easily excluded.
Cho et al. [33] retrospectively analysed 84 patients with bronchial obstruction who underwent both contrast-enhanced CT and PET-CT and demonstrated that CT had high sensitivity (95%) but low specificity (48%), and that high metabolic activity at the site of obstruction was a better diagnostic indicator (sensitivity 95%, specificity 91%); these results, obtained for staging, may also be applied to bronchial recurrence (Fig. 2).
Bronchial stump and nodal recurrence. A 71-year-old patient affected by adenocarcinoma who underwent right lower lobe (RLL) lobectomy and chemotherapy presented at 1-year follow-up CT A with solid tissue at the bronchial stump arising in the intermediate bronchus (arrow). Four months later, a contrast-enhanced CT B again demonstrated solid tissue at the stump but also increased peri bronchial soft tissue thickening (C arrowhead). PET-CT D, E demonstrated FDG uptake at the bronchial stump and station 7 node
Apart from recurrence in the bronchial stump, tumours can also recur in the pulmonary artery stump. This is a more difficult area to deal with and only limited data exist on imaging findings. Kim et al. [34] investigated the presence of filling defects in the pulmonary arterial stump in 18/147 patients following pneumonectomy and concluded that in the absence of contrast enhancement or significant growth on serial CT studies, these findings can be considered as in situ thrombi due to stasis of blood, rather than tumour recurrence.
Early-stage (stage I) NSCLC that do not undergo surgery may be treated with minimally invasive techniques including SBRT and RFA. The use of external beam radiotherapy alone for curative intent has been abandoned due to the high local recurrence rate of up to 70% [35]. Different imaging strategies are employed to identify recurrence in these patients.
Stereotactic body radiotherapy is an emerging technique for the treatment of stage I lung cancer, allowing the delivery of a high radiation dose to the target, while sparing the adjacent tissues [36]. SBRT causes radiation-induced lung parenchymal damage leading to fibrosis; early and late radiographic changes have been described (Table 1) [37]. Distinguishing recurrence in these patients can therefore be challenging and requires experienced readers, also considering the fact that radiation-induced fibrosis can appear more than 1 year after the end of therapy [38]. Detection of recurrence may be difficult within 9 months of the delivery of SBRT because of secondary radiation pneumonitis [39]. Matsuo et al. [40] reported that, among other factors, only growth of consolidation after 12 months on CT was a statistically relevant predictor of recurrence. Otherwise, other findings already described as signs of recurrence after conventional radiotherapy can be used to suspect recurrence after SBRT; these include the filling of radiation-induced ectatic bronchi [41], development of bulging at margins of radiation-induced consolidation, and new pleural effusion (Figs. 3 and 4) [39]. PET-CT is also a helpful technique for early detection of recurrence after SBRT, even though it needs to be applied in conjunction with other techniques [42]. As for conventional radiotherapy, to avoid false–positive results from residual inflammatory activity, PET should be used at least 3 months after the completion of SBRT. It has been reported that moderate 18-FDG uptake may persist for up to 2 years following the end of treatment [43]. However, the combination of PET and follow-up CT has been the mainstay of recurrence evaluation in North American SBRT trials [39].
Recurrence after stereotactic body radiotherapy (SBRT). A 75-year-old woman underwent SBRT for an adenocarcinoma of the right middle lobe (A). Chest CT obtained 4 months after treatment completion (B): an ill-defined consolidation was observed (arrow), likely tumour shrinkage and initial fibrosis after SBRT, with surrounding ground-glass areas. Follow-up chest CT performed 10 months later (C) showed a nodule (arrowheads) within radiation-induced changes with high metabolic activity on PET-CT (D). As findings showed suspected NSCLC recurrence, a transthoracic needle biopsy was performed confirming recurrence
Recurrence after SBRT. A 68-year-old man treated with SBRT for an adenocarcinoma of the left upper lobe (A). Follow-up PET-CT 6 months after the end of the treatment (B) showed an area of retraction with no FDG uptake, likely radiation-induced fibrosis. A contrast-enhanced chest CT performed 15 months after treatment completion (C) showed a hypodense nodular area within radiation-induced fibrosis (white arrow), with peripheral contrast enhancement showing suspected NSCLC recurrence. A PET-CT performed thereafter (D) showed high metabolic activity within the nodular area observed on CT, confirming recurrence
Expected changes following radiofrequency ablation of lung cancer. A 76-year-old patient diagnosed with T1bN0 squamous cell carcinoma of the right upper lobe showing high FDG uptake (A) underwent RFA. CT during RFA (B) showed correct positioning of the needle in the tumour. CT performed immediately after the RFA (C) showed presence of ground glass attenuation areas surrounding the treated nodule (arrows); please note a linear hypodensity in the nodule corresponding to the needle path (arrowhead). CT and PET-CT performed 4 months after RFA (D) showed a diffuse hypodensity after administration of contrast medium and no evidence of increased metabolic activity at the post-ablation site
Recurrence of lung cancer following radiofrequency ablation. A 71-year-old patient presented with a lobulated mass in the right upper lobe showing increased metabolic activity at PET-CT which resulted in an adenocarcinoma (A). Patient underwent RFA because of concurrent cardiovascular disease. A PET-CT performed two months after the RFA (B) showed increased axial diameter of the right upper lobe mass with persistence of focal uptake in the medial part of the mass (arrow), suspicious for residual disease. A follow-up PET-CT at 5 months after RFA (C) showed an increase of axial and cranio-caudal diameter of the mass with evidence of increased metabolic activity of the whole mass, indicative for recurrent disease
Radiofrequency ablation is a technique that permits delivery of heat into neoplasms as a means of coagulating tissues using the flow of high kV alternate current through a needle introduced percutaneously under CT guidance [44]. Recurrences after RFA usually occur locally within the first 2 years. RFA alone or in combination with other therapeutic strategies such as adjuvant radiation, may offer improvement in outcomes [45]. As for SRBT, RFA causes focal lung changes such as ground glass opacities surrounding the tumour [46]. This represents a transition from the ablated area to a reactive pneumonia in the nearby normal lung tissue [40]; it also represents a “safety boundary”: a ground-glass margin of 5 mm or more, is reported to indicate full ablation [47] (Fig. 5).
