Abstract
Objective
The objective of the present study was to evaluate the role of 68Ga-DOTA(0)-Phe(1)-Tyr(3)-octreotide (68Ga-DOTATOC) positron emission tomography computed tomography (PET-CT) for detection and staging of pancreatic neuroendocrine tumours (NETs).
Methods
Twenty patients with clinically suspected and/or histopathologically proven pancreatic NET underwent 68Ga-DOTATOC PET-CT imaging for staging and /or localisation of primary lesion. They also underwent contrast enhanced CT (CECT) and 8 patients underwent 18F-FDG PET-CT. SUVmax of primary and metastatic lesions were measured. Results were verified with histopathology for primary tumour and with clinical follow up/MRI and /or biopsy for metastatic disease. Results of 68Ga-DOTATOC PET-CT were compared to CECT and 18F-FDG PET-CT.
Results
68Ga-DOTATOC PET-CT correctly localised primary in all 20, CECT in 15 and 18F-FDG PET-CT in 2 patients. 68Ga-DOTATOC PET-CT demonstrated metastases in 13 patients, CECT in 7 and 18F-FDG PET-CT in 2. 68Ga-DOTATOC PET-CT emerged as the best investigation with 100% sensitivity and PPV for detecting primary tumour and metastatic disease. The detection rate of CECT was lower than 68Ga-DOTATOC PET-CT, both for primary tumour (20vs.15) or metastatic disease (13vs.7). 18F-FDG PET-CT performed poorly for primary and metastasis.
Conclusion
Ga-DOTATOC PET-CT is a very useful imaging investigation for diagnosing and staging pancreatic NET.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic neuroendocrine tumours (NETs) are substantially rarer than adenocarcinoma with a reported incidence of about 2·5 to 5 cases per 100 000/annum [1]. Clinical behaviour of pancreatic NETs varies strikingly, both in terms of symptoms and outcome. It depends on the site of the primary tumour as well as whether they are functioning tumours or not—i.e. whether the peptides secreted produce symptoms. Pancreatic NETs are large in most cases, with up to 40% being non-functioning, and about 50% presenting with hepatic metastases at diagnosis [2, 3]. Functioning pancreatic NETs might secrete several peptide hormones and lead to diverse symptomatology. All these tumours are difficult to diagnose because of intermittent peptide release, unusual symptoms, and fluctuating plasma hormone levels [2, 4].
Diagnosis of pancreatic NETs is usually based on clinical presentation, hormone assays, and pathology. Some biochemical markers that are identifiable in body fluids suggest specific tumours, whereas others are common to several pancreatic NET subtypes [2, 3]. Correlation of level of serum markers with symptomatology and lesion location is important to facilitate correct diagnosis. Assessment of the location and extent of pancreatic NETs is crucial for accurate management. Commonly used imaging investigations for pancreatic NETs include: conventional transabdominal ultrasonography [USG], computed tomography [CT], and magnetic resonance imaging [MRI]; endoscopic ultrasonography; selective angiography, with or without hormonal sampling and radionuclide imaging (somatostatin-receptor scintigraphy [SRS] with single photon emission CT [SPECT]). Conventional imaging for pancreatic NETs localises only 10–60% of primary tumours.
NETs over-express somatostatin receptors (SSTRs) with heterogeneous tissue distribution [5, 6]. SSTRs appear in five subtypes, SSTR 1–5. Besides their affinity to somatostatin, SSTR2, SSTR3 and SSTR5 also bind to the somatostatin analogues like octreotide and are expressed in 85%–100% pancreatic NETs [7, 8].This receptor overexpression can be exploited for functional imaging of NET by radiolabeled somatostatin analogues binding to the SSTRs[9] and thus tumour detection, depending on both density of SSTR subtype expression and receptor affinity of the radioligand [10, 11]. SRS can be used to identify surgically resectable tumour as well as metastatic tumours which are suitable for peptide receptor radionuclide therapy (PRRT).The most widely used tracer for SRS is 111In-DTPA-octreotide (111In-DTPAOC, Octreoscan), which is commercially available. The sensitivity of 111In-DTPAOC for the detection of NETs varies between 67% and 100% [12, 13]. In comparison to scintigraphy, positron emission tomography (PET) has a two-to threefold higher spatial resolution (3–6 mm versus 10–15 mm) and also facilitates quantification of tracer uptake. 68Ga-DOTA(0)-Phe(1)-Tyr(3)-octreotide (68Ga-DOTATOC) is a promising PET tracer for SSTR imaging [14]. 68Ga-DOTATOC-PET is superior to conventional SRS [15, 16] and also to CT with a reported sensitivity of 97% and a specificity of 92% [17] for PET alone. The objective of the present study was to evaluate the role of 68Ga-DOTATOC-PET-CT for detection and staging of pancreatic NETs.
