Abstract
Purpose
The aim of this study was to compare ultrasound-guided access of the superficial femoral artery and the common femoral artery.
Material and methods
100 patients were randomized to ultrasound-guided access either into the SFA or the CFA. The two groups were compared with respect to technical success, access time and complications. In addition, a subgroup analysis was performed to compare the complication rate using manual compression versus closure devices for haemostasis.
Results
In the SFA group 49/50 patients were successfully accessed in the assigned location, compared to 41/50 in the CFA group (p = 0.016). The median access time was significantly faster in the SFA group (3 min 25 s) compared to the CFA group (5 min 26 s) (p < 0.001). The most frequent complications in the SFA group were pseudoaneurysms (16.3%) whereas access site haematomas (14.6%) were the most common complication in the CFA group. However, when looking at subgroup with closure devices there was no difference between the SFA group compared to CFA group (p = 1.000).
Conclusion
Accessing the SFA was more often successful and significantly faster than puncturing the CFA. The pseudoaneurysm rate was higher in the SFA group when using manual compression, but similar when using closure devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peripheral vascular disease (PVD) is a common medical problem in the western world, with a high impact in medical care, especially in elderly patients. In the USA, up to 8 million people per year suffer from this disease [1, 2].
For the constantly increasing rate of endovascular interventional treatment of peripheral arterial disease of the lower extremity a femoral approach is the most important access for interventional procedures. Endovascular therapy often prevents or delays the necessity of a bypass operation or amputation of the affected lower extremity [3–5]. There are two basic options to perform access to the lower extremity arteries: Either a retrograde contralateral approach with cross-over advancement of the sheath or an antegrade ipsilateral approach. Most interventional groups favour arterial access into the common femoral artery (CFA) by palpation for antegrade interventions. For SFA access ultrasound guidance is necessary. The direct antegrade puncture of the superficial femoral artery has already been described in small studies [6–8]. In these studies, it was assumed that antegrade access via the SFA could be an alternative access approach, for example, in special situations like “hostile groin”, when puncture of the CFA is impossible [8].
A first recently published prospective feasibility study investigated safety in antegrade puncturing of the SFA in 100 patients. They found that there was a comparable rate of minor complications in comparison to the few older studies, which investigated safety in antegrade puncturing of the CFA [9].
The aim of the study was to compare ultrasound-guided access between SFA and CFA in a general population.
Materials and methods
Patients
This prospective clinical study was performed with approval of the institutional review board and informed consent from all patients. Between May 2009 and December 2009 we screened 139 patients in our institute who were referred for lower extremity arterial angiography and intervention (Fig. 1). Patients from other hospitals were not screened in order to have a consistent follow-up at our own institution.
Flowchart image showing the excluded patients in this study. 39 patients were excluded: Seven patients had a relevant stenosis of a CFA, 30 patients had a proximal SFA stenosis and two patients were excluded for emergency reasons. Finally, we included 100 patients who were randomized into two groups (50 patients SFA group/50 patients CFA group)
Inclusion criteria were at least 18 years of age and planned antegrade access for lower extremity angiography and intervention. Exclusion criteria were a relevant stenosis (≥50%) of the CFA or the proximal SFA, documented by ultrasound or MRI before intervention and emergency angiography. Besides age and sex, the following parameters were recorded for every patient: Body mass index (BMI); antiplatelet medication and anticoagulation; laboratory findings such as platelet count and international normalized ratio (INR).
Intervention technique
All patients were placed in supine position with feet first on the table. Standard preparation of the groin access site was performed.
The CFA and the proximal SFA were examined using ultrasound B-mode (Zonare 1, Medical Systems, California, USA) to determine if both vessels were amendable for access. The diameter of the SFA and CFA and the soft tissue layer above the SFA and CFA were measured. If no contraindications were found, the patients were randomized into one of two groups (SFA or CFA group) and the interventional radiologist accessed the assigned vessel. All radiologists have interventional experience (especially vessel interventions) more than 12 years.
For the purpose of the study, only ultrasound guidance was recommended for introducing the sheath. The use of fluoroscopy for sheath placement was recorded separately.