Most authors perform contrast-enhanced CT with densitometry after 1, 2, 3 and 4 min following administration of contrast media to capture the characteristics of the baseline tumour [39, 48]. There is no consensus on a standard protocol for post-RFA follow up. CT is commonly used as the first-line investigation because of its wide availability [39]; however, many authors combine CT and PET-CT in the post-RFA follow up.
Eradat et al. [39] proposed CT follow-up 1–2 months after RFA with densitometry after 1, 2, 3 and 4 min following administration of contrast media, it was suggested that contrast enhancement greater than 15 HU compared with un-enhanced imaging should be considered as incomplete ablation of the tumour. This is then followed by a PET-CT at 3 months and thereafter alternated with contrast-enhanced CT with nodule densitometry every 3 months for 2 years.
Beland et al. [45] used contrast-enhanced CT at 3 weeks and 3 months followed by PET/CT at 6 months; alternating CT and PET/CT examinations were then performed every 3 months.
CT and PET-CT imaging findings of residual versus recurrent NSCLC after RFA have been described [39]; authors divided them into early (up to 1 week post-RFA), intermediate (1 week to 2 months), and late phase (months or years). Early phase findings suspecting persistence of disease included absence of tumour size increase after RFA, or absence of expected ground-glass margin, as well as the presence of peripheral nodular enhancement on positive dynamic contrast CT. Intermediate phase findings included growth to more than the immediate post-ablation size, and post-contrast enhancement similar to that described in early phase findings. Late phase findings include new mass effects, spiculation, higher enhancement values compared to pre-RFA, and positive contrast-enhanced CT densitometry and/or PET-CT tracer uptake (Fig. 6).
Singnurkar et al. [49] also described patterns of FDG uptake post-RFA. Favourable uptake patterns included:
-
diffuse uptake (homogeneous FDG uptake, with smooth tapering borders around the ablation site and the surrounding lung parenchyma)
-
heterogeneous uptake, defined as an area of increased FDG uptake interspersed by areas of lesser uptake circumferentially
-
Rim uptake (ring-like uptake in the periphery of the ablation site)
-
Rim uptake with additional focal uptake at a site different from the ablated tumour nodule, indicating heterogeneous inflammation
Unfavourable uptake patterns included:
-
Focal uptake, defined as an area of FDG uptake surrounded by a photopenic area, involving the entire post-ablation site or a portion of it
-
Rim uptake with additional focal uptake at the site corresponding to the original tumour nodule
The authors also considered that a higher rim ratio (described as the ratio of the outer edge of FDG uptake surrounding an ablated lesion to the diameter of original tumour) had a trend in predicting tumour-free survival.
Nodal recurrence
Imaging surveillance after curative intent therapy is recommended to exclude nodal recurrence of NSCLC. Jang et al. [25] followed 379 surgically resected NSCLC patients of whom 75 had thoracic recurrences of which 39 (52%) were hilar-mediastinal nodal metastases. Unlike loco-regional recurrences or new primary lung tumours, nodal recurrences, in particular mediastinal, are not surgically treated and non-curative medical therapies aiming to prolong life or to sustain a level of quality of life are offered [50, 51].
CT criteria for primary nodal staging in NSCLC patients are mostly based on measurement of the nodal diameter on the short axis, using a 1-cm cross-sectional diameter cut-off value [52]. A review by Toloza et al. [53] analysed 20 studies with 3438 evaluable patients and showed a sensitivity of 57% and a specificity of 82% for CT in the detection of nodal metastases for staging purposes, with marked heterogeneity amongst studies. Similar results are confirmed by more recent studies demonstrating even lower specificity values (70% and 74%) [54, 55]. Poor sensitivity of CT occurs because micrometastases can be present in nodes smaller than 1 cm [56]. Enlarged non-pathological nodes (i.e. inflammatory nodes) can explain false–positive findings (Fig. 7). Similarly, the accuracy of CT is low for restaging after neoadjuvant treatment, with sensitivity of 14–41% and specificity of 62–79% [57, 58]. Pitfalls for CT in restaging are represented by false–positives due to nodal enlargements revealing themselves as sclerosis and fibrosis at histopathology [59]. Comparison with previous CT examinations can be useful: evidence of growth in a previously normal node is regarded as suspicious for relapse and will need further investigation.