Material and methods
Patients
The study was conducted after taking approval from the institutional review board. Between September 2006 and July 2010, 20 patients with clinically suspected and/or histopathologically proven pancreatic NET who underwent 68Ga-DOTATOC PET-CT imaging for staging and/or localisation of primary lesion were included in the study. All the patients also underwent contrast enhanced CT and 8 patients underwent 18F-FDG PET-CT. Written informed consent was taken from all the patients.
68Ga-DOTATOC synthesis
68Ga-DOTATOC DOTA0-D-Phe1-Tyr3-octreotide was synthesised as described in the literature [18, 19]and is briefly summarised here. 30–50 mCi 68Ge/68Ga generator was eluted using 0.1 M HCL. The eluent was loaded onto a cation exchange cartridge to pre concentrate and pre purify (using 80% acetone/0.15 M HCL). Purified 68Ga (half-life, 68.3 min; β +88%; Eβ +maximum, 1.9 MeV)was directly eluted with 97.7% acetone/0.05 M HCL into the reaction vial containing 30–50 μg of DOTATOC. Synthesis was carried out at approximately 1260 C for 10–15 min. This was followed by removal of labelled peptide from unlabelled peptide using reverse phase C-18 column, using 400 μl of ethanol. This was further diluted with normal saline and passed through 0.22 μm filter to get sterile preparation for injection.
68Ga-DOTATOC PET-CT acquisition
The studies were performed with dedicated PET-CT equipment (Biograph 2, Siemens, Erlangen, Germany). A dose of 132–222 MBq (4–6 mCi) of 68Ga-DOTATOC was injected intravenously. After a 30–45minute uptake period the patients were taken for PET-CT. Oral contrast agent was used. No intravenous contrast agent was used. In the PET-CT system, CT acquisition was performed on spiral dual slice CT with a slice thickness of 4 mm and a pitch of 1. Image was acquired using a matrix of 512 × 512 pixels and pixel size of 1 mm. After CT, 3D PET acquisition was done from skull to mid thighs. PET data was acquired using matrix of 128 × 128 pixels with a slice thickness of 1.5 mm. CT based attenuation correction of the emission images was employed. PET images were reconstructed by iterative method ordered subset expectation maximization (OSEM; 2 iterations and 8 subsets). After CT acquisition, the table was moved toward the field of view of PET and PET acquisition of the same axial range was started with the patient in the same position on table. After completion of PET acquisition, the reconstructed attenuation corrected PET images, CT images and fused images of matching pairs of PET and CT images were available for review in axial, coronal and sagittal planes,as well as in maximum intensity projections,three dimentional cine mode.
18F-FDG PET-CT acquisition
The studies were performed with dedicated PET-CT equipment (Biograph 2, Siemens, Erlangen, Germany). All patients fasted for at least 4-hours. Blood glucose was less than 7.8 mmol/l. A dose of 370 MBq (10 mCi) of FDG was injected intravenously. The patients rested in a quiet room and after a 45–60 minute uptake period, were taken for PET-CT. PET-CT were acquired from base of the skull to mid thigh. The remaining acquisition parameters were similar to that of 68Ga-DOTATOC PET-CT as detailed above.
