Abstract
We aimed to demonstrate that coronary CT angiography (cCTA) can be used to non-invasively study the effect of hemodynamic factors in the pathophysiology of plaque formation. cCTA data of 73 patients were analyzed. All detected plaques were classified according to location (bifurcation, non-branching segment), configuration (eccentric, concentric), orientation (myocardial, lateral, epicardial side of the vessel wall), and composition (calcified, mixed, non-calcified). Bifurcation lesions were further characterized using the Medina classification. Of 382 plaques, 8.1% were in the LM, 46.3% in the LAD, 18.3% in the LCx, and 25.9% in the RCA. Also, 25.1% were completely calcified, 72.3% were mixed, and 2.6% were purely non-calcified. Of the plaques, 51.3% were bifurcation lesions. The most frequent (40%) Medina pattern was 1.1.0 (lesion starts before, extends beyond bifurcation, sparing the side branch). Eighty percent of plaques were eccentric. A significant (p < 0.01) majority (55%) were on the myocardial side, while 17.3% were lateral, and 27.7% epicardial. Of all non-calcified and mixed plaques, 45.1% (p < 0.01) were myocardial, whereas only 14.3% were lateral, 20.6% epicardial, and 19.9% concentric. We conclude that cCTA can non-invasively study the effect of vascular hemodynamics, such as turbulent flow (bifurcations) and low shear stress (myocardial vessel wall), on the distribution and composition of atherosclerotic plaque deposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The primary cause of myocardial infarction (MI) is coronary artery atherosclerosis. Atherosclerotic coronary artery plaques are widely, but not uniformly distributed within the coronary tree, which suggests that, beside systemic risk factors, there are other local conditions that influence the formation and progression of atherosclerosis. Based on invasive coronary angiography [1] as well as intravascular ultrasound (IVUS) [2], it has been previously shown that the walls of coronary arteries are exposed to varying shear stresses and that the majority of atherosclerotic plaques are located in regions of low shear stress or in areas with turbulent flow, e.g., in the vicinity of vessel bifurcations. It has also been noted that there are differences in the progression of coronary artery atherosclerosis among the three major coronary arteries [3]. Plaques located in the right coronary artery (RCA) have shown the highest degree of progression, followed by the left anterior descending (LAD) distal to the first septal branch (SB), the first diagonal (D) branches, and the obtuse marginal (OM) branches of the left circumflex (LCx) artery [3]. Investigating the spatial distribution of coronary artery plaques has relevance in light of earlier studies using invasive tests [4, 5], which showed that eccentric and long lesions were more likely to predict a worse outcome following coronary angioplasty procedures and indicate a higher risk of coronary dissection when compared to lesions that are concentric and short, which is likely related to the greater technical difficulties with navigating the catheter guide wires beyond eccentric lesions. A recent study [6] has also demonstrated that plaque ruptures do not have a uniform distribution within the coronary artery tree, and it has been found that the most vulnerable plaques are located in the proximal third of each of the three major coronary vessels, enhancing the hypothesis that in the complex systemic framework of plaque etiology and pathogenesis certain local factors may contribute to the progression of atherosclerotic disease. Most importantly, acute coronary syndromes (ACS) and the mortality related to them have been associated with acute changes in plaques, such as ulceration, thrombosis, or intra-plaque hemorrhage, which produce a “vulnerable plaque” [7–10].
Coronary CT angiography (cCTA) can non-invasively assess luminal integrity as well as atherosclerotic changes in the vessel wall in vivo. The primary aim of this study therefore was to demonstrate that the same atherogenetic phenomena that have traditionally been studied invasively or ex vivo can be non-invasively explored by cCTA.
Materials and methods
Patients
This retrospective study was performed following approval by our institutional IRB. The need for informed patient consent was waived. We analyzed data of 73 consecutive patients (27 women, mean age 59 ± 10 years) who had findings of coronary atherosclerosis at contrast medium-enhanced cCTA on a 64-slice multi-detector-row CT (MDCT) system (SOMATOM Sensation 64 Cardiac, Siemens, Forchheim, Germany) using retrospective ECG gating. All patients had been clinically referred for cCTA because of suspected coronary artery disease in the setting of atypical chest pain and a non-diagnostic or equivocal prior study (e.g., ergometric stress testing, nuclear myocardial perfusion study). None of the patients had a known history of coronary heart disease.