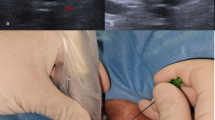
The artery was punctured using a vascular access needle (COOK, SDN-19UT-7.0, 19 gauge, Bloomington, USA) under ultrasound guidance (Fig. 2). Using a J-tip guide wire, a sheath (Cordis, brite tip sheath, 4–10 F, Cordis cooperation, Miami, USA) was inserted into the CFA or SFA. After introduction of the sheath, angiography in an ipsilateral oblique view of the femoral bifurcation artery was obtained in every patient, which allowed evaluation of the exact entry point into the artery (Fig. 3a and b). The time needed to gain access was defined as the time from the injection of a local anesthetic until sheath placement into the SFA or CFA (confirmed by blood aspiration).
a. Femoral arterial bifurcation and the lower margin of the femoral head were measured in relation to the entry site of the sheath after application of contrast medium through the sheath. b. According to Fig. 3a, measurement of the entry site according to the femoral bifurcation and the lower border of the femoral head was documented, in this case after antegrade access over the right SFA
At the end of the procedure, the sheath was removed and hemostasis was achieved either by manual compression or a closure device (Starclose, Abbott, Redwood City, USA). Manual compression was performed for at least 20 min. The use of a closure device was at the discretion of the operator. After finishing the intervention all patients received a compression bandage for 6 hours.
Follow-up
All patients were followed by duplex ultrasound. The follow-up of the access site was performed in all patients after 6 hours (outpatients) or 24 hours (inpatients). During the ultrasound, we looked for the following complications: stenosis, pseudoaneurysm, dissection and occlusion. If there was a haematoma or a pseudoaneurysm, exact measurements including calculation of the maximum size were performed.
Statistical analysis
Statistical analyses were performed using the statistical program PASW Statistics 18. The data were analyzed descriptively and described with the median and the interquartile range for continuous data or the number of subjects and percentage of the total for categorical variables. Comparisons between all groups were performed with a Mann-Whitney U test or a Chi2-test (Fisher’s exact test).
Results
A total of 139 patients were screened for the study. Thirty-nine patients were excluded for the following reasons: 7 patients had a relevant stenosis of the CFA, 30 patients had a proximal SFA stenosis and 2 patients were excluded because the procedure became an emergency.
Finally, we included 100 patients (67 men) with a mean age of 73.8 years (range: 22–95 years), who were randomized into two groups (50 patients SFA group/50 patients CFA group). Patient enrollment is summarized in Fig. 1. Demographics and clinical data of the included patients are summarized in Table 1.
Successful accesses
Of the 50 patients in the randomized CFA group, access into the assigned vessel was successfully performed in only 41 (82%) patients. Of the 9 “incorrect” punctures, 2 patients were accessed accidentally in the other location (SFA) not related to a clinical problem. In the other 7, accessing the CFA was technically unfeasible because it was not possible to advance the wire into the SFA. In all 7 cases intentional access into the SFA was possible afterwards. The 7 frustrated access attempts had a median duration of 15 min 01 s (range 6 min 50 s–39 min 16 s). We did not find a clear statistical indication for the relatively high number of puncture failures in the CFA group. The only determinable index was the fact that the patients with failed puncture of the CFA had a higher BMI than the CFA patients with successful access. However, the relationship was not statistically significant (p = 0.078).
Access was successful in 49 of the 50 patients (98%) who were randomized into the SFA group. Only one patient had to be accessed in the CFA because the vessel (SFA) was so rigid that insertion of the wire was impossible. In this case, we changed the approach to the CFA after a frustration access time of 20 min 27 s.
Access time and radiation exposure
The median time for successful arterial access into the assigned location was 5 min 26 s (IQR 3 min 35 s–9 min 53 s) in the CFA group and 3 min 25 s (IQR 2 min 37 s–4 min 53 s) in the SFA group. Arterial access was thus significantly faster in the SFA than in the CFA group (p < 0.001).