Contrast enhanced CT
All patients underwent contrast enhanced abdominal CT (Somatom plus 4, Seimens, Germany). CT was performed using a triple-phase protocol (mAs 230 eff., kV120) following intravenous contrast medium. Slice thickness was 0.75 mm for the arterial (bolus tracking; approximately 24 s delay) and portal-venous phase (45 s delay) and 1.5 mm for the venous phase (70 s delay) using 70–100 ml of intravenous contrast medium (Ultravist 370; Bayer Schering Pharma, Berlin, Germany). The protocol did not include radio-opaque oral contrast media. For evaluation purposes, all CT data-acquisition phases were counted as a whole CT examination. These CT examinations were assessed by experienced radiologists for evidence of primary/metastatic disease.
Image interpretation
68Ga-DOTATOC and 18F-FDG PET-CT studies were evaluated by two experienced Nuclear medicine physicians. They were blinded to findings of the structural imaging. PET images were evaluated both qualitatively and semi-quantitatively. Any non physiological focal area of increased 68Ga-DOTATOC or 18F-FDG uptake was looked for, keeping physiological tracer distribution in perspective. For 68Ga-DOTATOC PET-CT any non physiological uptake more than surrounding tissue was taken as positive. Positive findings on 68Ga-DOTATOC and 18F-FDG PET were localized to anatomic images from the non-enhanced CT. Criteria for a correct detection by PET-CT are both positive 68Ga-DOTANOC/18F-FDG uptakes as well as the correct anatomic localization of the tumour. The PET-CT findings were grouped as primary and metastatic disease. The maximum standardised uptake values (SUVmax) of primary and metastatic lesion was calculated. For final analysis lesion with the highest pathological tracer accumulation within each region in each patient was used.
Reference standard
Results of 68Ga-DOTATOC, 18F-FDG PET-CT and CECT were verified with biopsy/ histopathology for primary tumour. For metastatic disease clinical follow up/magnetic resonance imaging (MRI) and /or biopsy was used to verify the findings. The PET-only positive lesions that showed a morphological lesion in the follow-up were classified as NET lesions. The establishment of a true histopathology-based gold standard for all lesions was methodically and ethically not feasible.
Statistical analysis
Continuous variables, which might be not normally distributed because of the small numbers of patients, are reported as the median and interquartile ranges. Sensitivity, specificity, accuracy, positive (PPV) and negative predictive value (NPV) of 68Ga-DOTATOC PET-CT, 18F-FDG PET-CT (when available) and CECT were calculated. Mc Nemar test was used to compare the diagnostic abilities of different investigations. Spearman correlation coefficients (two tailed) were used to evaluate any correlation in SUVmax of FDG and DOTANOC PET-CT. The p value <0.05 was considered as significant. All the data analyses were performed using the statistical software packages SPSS 11.5(SPSS Inc., Chicago, Illinois, USA).
Results
Patient characteristics
Twenty patients with known/suspected NETs were evaluated in the study. Patient characteristics including age, sex, serum chromogranin levels, indication of PET-CT, and final diagnosis are detailed in Table 1.
68Ga-DOTATOC PET-CT findings
68Ga-DOTATOC PET-CT localised primary tumour in all 20 patients. The primary tumour was localised to head of pancreas in 7 patients, in body of pancreas in 9 patients, in tail of pancreas in 3 patients and multiple masses in 1 patient. The median SUVmax of primary tumour on 68Ga-DOTATOC PET-CT was 12.6 (interquartile range-8.8–27.6). The sensitivity was 100% (95% CI-83.01–100) and PPV 100% (95% CI:-83.01–100).The mean tumor size was 3.3 ± 1.7 cm (range: 1–8 cm). No significant correlation was found between size of primary tumor and the SUVmax (r −0.037; p 0.870; Fig. 1).Metastatic disease was seen in 13 patients. [Sensitivity-92.8% (95% CI: 66–98.8), specificity-100% (95% CI: 54–100), PPV-100% (95% CI: 75.1–100) and NPV-85.7% (95% CI: 42.2–97.6)]. Liver metastasis was seen in majority of patients (10/20). Retroperitoneal lymph node metastasis was seen in 5 patients and bone metastasis in 1 patient. The median SUVmax of metastatic lesions was 15.1 (interquartile range-8.6–22.2).