Nodal recurrence: false–positive CT. A 68-year-old patient with pT2N0 squamous carcinoma of the right upper lobe (A) with invasion of the horizontal fissure (arrow) underwent right upper lobe (RUL) and middle lobe bilobectomy. PET-CT showed an enlarged 4R node (short axis longer than 1 cm) but no FDG uptake. The patient refused adjuvant treatment following surgery. After 1 year, because of elevation of the tumour marker CEA, a PET-CT was performed (B). Increased FDG uptake of the enlarged station 7 node was demonstrated (arrow) but no activity in the enlarged 4R node. EUS-NA demonstrated metastasis to the station 7 node but not the 4R node
PET-CT evidence of high metabolism in a previously silent node is useful to confirm recurrence (Fig. 8). Vansteenkiste et al. [51] reviewed 10 studies (403 patients) in which PET-CT was performed in the restaging of NSCLC patients following induction therapy. The reported sensitivity and specificity ranged from 20 to 77%, and 61 to 99%, respectively. These results demonstrated high specificity of PET-CT in restaging, but low sensitivity compared with primary staging PET-CT [7, 51]. PET-CT false–negatives can be due to presence of a central tumour, or of centrally located N1 nodes, both of which may obscure nearby existing mediastinal nodal metastases. PET-CT false–positives can be explained through the increase of glycolysis, and consequently of FDG uptake, due to macrophage infiltration when inflammation is present (Fig. 9) [59]; for the same reason PET-CT should be performed at 3–6 months after irradiation, as radiation-induced inflammatory reactions can persist during this period [60]. When recurrence is suspected by CT or PET-CT, pathological confirmation may be achieved by mediastinoscopy or endo-oesophageal / endo-bronchial needle aspiration techniques (EUS-NA and EBUS-NA) sampling.
Nodal recurrence. A 74-year-old patient underwent SBRT for T1bN0 adenocarcinoma of the superior segment of the lingula (A); staging CT demonstrated ill-defined margins and PET-CT showed high metabolic activity. No other parenchymal findings or nodal enlargement were demonstrated. PET-CT performed 3 months after completion of treatment (B) showed shrinkage of the primary tumour with lower FDG uptake (arrows) and two new consolidations of lingula, both with FDG uptake (*,°). Enlarged nodes of stations 4R and 4 L showing high metabolic activity were also present (arrowheads). A 1-month follow-up CT (C) showed resolution of lingular consolidations (*,°), with residual septal thickening and scars, owing to resolved inflammatory events. No significant modification in shape or dimensions of the primary tumour was observed (arrow). Further enlargement of mediastinal nodes was also observed (arrowheads). Endo-oesophageal ultrasound-guided needle aspiration (EUS-NA) confirmed nodal metastases from adenocarcinoma
Nodal recurrence pitfall: false–positive PET-CT. A 78-year-old patient underwent LUL lobectomy for a T1a adenocarcinoma. A CT performed after 2 months (A) demonstrated a 2R node with a short-axis diameter of less than 1 cm (arrow). Follow-up PET-CT (B) demonstrated high nodal FDG uptake (arrow). The endo-bronchial ultrasound-guided needle aspiration (EBUS-NA) performed was negative for malignant cells (reactive 2R lymph node at cytology). 12-month CT (C) demonstrated no nodal size change (arrow)
Pleural recurrence
Pleural invasion from NSCLC is known to worsen survival; T1 tumours are upgraded to T2 when the elastic layer of the visceral pleura is invaded [61], while pleural tumour seeding is regarded as M1a [62].
It is not always easy to demonstrate pleural recurrence on CT or PET-CT. Pleural recurrences are usually associated with malignant pleural effusion, but pleural metastases without pleural effusions have also been reported [61, 63, 64]. Pleural effusion in NSCLC patients not disappearing in serial examinations is regarded as suspicious for recurrence and must be further investigated by aspiration and cytology.
Pleural thickening, intrapleural nodules, enhancement of the pleura or soft tissues within the pleural space after contrast media administration and high SUV indicate suspected pleural recurrence (Fig. 10). Identification of pleural thickenings on CT or PET-CT can be secondary to scarring following a previous surgical procedure; uptake at PET-CT can be related to post-surgical inflammation especially when PET-CT is acquired within 3 months of surgery. Follow up studies looking for interval growth will then be beneficial.
Pleural and nodal recurrence after SBRT. A 72-year-old patient underwent SBRT for a pT2N0 adenocarcinoma with visceral pleural invasion (arrow), as shown by pre-operative imaging (A). 1-year follow up PET-CT (B) demonstrated no FDG-uptake of the radiation-induced fibrosis area, where the maximum dose was delivered, but showed malignant pathological activity of the 4R node and pleural thickening, which was also demonstrated by CT (arrowheads)
Distant metastases
Distant metastasis is the most common form of NSCLC recurrence. Depending on the stage of disease at primary diagnosis and treatment administered, metastatic recurrence comprises 39 to 65.5% of all recurrences [10, 11, 14, 45, 65].
Yano et al. [66] analysed the first site of recurrence in patients who underwent major surgery (lobectomy or pneumonectomy with total dissection of the ipsilateral hilar and mediastinal nodes) for NSCLC and who were staged as pN0 and pN2. The difference in the pattern of distant metastases between pN0 disease and pN2 disease was significant, with pulmonary metastases more prevalent in pN2 patients. The authors explained this by the presence of drainage routes from the N2 nodes into the superior vena cava leading to pulmonary metastasis.