The median radiation exposure for sheath placement in the CFA group was 26 s (IQR 0 s–1 min 13 s), which was significantly longer compared to 0 min (IQR 0–0 min) in the SFA group (p < 0.001) (Tab. 1). In 38 of 49 SFA cases no fluoroscopy was used for sheath placement compared to 11 of 41 CFA cases.
Complications
In the CFA group, 8/41 patients (19.5%) encountered a complication (Tab. 2). Overall, 6 patients had small haematomas (14.6%) with a median size of 33 mm and 1 patient showed a small pseudoaneurysm (2.4%) with a maximum size of 11 mm. It was treated successfully with a thrombin injection. One patient showed occlusion of the SFA after a difficult access of the CFA and repeated malpositioning of the wire into the deep femoral artery. The occlusion was treated successfully with local thrombolysis.
In the SFA group, 13/49 patients (26%) encountered a complication (Tab. 2). Overall, four patients (8.1%) had small haematomas and 8 patients (16.3%) showed pseudoaneurysms with a medium size of 18 mm (range 7–45 mm). One patient showed a partial SFA occlusion after insertion of the closure device, which was treated with PTA using a contralateral approach just after the first intervention. Two of the 8 patients with pseudoaneurysms were treated successfully with manual compression (10 min compression under ultrasound guidance) and 5 patients were treated successfully with a thrombin injection. One small pseudoaneurysm (7 mm) regressed spontaneously without any intervention within one day. No patient with a complication in either group had any long term problems. No patient stayed longer in the hospital because of a complication.
Looking at the entire population of SFA and CFA access, the complication rate was similar in both groups (p = 0.619). However, the rate of pseudoaneurysms was significantly higher in the SFA than in the CFA group (p = 0.036). There was no difference regarding the development of haematoma (p = 0.503).
Subgroup analysis for complications after using hemostasis with manual compression or a closure device
Overall, 35 patients from the CFA group and 39 patients in the SFA group had haemostasis with manual compression (Table 2). There was a lower rate of pseudoaneurysms in the CFA group (1 patient) than in the SFA group with 7 pseudoaneurysms using manual compression (p = 0.059). This supports the general opinion that manual compression seems to be more difficult after SFA access than CFA access.
Overall, 6 patients from the CFA and 10 patients from the SFA group had haemostasis with a closure device (Table 2). The complication rate was equally low in both groups when using a closure device (p = 1.000). Noteworthy in the SFA group, there was only 1 pseudoaneurysm in contrast to the higher rate of pseudoaneurysms in the SFA group using manual compression. Looking at the subgroup with closure devices there was no difference between the SFA group (1 aneurysm/10 cases) compared to CFA group (0 aneurysm/6 cases) (p = 1.000).
Discussion
Traditional access for endovascular treatment of the lower limb is performed either by a retrograde contralateral or an antegrade ipsilateral approach to the common femoral artery. Due to the absence of bony counter fort during manual compression and the resulting higher risk for pseudoaneurysm formation, the antegrade approach of the SFA was considered less suitable by the interventional radiological community. However, to our knowledge, the exact risk of SFA access in comparison to the traditional access over the CFA has not yet been investigated scientifically. So far, a smaller study could demonstrate that the antegrade approach of the SFA can be an advantageous alternative in difficult situations such as “hostile groin” compared to the traditional access route via the CFA [8]. A recently published study by the group of Gutzeit et al. in 2010 showed, that a direct antegrade approach in the SFA in a general population is feasible [9].
In this study, we were able to show that ultrasound-guided antegrade access into the SFA with a success rate of 98% is better than the success rate of antegrade CFA puncture of 82% in a general study population. Failure to use CFA access was often caused by an inability to advance wire and/or sheath into the SFA. There was no statistical significance between the patients with successful CFA access compared to the failures, but there was a trend to a higher BMI in the failure group (p = 0.078).
SFA access was also faster when compared to CFA access with a mean access time of 3 min 25 s versus 5 min 26 s for CFA. Also less fluoroscopy and ultrasound was needed in the SFA group compared to the CFA group. In 38/49 cases no fluoroscopy was used for SFA access compared to no fluoroscopy in only 11/41 cases with CFA access. This saves radiation exposure to the operator’s hand.