18F-FDG PET-CT findings
18F-FDG PET-CT was available for 8 patients. It localised primary tumour in 2 of these 8 patients. In both of these patients the primary tumour was localised in the body of pancreas (SUVmax-4.8 and 5.6). The sensitivity was 25% (95% CI: 3.9–64.9) and PPV was 100% (95% CI: 19.2–100). Metastatic disease was also identified in these two patients only. [Sensitivity-20% (95% CI: 3.1–55.5), specificity-100% (95% CI: 16.5–100), PPV-100% (95% CI: 19.2–100) and NPV-11.1% (95% CI: 1.8–48.2)]. Liver was the site of metastasis in both of them (SUVmax-5.6 and 7.9 respectively). 18F-FDG PET-CT failed to localise primary or metastatic disease in remaining 6 patients.
CECT findings
CECT demonstrated pancreatic mass, likely to be primary tumour in 16 patients and diffusely bulky pancreas in 1 patient. CECT was false positive in 2 patients including the later, where the primary tumour was localised to body of pancreas. The primary lesion was contrast enhancing in all the patients. In 3 patients it failed to show primary tumour. [Sensitivity-83.3% (95% CI: 58.5–96.2) and PPV 88.2% (95% CI: 63.5–98.2)].It demonstrated metastatic disease in 7 patients (all liver). [Sensitivity-57.1% (95% CI: 28.9–82.2), specificity-100% (95% CI: 54–100), PPV-100% (95% CI: 62.9–100) and NPV-50 (95% CI: 21.2–78.7)].Metastatic lesions also were contrast enhancing.
Comparison of 68Ga-DOTATOC PET-CT, 18F-FDG PET-CT and CECT
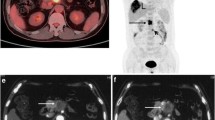
Table 2 compares the performance of each investigation in detection of primary as well as metastatic disease. 68Ga-DOTATOC PET-CT emerged as the best investigation for localising the primary tumour as well as detecting metastasis (Figs. 2, 3, 4 and 5). 18F-FDG PET-CT performed poorly in both the aspects. It failed to detect primary tumour and metastasis in most of the patients. On Mc Nemar analysis there was significant difference 68Ga-DOTATOC and 18F-FDG PET-CT for detecting primary (p 0.030) but not metastatic tumour (p 0.218). No significant correlation was found between SUVmax of primary (r- 0.126; p 0.764) or metastatic tumour (r −0.251; p 0.547) on 68Ga-DOTATOC and 18F-FDG PET-CT. CECT showed pancreatic lesion in majority of patients but was unable to characterise the lesions. Moreover, it missed metastasis in most patients. No significant difference was found between 68Ga-DOTATOC PET-CT and CECT for primary tumour (p 0.062) and metastatic tumour (p 0.125). There were 68Ga-DOTATOC PET-CT positive and CECT negative liver lesions in 4 patients (Fig. 6). All liver lesions that were missed by CT were sub-centimetre in size (range: 0.5–0.8 cm). The SUVmax of these liver lesions ranged from 2.5 to 14.6. Only one of the lesions, which measured 0.8 cm and had SUVmax of 13 was retrospectively visible on CECT. 68Ga-DOTATOC PET-CT positive and CECT negative lymph node lesions were seen in 5 patients. All of these were small in size (range: 0.4–0.6 cm) and showed high 68Ga-DOTATOC uptake (SUVmax range: 4.8–36).