The pattern of recurrent metastatic involvement is commonly similar to distant metastasis at first presentation (i.e. lung, liver, bone, brain and adrenals) [11, 17]. Extrathoracic metastases are reported to be better demonstrated by PET-CT than by either CT or PET alone [67, 68]. However, a review of the role of PET in lung cancer imaging [69] stated that indications for PET-CT in NSCLC recurrences needed further prospective investigations. Two studies published in 2010 enrolled 92 and 121 patients who underwent NSCLC resection [70, 71] and stated that PET-CT accuracy in assessing intra- and extra-thoracic recurrences was equivalent to or better than standard radiological examinations. Kanzaki et al. [4] proposed that conventional imaging for the detection of extrathoracic metastases in patients who underwent potentially curative surgery for NSCLC (with the exception of brain MRI) can be omitted if PET-CT is negative, because of its high negative predictive value.
In spite of the excellent results of PET-CT for the staging of NSCLC, the 2nd edition of the American College of Chest Physicians (ACCP) evidence-based guidelines [72] on the follow-up and surveillance of lung cancer patients did not recommend PET in standard surveillance. The reason given was the lack of evidence that follow-up PET improved either survival or quality of life following curative intent therapy for NSCLC.
Lung metastases often present as nodules. As discussed earlier, it is difficult to determine whether a pulmonary lesion presenting in an already treated NSCLC patient represents a recurrence or a second primary tumour [10, 20, 69] and any new nodule should be properly investigated. Previous studies demonstrated that CT findings of multiple pulmonary nodules with the feeding vessel sign and well-defined and smooth margins in the lower lobes, especially when they were not present on previous images must be suspected of being metastases [69, 70, 73]. Solitary pulmonary nodules in already treated lung cancer patients may be either recurrent disease or a second primary. When possible, surgical resection is reported to be the best approach [18]. Other authors would only consider re-intervention when the primary tumour was determined to be stage I disease [74].
When diagnosis is uncertain (i.e. the radiologist cannot obtain previous studies), a dynamic CT nodule enhancement study, or volumetric evaluation of nodule growth on two consecutive CT can help in excluding other aetiologies (i.e. infection, if calculated 3D-volume doubling time is shorter than 30 days, or benign nodule, if it is longer than 500 days) (Fig. 11) [73, 75, 76].
New RUL nodule in a 50-year-old patient who previously underwent a segmental nodule resection of RLL for a T1aN0 adenocarcinoma. Volumetric evaluation of nodule growth comparing two consecutive CT performed 2 months apart (A) demonstrated a volume doubling time of 26 days (arrowhead) with suspected inflammation. A CT dynamic nodule study performed 2 months later (B) demonstrated significant enhancement (>15 HU). The patient then underwent a segmental resection, which demonstrated a parenchymal recurrence
PET-CT can also be a useful tool in detecting pulmonary metastases presenting as nodules. However, non-solid nodules or nodules smaller than 1 cm may appear FDG-negative (Fig. 12) [70]. Moreover, certain pathological conditions such as BAC may be FDG-negative and need follow-up by CT [27, 77]. For brain metastases, contrast-enhanced CT is commonly used, although it is widely recognised that MRI is the reference imaging technique [78]. PET/CT is limited in the detection of brain metastases because of the high FDG avidity of normal brain parenchyma obscuring cerebral metastases [79]. Lee et al. [80] stated that a combined brain MRI and total body PET/CT significantly increased detection of extrathoracic metastases in patients with lung adenocarcinoma.
Potential CT pitfall. A 57-year-old patient who underwent neoadjuvant therapy followed by surgery for a cT3N1M0 adenocarcinoma of RUL (pT2N0) underwent a 2-year follow-up CT (A) that showed two non-solid nodules of the left upper lobe (arrow, arrowhead). PET-CT showed high FDG uptake in the larger nodule (arrow) and no uptake in the smaller nodule (arrowhead). The patient then underwent a left upper lobectomy. Histopathology demonstrated a G2 adenocarcinoma in the larger nodule (recurrence) but no evidence of a smaller nodule (considered a resolved inflammatory event)
Adrenal metastases are common and the adrenal glands should always be investigated as part of staging in an NSCLC patient [81]. CT criteria for excluding a benign mass are summarised in Table 2 [82]; otherwise, further evaluation by MRI, PET-CT or biopsy is recommended. Recent studies described two PET-CT criteria to determine adrenal metastases in NSCLC patients. One study [83] demonstrated that an SUV max value of greater than 3.1 had a sensitivity of 100% for malignancy. Another study focused on the SUV ratio between adrenals and liver; when adrenal uptake was greater than that of liver by a ratio of 1.0, it was 100% sensitive and 98% specific for malignancy [84]. A recent study compared those criteria and stated that SUVmax threshold was more specific and fewer false-positives were observed. CT criteria (mean attenuation >10 HU) in conjunction with SUVmax threshold increase specificity (86.2% vs. 75.9%) without affecting sensitivity. The authors then proposed a systematic approach in which CT criteria should be followed by SUV max value greater than 3.1 and then by application of an SUV ratio between the adrenals and the liver of 2.5, which helps in excluding false-positive FDG-avid adenomas [85].