A disadvantage of antegrade access to the SFA was a higher rate of pseudoaneurysms. A subgroup analysis showed that a higher rate of pseudoaneurysms only occurred for manual compression but not for hemostasis with closure devices. An explanation for the higher rate of pseudoaneurysms could be the lack of a bony resistance to push against. Interestingly however, there was no correlation between the distance between the access site and the femoral head and the pseudoaneurysm formation. Also there was no statistical correlation with BMI or anticoagulation.
Interestingly our pseudoaneurysm rate of 2.4% in the CFA group was lower than other studies [10–12], especially in a study by Mlekusch et al. [11], who reported pseudoaneurysm rates after antegrade access of the CFA between 7% and 9%. One possible explanation for this difference could be the use of ultrasound guidance in our study. By using ultrasound guidance repeat puncture can be minimized compared to the palpation technique performed by Mlekusch et al. [11].
A limitation to the present study is that the use of manual compression versus closure devices was not standardized, but rather was at the discretion of the operator. Nevertheless, the use of closure devices was mostly dependent on the sheath size. Therefore a bias seems unlikely.
In conclusion, ultrasound-guided antegrade access into the SFA was more often successful than CFA access. It seems that SFA puncture is easier, especially in obese patients. Also the time to access the SFA was shorter SFA group compared with accessing the CFA. On the other hand, SFA access showed a higher rate of pseudoaneurysms when using manual compression. However, no differences in pseudoaneurysm formation was observed when using closure devices.
References
Rosamond W, Flegal K, Friday G et al (2006) Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 115:69–71
McDermott MM, Tian L, Liu K et al (2008) Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol 15:1482–1489
Arain SA, White CJ (2008) Endovascular therapy for critical limb ischemia. Vasc Med 13:267–279
Suding PN, McMaster W, Hansen E, Hatfield AW, Gordon IL, Wilson SE (2008) Increased endovascular interventions decrease the rate of lower limb artery bypass operations without an increase in major amputation rate. Ann Vasc Surg 22:195–199
Rogers JH, Laird JR (2007) Overview of new technologies for lower extremity revascularization. Circulation 116:2072–2085
Nice C, Timmons G, Bartholemew P, Uberoi R (2003) Retrograde vs. Antegrade puncture for infra-inguinal angioplasty. Cardiovasc Intervent Radiol 26:370–374
Blais C (1993) Antegrade puncture of the superficial femoral artery: a pilot project. Can Assoc Radiol J 44:253–256
Marcus AJ, Lotzof K, Howard A (2007) Access to the superficial femoral artery in the presence of a “hostile groin”: a prospective study. Cardiovasc Intervent Radiol 30:351–354
Gutzeit A, Schoch E, Sautter T, Jenelten R, Graf N, Binkert CA (2010) Antegrade access to the superficial femoral artery with ultrasound guidance: feasibility and safety. J Vasc Interv Radiol 21:1495–1500
Katzenschlager R, Ugurluoglu A, Ahmadi A et al (1995) Incidence of pseudoaneurysm after diagnostic and therapeutic angiography. Radiology 195:463–466
Mlekusch W, Minar E, Dick P et al (2008) Access site management after peripheral percutaneous transluminal procedures: neptune pad compared with conventional manual compression. Radiology 249:1058–1063
Gabriel M, Pawlaczyk K, Waliszewski K, Krasiński Z, Majewski W (2007) Location of femoral artery puncture site and the risk of postcatheterization pseudoaneurysm formation. Int J Cardiol 120:167–171
Acknowledgements
We would like to thank the Department of Angiology of the Cantonal Hospital of Winterthur/Switzerland, especially Yvonne Fuchs, Patrick Hodel MD and Martin Kliem MD for their support with patient care during the study. We would like to express our thanks to Philips Healthcare Switzerland for their effective technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gutzeit, A., Graf, N., Schoch, E. et al. Ultrasound-guided antegrade femoral access: comparison between the common femoral artery and the superficial femoral artery. Eur Radiol 21, 1323–1328 (2011). https://doi.org/10.1007/s00330-010-2032-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-010-2032-z