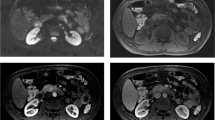
Transaxial CT (a), 68Ga-DOTATOC PET (b) and 68Ga-DOTATOC PET-CT (c) images of a 40 year old female patient presenting with pain abdomen. CT images revealed a mass lesion in the head of pancreas. 68Ga-DOTATOC PET-CT images revealed intense tracer uptake in the mass suggesting SSTR receptor expression. A diagnosis of NET was made. On histopathology the mass turned out to be a well differentiated NET
PET-CT images of a 35 year old female patient presenting with pain abdomen. Transaxial CT, 68Ga-DOTATOC PET and 68Ga-DOTATOC PET-CT images (upper row; left to right) reveal a mass lesion in body of pancreas showing intense 68Ga-DOTATOC uptake. Transaxial CT, 18F-FDG PET and 18F-FDG PET-CT images (lower row; left to right) show the pancreatic mass. However, no significant FDG uptake was noted and the study was interpreted as negative. On biopsy the pancreatic mass was found to be a well differentiated NET
A 26 year old female patients presented with recurrent episodes of hypoglycaemia. Transaxial CT, 68Ga-DOTATOC PET and 68Ga-DOTATOC PET-CT images (left to right) reveal a soft tissue lesion in the tail of pancreas showing intense uptake of 68Ga-DOTATOC. A diagnosis of SSTR expressing tumour was made. The patient underwent resection of the mass and histopathology turned out to be insulinoma
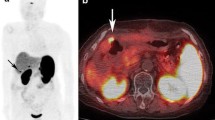
PET-CT images of a 40 year old male patient presenting with pain abdomen and diarrhoea. Transaxial CT, 68Ga-DOTATOC PET and 68Ga-DOTATOC PET-CT images (upper row; left to right) reveal a mass lesion in head of pancreas (arrowhead) showing intense tracer uptake. Also noted hypodense lesion in liver (bold arrow) showing increased 68Ga-DOTATOC uptake suggesting metastasis. Transaxial CT, 18F-FDG PET and 18F-FDG PET-CT images (lower row; left to right) show the pancreatic mass (arrowhead) and hypodense liver lesion (bold arrow). However, no significant FDG uptake was noted and the scan was interpreted as negative. On biopsy the pancreatic mass was found to be a well differentiated NET
A 47 year old female patients presented with pain abdomen and jaundice. Neuroendocrine tumor was suspected because of elevated levels of serum chromogranin and the patient underwent 68Ga-DOTATOC PET-CT. A mass lesion was noted with intense uptake of 68Ga-DOTATOC (not shown). Transaxial non contrast CT (a) images of the liver were unremarkable. 68Ga-DOTATOC PET (b) and 68Ga-DOTATOC PET-CT (c) images revealed foci of intense uptake of 68Ga-DOTATOC. A diagnosis of metastatic NET was made on 68Ga-DOTATOC PET-CT and was confirmed on histopathology
Discussion
Although neuroendocrine tumours (NETs) have been regarded as fairly rare diseases, the US Surveillance Epidemiology and End Results (SEER) database suggests that their prevalence has increased substantially over the past three decades as awareness and diagnostic techniques have improved [20]. This may partly reflect increased diagnosis of benign and incidentally identified lesions due to increased availability of advanced endoscopic and radiological imaging. Diagnosis of NET primaries and metastases is difficult because they frequently present as small lesions and at variable anatomical locations. Diagnosis is based on clinical symptomatology and assay of tumour markers. Some biochemical markers that are identifiable in body fluids suggest specific tumours, whereas others are common to several GEP NET types [2, 3]. Markers common to many types of pancreatic NETs include chromogranin A and pancreatic polypeptide. Levels of circulating chromogranin A are increased in 60–80% of pancreatic NETs and are especially useful in the diagnosis of non-functioning tumours [2, 3, 21]. In the present study serum chromogranin levels were elevated in 95% patients (median chromogranin A level was 316).