Follow up studies may demonstrate liver and bone metastasis. When CT is inconclusive, further investigation by PET-CT, MRI, bone scintigraphy and/or guided biopsy may be necessary [81].
Criteria for prediction of recurrence
Tumour size is an independent prognostic factor for recurrence when the tumour is greater than 5 cm (T2b or T3 - N0M0, stage IIA or IIB) [86]. In stage I patients (T1aN0 and T1bN0) tumour size is not a significant risk factor for recurrence, as demonstrated by Maeda et al. [61], who enrolled 713 consecutive stage I patients who underwent surgery. The authors otherwise identified three independent risk factors in a multivariate analysis: histological differentiation (hazard ratio: 2.3), presence of intra-tumoural vessel invasion (hazard ratio: 2.9), and presence of pleural invasion (hazard ratio: 1.8). The latter is already included in the Union for International Cancer Control (UICC)’s seventh edition of the TNM classification (Fig. 13). The authors stated that patients who present with two of these factors can be identified as a high-risk subgroup of stage IA NSCLC patients who may benefit from adjuvant chemotherapy.
Pleural recurrence. A 61-year-old patient who underwent RUL lobectomy for adenocarcinoma (pT2N0). Preoperative CT (A) and PET-CT (B) demonstrated a 2-cm nodule with visceral pleural invasion (black arrow) and high FDG uptake (white arrow). 1-year follow-up CT (C) demonstrated pleural thickening with enhancement after contrast medium administration (black arrowhead). PET-CT (D) demonstrated high metabolic activity (white arrowhead) indicating pleural recurrence
Although tumour size is generally seen as a main predictor for outcome, location plays a significant role as well. Al-Kattan et al. [14] reported that stage I patients who required a pneumonectomy instead of a lobectomy for a hilar tumour or a tumour crossing a major fissure have a lower survival rate (P = 0.03; risk ratio 1.62) and a major incidence of recurrence (P = 0.02). However, the presence of anatomical variation such as incomplete fissures does not appear to play a role in prognostic terms. Kamiyoshihara et al. [87] retrospectively compared 239 patients who underwent lobar resection for stage I or II NSCLC (excluding T3N0); 74 of them had a surgical finding of an incomplete interlobar fissure. Three to sixteen years follow up demonstrated that finding of an incomplete interlobar fissure has no influence on recurrence and survival.
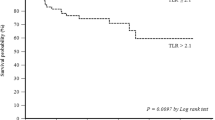
Two studies evaluated the prognostic value of PET. The first stated that 18F-FDG uptake in patients with pathological stage I primary NSCLC was independently associated with worse survival [88]. The other study, performed in a relatively small group of selected patients suggested that PET might also have independent prognostic value for recurrence, being superior to pathological staging in predicting recurrence (Fig. 14) [89].
PET-CT as clinical predictor of recurrence in stage IA NSCLC. Two patients, aged respectively 50 and 58 years presented with T1a adenocarcinoma of RUL. The first patient’s tumour presented an SUV max of 6.6 (A); the second patient’s tumour had an SUV max of 2.0 (B). No adjuvant therapy was administered to either of the patients. One-year follow-up demonstrated parenchymal recurrence in only the first patient (C) and not the second (D)
Locally advanced NSCLC include several factors that influence risk of recurrence. Moretti et al. [90] evaluated the role of post-operative radiotherapy in 84 patients staged as pN2 and described an improved survival rate in patients without nodal extracapsularity at histopathology but not in patients with extracapsularity. They also noted that negative surgical margins were predictive of better overall survival rate but did not influence local or distant recurrence-free survival; they hypothesised that bulky nodal involvement at histopathology was a major indicator of recurrence.
Maeda et al. [11] analysed late recurrences (after 5 years of curative intent-surgery) and found that nodal involvement and intravascular invasion at histopathology significantly influenced recurrence, and suggest that for selected populations of NSCLC patients 5 years’ follow up was not sufficient.
In conclusion imaging plays an important role in investigation of NSCLC recurrence, as it can accurately identify thoracic and extrathoracic relapse and also indicate predictive criteria. However radiologists should be aware of potential imaging pitfalls.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Ferlay J, Shin HR, Bray F (2008) GLOBOCAN Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Lyon, France International Agency for Research on Cancer. Available at: http://globocan.iarc.fr. Accessed 16/02/2011
Altekruse S, Kosary CL, Krapcho M et al (2010) SEER Cancer Statistics Review, 1975–2007, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010. Accessed 3/4/2011
Kanzaki R, Higashiyama M, Maeda J et al (2010) Clinical value of F18-fluorodeoxyglucose positron emission tomography-computed tomography in patients with non-small cell lung cancer after potentially curative surgery: experience with 241 patients. Interact Cardiovasc Thorac Surg 10:1009–1014
Bryant A, Cerfolio RJ (2007) Differences in epidemiology, histology, and survival between cigarette smokers and never-smokers who develop non-small cell lung cancer. Chest 132:185–192
Mountain CF (1997) Revisions in the international system for staging lung cancer. Chest 111:1710–1717
Van Schil P (2003) The restaging issue. Lung cancer (Amsterdam, Netherlands) 42:S39–S45
Bueno R, Richards WG, Swanson SJ et al (2000) Nodal stage after induction therapy for stage IIIA lung cancer determines patient survival. Ann Thorac Surg 70:1826–1831
Virgo KS, Johnson FE, Naunheim KS (1999) Follow-up of patients with thoracic malignancies. Surg Oncol Clin N Am 8:355–369
Rubins J, Unger M, Colice GL (2007) Follow-up and surveillance of the lung cancer patient following curative intent therapy: ACCP evidence-based clinical practice guideline (2nd edition). Chest 132:355S–367S
Maeda R, Yoshida J, Hishida T et al (2010) Late recurrence of non-small cell lung cancer more than 5 years after complete resection: incidence and clinical implications in patient follow-up. Chest 138:145–150
Ichinose Y, Kato H, Koike T et al (2001) Overall survival and local recurrence of 406 completely resected stage IIIa-N2 non-small cell lung cancer patients: questionnaire survey of the Japan Clinical Oncology Group to plan for clinical trials. Lung Cancer 34:29–36
Moldvay J, Scheid P, Wild P et al (2000) Predictive survival markers in patients with surgically resected non-small cell lung carcinoma. Clin Cancer Res 6:1125–1134
al-Kattan K, Sepsas E, Fountain SW, Townsend ER (1997) Disease recurrence after resection for stage I lung cancer. Eur J Cardiothorac Surg 12:380–384
Martini N, Bains MS, Burt ME et al (1995) Incidence of local recurrence and second primary tumours in resected stage I lung cancer. J Thorac Cardiovasc Surg 109:120–129
Crino L, Weder W, van Meerbeeck J, Felip E (2010) Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21:v103–v115
Bogot NR, Quint LE (2004) Imaging of recurrent lung cancer. Cancer Imaging 4:61–67
Ponn RB (2000) Lightning can strike twice: second primary lung cancers. Chest 118:1526–1529
Johnson BE (1998) Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst 90:1335–1345
Martini N, Melamed MR (1975) Multiple primary lung cancers. J Thorac Cardiovasc Surg 70:606–612
Seo JB, Im JG, Goo JM, Chung MJ, Kim MY (2001) Atypical pulmonary metastases: spectrum of radiologic findings. Radiographics 21:403–417
Yano T, Hara N, Ichinose Y et al (1994) Local recurrence after complete resection for non-small-cell carcinoma of the lung. Significance of local control by radiation treatment. J Thorac Cardiovasc Surg 107:8–12
Hermanek P, Wittekind C (1994) The pathologist and the residual (R) tumour classification. Pathol Res Pract 190:115–123
de Geus-Oei LF, van der Heijden HF, Corstens FH, Oyen WJ (2007) Predictive and prognostic value of FDG-PET in nonsmall-cell lung cancer: a systematic review. Cancer 110:1654–1664
Jang KM, Lee KS, Shim YM et al (2003) The rates and CT patterns of locoregional recurrence after resection surgery of lung cancer: correlation with histopathology and tumour staging. J Thorac Imaging 18:225–230
Gaeta M, Blandino A, Pergolizzi S et al (2003) Patterns of recurrence of bronchioloalveolar cell carcinoma after surgical resection: a radiological, histological, and immunohistochemical study. Lung Cancer 42:319–326
Heyneman LE, Patz EF (2002) PET imaging in patients with bronchioloalveolar cell carcinoma. Lung Cancer 38:261–266
Glazer HS, Aronberg DJ, Sagel SS, Emami B (1984) Utility of CT in detecting postpneumonectomy carcinoma recurrence. AJR Am J Roentgenol 142:487–494
Gorich J, Beyer-Enke SA, Flentje M, Zuna I, Vogt-Moykopf I, Van Kaick G (1990) Evaluation of recurrent bronchogenic carcinoma by computed tomography. Clin Imaging 14:131–137
Hellwig D, Groschel A, Graeter TP et al (2006) Diagnostic performance and prognostic impact of FDG-PET in suspected recurrence of surgically treated non-small cell lung cancer. Eur J Nucl Med Mol Imaging 33:13–21
Chae EJ, Seo JB, Kim SY et al (2006) Radiographic and CT findings of thoracic complications after pneumonectomy. Radiographics 26:1449–1468
Hollaus PH, Wurnig PN, Pridun NS (2003) The natural history of recurrence after bronchoplastic procedures for non-small cell lung cancer. Ann Thorac Surg 76:363–369