Clinical behaviour of pancreatic NETs varies strikingly, both in terms of symptoms and outcome. Insulinomas are typically small benign, functioning tumours, and patients present with hypoglycaemia [2, 4]. Pancreatic gastrinomas are usually malignant; about 25% are associated with MEN1 [2, 4]. Glucagonomas, which cause diabetes and a characteristic rash (necrolytic migratory erythema), and VIPomas, which are associated with severe diarrhoea, are large tumours with metastases when diagnosed [2]. Other rare functioning tumours that secrete adrenocorticotropic hormone, growth hormone releasing hormone, parathyroid hormone-related protein, and somatostatin have been reported [2]. As most pancreatic NETs are non-functioning they present fairly late, with symptoms of mass effects or distant (usually hepatic) metastases, or both [2, 22]. Under these circumstances early and accurate localisation of tumour, primary or metastatic is essential. Endoscopic ultrasonography can detect 90–100% of pancreatic lesions [23]. But its ability to detect metastatic disease is limited. Angiography locates about 40–75% of lesions while assessment of hormonal gradients during angiography is 80–100% sensitive [24].Generally, detection frequency for MRI or CT is about 22–45%, which is higher than ultrasonography (13–27%)[25]. The main limitation of CECT is its inability to characterise the pancreatic tumour as NET or adenocarcinoma, as contrast enhancement can be seen in both of them. In present series CECT had sensitivity of 83.3% for detection of primary tumour and there were 2 false positives. The sensitivity CECT for metastatic disease was 57.1% while the specificity was 100%. As there was only one extra abdominal metastatic disease in present patient population these results might not reflect the overall true picture.
SSTR2, SSTR3 and SSTR5 are over expressed in 85%–100% of gastrointestinal and pancreatic NETs [7, 8].This forms the basis of SRS in pancreatic NETs. In human pancreas, up to 89% of α-cells and up to 87% of β-cells express SSTR 2–5[26], which increases the risk of false positive findings. The most widely used tracer for SRS is 111In-DTPA-octreotide (111In-DTPAOC, Octreoscan, Mallinckrodt Inc, St. Louis, MO, USA), which is commercially available. Its sensitivity for the detection of NETs varies between 67% and 100% [12, 13]. 111In-DTPA-octreotide SPECT however suffers from the usual limitations of limited resolution and long study time of SPECT technology.
68Ga-DOTATOC PET has the advantage of better resolution of PET technology when compared to 111In-DTPA-octreotide SPECT. Improved resolution is very useful in carcinoma patients; 7–71% of suspicious nodes with a size below 1 cm is infiltrated by tumour cells [27–29] and might be imaged with PET. Moreover, the affinity of 68Ga-DOTATOC in binding SSTR2 is 2.5 ± 0.5 nM and tenfold higher than that of 111In-DTPAOC (22 ± 3.6 nM) [30]. In comparison to conventional SRS, 68Ga-DOTATOC PET have higher sensitivity for the detection of NET lesions [15, 16], and in that aspect was also superior to computed tomography. It is superior to conventional SRS [15, 16] and also to CT with a reported sensitivity of 97% and a specificity of 92%[17] for PET alone. Buchmann et al also found 68Ga-DOTATOC PET to be better than 111In-DTPAOC SPECT in patients with NETs in a mixed patient population [31]. Ruf et al evaluated the impact of 68Ga-DOTATOC PET-CT on patients with management of NET (including 14 patients with pancreatic NET) and found that it has impact on management of one-third of patients [32]. In the present study 68Ga-DOTATOC PET-CT showed a very high sensitivity for detection of primary tumour (100%). For metastatic disease too 68Ga-DOTATOC PET-CT was very useful with a sensitivity 92.8% and specificity of 100%. Although 68Ga-DOTATOC PET-CT showed higher detection rate compared to CECT ,on Mc Nemar analysis it only showed borderline significance for primary tumour (p 0.062) and no significant difference was found for metastatic tumour (p 0.125). The mean SUVmax of primary pancreatic tumour on 68Ga-DOTATOC PET-CT was 19.4 ± 16.9.This is similar to the values reported by Prasad et al −18.6 ± 9.8 for previously unknown primary pancreatic tumours [33].
Uptake of 18F-FDG in tumour cell depends on the metabolic rate of tumours and the expression on glucose transporter (GLUT-1). As NET are slow growing tumours with low metabolic rate 18F-FDG PET-CT is not a suitable investigation for imaging NETs. Kayani et al in their study on NET compared the efficacy of 18F-FDG PET-CT and 68Ga-DOTANOC PET-CT [34]. Their patient population included 9 patients with pancreatic NET. In these 9 patients 68Ga-DOTANOC uptake was higher than 18F-FDG in low grade tumors, while the reverse was true for high grade tumors. They however had not evaluated the diagnostic efficacy especially for pancreatic NET. In present study 18F-FDG PET-CT showed very poor sensitivity for detecting primary tumour (sensitivity-18.1%) and metastatic disease (sensitivity-20%). This was much lower than 68Ga-DOTANOC PET-CT (100%). 68Ga-DOTANOC PET-CT showed significant difference from 18F-FDG PET-CT for detecting primary tumour (p 0.03) but not metastatic disease (p 0.218). However, 18F-FDG PET-CT study can prognosticate these patients. A high FDG uptake denotes a shift in differentiation from well to poorly differentiated, thereby poorer prognosis. These patients require a different approach to therapy as cold or radiolabeled SSTR analogues works poorly on these lesions.
The present study had certain limitations. Firstly, only 20 patients were included in the analysis. A more robust study with a larger patient population is needed. However, the incidence of pancreatic NET is so low that it is difficult to recruit large number of patients. Secondly, as metastatic lesions of NET can be missed by all of the imaging investigations; it is difficult to accurately evaluate the role of any of them for detecting metastasis. Furthermore, histopathological confirmation was available only for few of the metastatic lesions.
In conclusion 68Ga-DOTATOC PET-CT is a very useful imaging investigation for diagnosing and staging pancreatic NET. It can characterise the pancreatic mass seen on CECT as NET. It appears better than both 18F-FDG PET-CT and CECT for staging the pancreatic NET.
References
Modlin IM, Lye KD, Kidd M (2003) A 5-decade analysis of 13,715 carcinoid tumors. Cancer 97:934–959
Kaltsas GA, Besser GM, Grossman AB (2004) The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev 25:458–511
Eriksson B, Oberg K, Stridsberg M (2000) Tumor markers in neuroendocrine tumors. Digestion 62:33–38
Oberg K, Eriksson B (2005) Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol 19:753–781
Ueberberg B, Tourne H, Redman A, Walz MK, Schmid KW, Mann K et al (2005) Differential expression of the human somatostatin receptor subtypes sst1 to sst5 in various adrenal tumors and normal adrenal gland. Horm Metab Res 37(12):722–728
Papotti M, Kuma U, Volante M, Pecchiono C, Patel YC (2001) Immunohistochemical detection of somatostatin receptor types 1–5 in medullary carcinoma of the thyroid. Clin Endocrinol 54:641–649
OdaY TanakaY, Naruse T, Sasanabe R, TsubamotoM FH (2002) Expression of somatostatin receptor and effects of somatostatin analog on pancreatic endocrine tumors. Surg Today 32(8):690–694
Papotti M, Bongiovanni M, Volante M, Allia E, Landolfi S, Helboe L et al (2002) Expression of somatostatin receptor types 1–5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch 440(5):461–475
Reubi JC (1995) Neuropeptide receptors in health and disease: the molecular basis for in vivo imaging. J Nucl Med 36:1825–1835
Reubi JC, Schar JC, Waser B, Wenger S, Heppeler A, Schmitt JS, Macke HR (2000) Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med 27:273–282
Miederer M, Seidl S, Buck A, Scheidhauer K, Wester HJ, Schwaiger M, Perren A (2009) Correlation of immune-histopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging 36:48–52
Kaltsas G, Mukherjee JJ, Grossman AB (2001) The value of radiolabeled MIBG and octreotide in the diagnosis and management of neuroendocrine tumours. Clin Endocrinol 55:575–587
Kaltsas G, Rockall A, Papadogias D, Reznek R, Grossman AB (2004) Recent advances in radiological and radionuclide imaging and therapy of neuroendocrine tumours. Eur J Endocrinol 151:15–27
Hofmann M, Maecke H, Börner R, Weckesser E, Schöffski P, Oei L et al (2001) Biokinetics and imaging with the somatostatin receptor PET radioligand 68Ga-DOTATOC: preliminary data. Eur J Nucl Med 28:1751–1757
Buchmann I, Henze M, Engelbrecht S, Eisenhut M, Runz A, Schäfer M et al (2007) Comparison of 68Ga-DOTATOC PET and 111 In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging 34:1617–1626
Kowalski J, Henze M, Schuhmacher J et al (2003) Evaluation of positron emission tomography imaging using [68Ga]-DOTA- D- Phe(1)- Tyr(3)-octreotide in comparison to [ 111 In]- DTPAOC SPECT. First results in patients with neuroendocrine tumors. Mol Imaging Biol 5:42–48
Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C et al (2007) 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatinreceptor scintigraphy and CT. J Nucl Med 48:508–518
Heppeler A, Froidevaux S, Mäcke HR (1999) Radiometal-labeledmacrocyclic chelator derivatised somatostatin analogue with superb tumour-targeting properties and potential for receptormediated internal radiotherapy. Chem Eur J 5:1974–1981
Zhernosekov KP, Filosofor DV, Baum RP et al (2007) Processing of generator produced 68Ga for medical applications. J Nucl Med 48:1741–1748
US National Cancer Institute. SEER Database. http://seer.cancer.gov/ (accessed Nov 15, 2007).