Cho A, Hur J, Kang WJ et al (2010) Usefulness of FDG PET/CT in determining benign from malignant endobronchial obstruction. Eur Radiol 21:1077–1087
Kim SY, Seo JB, Chae EJ et al (2005) Filling defect in a pulmonary arterial stump on CT after pneumonectomy: radiologic and clinical significance. AJR Am J Roentgenol 185:985–988
Qiao X, Tullgren O, Lax I, Sirzen F, Lewensohn R (2003) The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer 41:1–11
Videtic GM, Stephans KL (2010) The role of stereotactic body radiotherapy in the management of non-small cell lung cancer: an emerging standard for the medically inoperable patient? Curr Oncol Rep 12:235–241
Trovo M, Linda A, El Naqa I, Javidan-Nejad C, Bradley J (2010) Early and late lung radiographic injury following stereotactic body radiation therapy (SBRT). Lung Cancer 69:77–85
Takeda A, Kunieda E, Takeda T et al (2008) Possible misinterpretation of demarcated solid patterns of radiation fibrosis on CT scans as tumour recurrence in patients receiving hypofractionated stereotactic radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys 70:1057–1065
Eradat J, Abtin F, Gutierrez A, Suh R (2011) Evaluation of treatment response after nonoperative therapy for early-stage non-small cell lung carcinoma. Cancer J 17:38–48
Matsuo Y, Nagata Y, Mizowaki T et al (2007) Evaluation of mass-like consolidation after stereotactic body radiation therapy for lung tumours. Int J Clin Oncol / Jp Soc Clin Oncol 12:356–362
Libshitz HI, Sheppard DG (1999) Filling in of radiation therapy-induced bronchiectatic change: a reliable sign of locally recurrent lung cancer. Radiology 210:25–27
Hadziahmetovic M, Loo BW, Timmerman RD et al (2010) Stereotactic body radiation therapy (stereotactic ablative radiotherapy) for stage I non-small cell lung cancer–updates of radiobiology, techniques, and clinical outcomes. Discov Med 9:411–417
Hoopes DJ, Tann M, Fletcher JW et al (2007) FDG-PET and stereotactic body radiotherapy (SBRT) for stage I non-small-cell lung cancer. Lung Cancer 56:229–234
Dupuy DE, Mayo-Smith WW, Abbott GF, DiPetrillo T (2002) Clinical applications of radio-frequency tumour ablation in the thorax. Radiographics, 22 Spec No:S259-269
Beland MD, Wasser EJ, Mayo-Smith WW, Dupuy DE (2010) Primary non-small cell lung cancer: review of frequency, location, and time of recurrence after radiofrequency ablation. Radiology 254:301–307
Bojarski JD, Dupuy DE, Mayo-Smith WW (2005) CT imaging findings of pulmonary neoplasms after treatment with radiofrequency ablation: results in 32 tumours. AJR Am J Roentgenol 185:466–471
Anderson EM, Lees WR, Gillams AR (2009) Early indicators of treatment success after percutaneous radiofrequency of pulmonary tumours. Cardiovasc Intervent Radiol 32:478–483
Suh RD, Wallace AB, Sheehan RE, Heinze SB, Goldin JG (2003) Unresectable pulmonary malignancies: CT-guided percutaneous radiofrequency ablation—preliminary results. Radiology 229:821–829
Singnurkar A, Solomon SB, Go M, Larson SM, Scho H (2010) 18F-FDG PET/CT for the prediction and detection of local recurrence after radiofrequency ablation of malignant lung lesions. J Nucl Med 51:1833–1840
Downey RJ (1999) Follow-up of patients with completely resected lung cancer. Chest 115:1487–1488
Vansteenkiste J, Dooms C, De Leyn P (2009) The multidisciplinarity of stage III non-small cell lung cancer. Eur J Cancer 45:92–105
De Leyn P, Lardinois D, Van Schil PE et al (2007) ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg 32:1–8
Toloza EM, Harpole L, McCrory DC (2003) Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest 123:137S–146S
Fritscher-Ravens A, Bohuslavizki KH, Brandt L et al (2003) Mediastinal lymph node involvement in potentially resectable lung cancer: comparison of CT, positron emission tomography, and endoscopic ultrasonography with and without fine-needle aspiration. Chest 123:442–451
Yang W, Fu Z, Yu J et al (2008) Value of PET/CT versus enhanced CT for locoregional lymph nodes in non-small cell lung cancer. Lung Cancer 61:35–43
De Leyn P, Vansteenkiste J, Cuypers P et al (1997) Role of cervical mediastinoscopy in staging of non-small cell lung cancer without enlarged mediastinal lymph nodes on CT scan. Eur J Cardiothorac Surg 12:706–712
Mateu-Navarro M, Rami-Porta R, Bastus-Piulats R, Cirera-Nogueras L, Gonzalez-Pont G (2000) Remediastinoscopy after induction chemotherapy in non-small cell lung cancer. Ann Thorac Surg 70:391–395
Lee KS, Shim YM, Han J et al (2000) Primary tumours and mediastinal lymph nodes after neoadjuvant concurrent chemoradiotherapy of lung cancer: serial CT findings with pathologic correlation. J Comput Assist Tomogr 24:35–40
De Leyn P, Stroobants S, De Wever W et al (2006) Prospective comparative study of integrated positron emission tomography-computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotherapy for mediastinoscopy-proven stage IIIA-N2 Non-small-cell lung cancer: a Leuven Lung Cancer Group Study. J Clin Oncol 24:3333–3339
Poettgen C, Theegarten D, Eberhardt W et al (2007) Correlation of PET/CT findings and histopathology after neoadjuvant therapy in non-small cell lung cancer. Oncology 73:316–323
Maeda R, Yoshida J, Ishii G et al (2010) Long-term survival and risk factors for recurrence instage I non-small cell lung cancer patients with tumours up to 3 cm in maximum dimension. Chest 138:357–362
Goldstraw P, Crowley J, Chansky K et al (2007) The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2:706–714
Yoshida J, Nagai K, Asamura H et al (2009) Visceral pleura invasion impact on non-small cell lung cancer patient survival: its implications for the forthcoming TNM staging based on a large-scale nation-wide database. J Thorac Oncol 4:959–963
Hwang JH, Song KS, Park SI, Lim TH, Kwon KH, Goo DE (2005) Subtle pleural metastasis without large effusion in lung cancer patients: preoperative detection on CT. Korean J Radiol 6:94–101
Nakagawa T, Okumura N, Ohata K, Igai H, Matsuoka T, Kameyama K (2008) Postrecurrence survival in patients with stage I non-small cell lung cancer. Eur J Cardiothorac Surg 34:499–504
Yano T, Yokoyama H, Inoue T et al (1994) The first site of recurrence after complete resection in non-small-cell carcinoma of the lung. Comparison between pN0 disease and pN2 disease. J Thorac Cardiovasc Surg 108:680–683
De Wever W, Ceyssens S, Mortelmans L et al (2007) Additional value of PET-CT in the staging of lung cancer: comparison with CT alone, PET alone and visual correlation of PET and CT. Eur Radiol 17:23–32
De Wever W, Vankan Y, Stroobants S, Verschakelen J (2007) Detection of extrapulmonary lesions with integrated PET/CT in the staging of lung cancer. Eur Respir J 29:995–1002
Vansteenkiste JF (2002) Imaging in lung cancer: positron emission tomography scan. Eur Respir J 35:49s–60s
Takenaka D, Ohno Y, Koyama H et al (2010) Integrated FDG-PET/CT vs. standard radiological examinations: comparison of capability for assessment of postoperative recurrence in non-small cell lung cancer patients. Eur J Radiol 74:458–464
Onishi Y, Ohno Y, Koyama H et al (2010) Non-small cell carcinoma: Comparison of postoperative intra- and extrathoracic recurrence assessment capability of qualitatively and/or quantitatively assessed FDG-PET/CT and standard radiological examinations. Eur J Radiol doi:10.1016/j.ejrad.2010.04.027
Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA (2007) Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 132:202S–220S
Revel MP, Merlin A, Peyrard S et al (2006) Software volumetric evaluation of doubling times for differentiating benign versus malignant pulmonary nodules. AJR Am J Roentgenol 187:135–142
Hishida T, Nagai K, Yoshida J et al (2006) Is surgical resection indicated for a solitary non-small cell lung cancer recurrence? J Thorac Cardiovasc Surg 131:838–842
Swensen SJ, Viggiano RW, Midthun DE et al (2000) Lung nodule enhancement at CT: multicenter study. Radiology 214:73–80
Girvin F, Ko JP (2008) Pulmonary nodules: detection, assessment, and CAD. AJR Am J Roentgenol 191:1057–1069
Poeppel TD, Krause BJ, Heusner TA, Boy C, Bockisch A, Antoch G (2009) PET/CT for the staging and follow-up of patients with malignancies. Eur J Radiol 70:382–392
Yokoi K, Kamiya N, Matsuguma H et al (1999) Detection of brain metastasis in potentially operable non-small cell lung cancer: a comparison of CT and MRI. Chest 115:714–719
Marom EM, McAdams HP, Erasmus JJ et al (1999) Staging non-small cell lung cancer with whole-body PET. Radiology 212:803–809
Lee HY, Lee KS, Kim BT et al (2009) Diagnostic efficacy of PET/CT plus brain MR imaging for detection of extrathoracic metastases in patients with lung adenocarcinoma. J Korean Med Sci 24:1132–1138
Munden RF, Swisher SS, Stevens CW, Stewart DJ (2005) Imaging of the patient with non-small cell lung cancer. Radiology 237:803–818
Mayo-Smith WW, Boland GW, Noto RB, Lee MJ (2001) State-of-the-art adrenal imaging. Radiographics 21:995–1012
Blake MA, Slattery JM, Kalra MK et al (2006) Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy–initial experience. Radiology 238:970–977
Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E (2006) 18-F-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med 47:32–37
Brady MJ, Thomas J, Wong TZ, Franklin KM, Ho LM, Paulson EK (2009) Adrenal nodules at FDG PET / CT in patients known to have or suspected of having lung cancer. A proposal for an efficient diagnostic algorithm. Radiology 250:523–530
Takeda S, Fukai S, Komatsu H, Nemoto E, Nakamura K, Murakami M (2005) Impact of large tumour size on survival after resection of pathologically node negative (pN0) non-small cell lung cancer. Ann Thorac Surg 79:1142–1146
Kamiyoshihara M, Kawashima O, Sakata S, Hirai T, Ishikawa S, Morishita Y (1999) Does an incomplete interlobar fissure influence survival or recurrence in resected non-small-cell lung cancer? Lung cancer (Amsterdam, Netherlands) 25:33–38
Nair VS, Barnett PG, Ananth L, Gould MK (2010) PET scan 18F-fluorodeoxyglucose uptake and prognosis in patients with resected clinical stage IA non-small cell lung cancer. Chest 137:1150–1156
Higashi K, Ueda Y, Arisaka Y et al (2002) 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med 43:39–45
Moretti L, Yu DS, Chen H et al (2009) Prognostic factors for resected non-small cell lung cancer with pN2 status: implications for use of postoperative radiotherapy. Oncologist 14:1106–1115
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caulo, A., Mirsadraee, S., Maggi, F. et al. Integrated imaging of non-small cell lung cancer recurrence: CT and PET-CT findings, possible pitfalls and risk of recurrence criteria. Eur Radiol 22, 588–606 (2012). https://doi.org/10.1007/s00330-011-2299-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-011-2299-8