Stridsberg M, Oberg K, Li Q et al (1995) Measurement of chromogranin A, chromogranin B (secretogranin I), chromogranin C (secretogranin II) and pancreastatin in plasma and urine from patients with carcinoid tumours and endocrine pancreatic tumours. J Endocrinol 144:49–59
Modlin IM, Kidd M, Latich I et al (2005) Current status of gastrointestinal carcinoids. Gastroenterology 128:1717–1751
Anderson MA, Carpenter S, Thompson NW et al (2000) Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am J Gastroenterol 95:2271–2277
Gibril F, Jensen RT (2004) Diagnostic uses of radiolabelled somatostatin receptor analogues in gastroenteropancreatic endocrine tumours. Dig Liver Dis 36:S106–S120
Gastrointestinal carcinoid tumours (2007) In: Modlin IM, Gustafsson BI, Kidd M, eds. Advances in digestive disease. Bethesda, MD: AGA Institute Press, pp 203–218.
Kumar U, Sasi R, Suresh S, Patel A, Thangaraju M, Metrakos P et al (1999) Subtype-selective expression of the five somatostatin receptors (hSSTR1–5) in human pancreatic islet cells: a quantitative double-label immunohistochemical analysis. Diabetes 48(1):77–85
Arita T, Kuramitsu T, Kawamura M, Matsumoto T, Matsunaga N, Sugi K et al (1995) Bronchogenic carcinoma: incidence of metastases to normal sized lymph nodes. Thorax 50:1267–1269
Einstein DM, Singer AA, Chilcote WA, Desai RK (1991) Abdominal lymphadenopathy: spectrum of CT findings. Radiographics 11:457–472
Gross BH, Glatzer GM, Orringer MB (1988) Bronchogenic carcinoma metastatic to normalsized nodes: frequency and significance. Radiology 166:71–74
Reubi JC, Schär JC, Waser B, Wenger S, Heppeler A, Schmitt JS et al (2000) Affinity profiles for human somatostatin receptor subtypes SST1–SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med Mol Imaging 27:273–282
Buchmann M, Henze S, Engelbrecht M, Eisenhut A, Runz M, Schäfer T et al (2007) Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging 34:1617–1626
Ruf J, Heuck F, Schiefer J, Denecke T, Elgeti F, Pascher A et al (2010) Impact of multiphase 68Ga-DOTATOC-PET/CT on therapy management in patients with neuroendocrine tumors. Neuroendocrinology 91:101–109
Prasad V, Ambrosini V, Hommann M, Hoersch D, Fanti S, Baum RP (2010) Detection of unknown primary neuroendocrine tumours (CUP-NET) using (68)Ga-DOTA-NOC receptor PET/CT. Eur J Nucl Med Mol Imaging 37:67–77
Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T et al (2008) Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (Dota-DPhe1, Tyr3-octreotate) and 18F-FDG. Cancer 112:2447–2455
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, R., Sharma, P., Garg, P. et al. Role of 68Ga-DOTATOC PET-CT in the diagnosis and staging of pancreatic neuroendocrine tumours. Eur Radiol 21, 2408–2416 (2011). https://doi.org/10.1007/s00330-011-2199-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-011-2199-y