Image acquisition
Acquisition delay time was determined by injection of a 20-ml test bolus at 5 ml/s, followed by 50 ml of saline using a dual-syringe injector (Stellant D, Medrad, Pittsburgh, PA). The peak time of test bolus enhancement was used as the delay time. Actual contrast medium enhancement was achieved with 50–75 ml of a non-ionic contrast medium (Isovue; 370 mgI/ml lopamidol; Bracco, Princeton, NJ) infused through an 18-G intravenous antecubital catheter at 5 ml/s, followed by a 50-ml saline chaser bolus. The contrast medium volume was individually computed according to the following formula: volume (ml) = image acquisition time (s) × 5. Contrast medium-enhanced coronary CTA was obtained in each patient, using the following parameters: 64 × 0.6 collimation, 0.33 s time, pitch of 0.2, 120 kV tube voltage, and 900 mAs tube current. Patients with average hearts rates >65 bpm and no contraindications to use of beta-blockers received up to three intravenous injections of 5 mg of Metroprolol Tartrate (Lopressor, Novaritis, East Hanover, NJ) immediately before the examination. Studies were acquired in a cranio-caudal direction with simultaneous recording of the patient’s ECG signal in order to allow for retrospective registration of image reconstruction to the desired cardiac phase. The examination range extended from the level of the carina to just below the diaphragm. Image reconstruction was performed using single-segment reconstruction and retrospective ECG gating. The reconstruction intervals were timed to the phase of the cardiac cycle with the least cardiac motion. This was determined based on a preview series, consisting of 20 images reconstructed at 20 different phases of the cardiac cycle in 5% increments (0–95% of the R-R interval) at the same z-position at the mid-level of the heart. Image reconstruction parameters comprised an individually adapted field of view encompassing the heart, a matrix size of 512 × 512 pixels, a medium soft-tissue convolution kernel (B25f), and a section thickness of 0.75 mm at an increment of 0.3 mm.
All CT datasets were transferred to a dedicated workstation (Circulation, Siemens) and analyzed using transverse sections and curved multiplanar reformats.
Image analysis
Each atherosclerotic plaque was analyzed by two experienced observers (one radiologist and one cardiologist) in consensus. Plaques were classified according to their proximity to a vessel bifurcation. Lesions located at least two times the vessel diameter away from bifurcations were classified as plaques in non-branching segments. Each plaque located at the level of bifurcation was further characterized according to the Medina classification [11]. The configuration (eccentric vs. concentric) was determined by calculating the Eccentricity Index as described by Mintz et al. [5]. The Eccentricity Index was defined as the ratio of maximum to minimum plaque (or vessel wall) thickness plus media thickness, and lesions were considered eccentric if the eccentricity index was ≥3 or if there was an arc of disease-free arterial wall when reviewing the cross section of the vessel. Eccentric plaques were further classified according to their spatial orientation in relation to the myocardium or epicardium: ‘myocardial,’ ‘lateral,’ or ‘epicardial.’ When the vessel wall was affected circumferentially, the orientation of the part of the lesion with the largest diameter was considered as a pointer. Regarding the composition of the plaques, three categories were used: calcified, purely non-calcified, or mixed plaques. No attempt was made to further differentiate non-calcified plaques with different tissue types (i.e., fibrous versus lipid-rich components), as there is a substantial overlap in attenuation of these two tissue types. [12]
Statistical analysis
The various categories of localization, orientation, configuration, and composition were cross referenced in several interactions. Thus, sub-categories were formed (e.g., non-branching, eccentric, mixed lesions) that were further divided according to the vessel segment affected. These were then expressed as percentages of either the total number of plaques or the number of plaques in a category or a sub-category (e.g., what percentage of the non-branching eccentric mixed lesions was found in the LAD territory). Data were statistically analyzed using chi-square test and Fisher’s exact test. A p value of less than 0.05 was considered indicative of statistically significant differences among vessel segments of plaque types.
Results
Of the 73 patients, 5 (7%) had very low risk of coronary heart disease according to the Framingham Prediction Score; 12 (16%) had low risk; 43 (59%) were at moderate risk; 13 (19%) had high risk of coronary heart disease.
A total of 382 plaques were analyzed. Of these, 8.1% were found in the LM, 46.3% in the LAD or its side branches, 1.3% in the Ramus Intermedius (RI), 18.3% in the LCx, and 25.9% in the RCA, (Fig. 1). As for each of the major vessels, 93.5% of LM, 80.2% of LAD, 78.8% of RCA, and 77.1% of LCx lesions were eccentric (Fig. 1). Interestingly, 45.2% of LM lesions were lateral, while 47.3% of LAD plaques and 48.5% of RCA plaques were myocardial.
Of all plaques the majority (80%) were eccentric (Fig. 2). A significant (p < 0.05) majority (55%) of eccentric plaques have been located at the myocardial side of the vessel wall, while 17.3% were lateral and 27.7% epicardial. Fig. 2 also shows examples for all types of concentric and eccentric plaques.
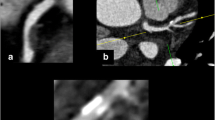
Distribution of plaques based on their configuration. (a) Transverse section of a concentric, non-side-branch, mixed plaque. (b) Curved MPR showing a long, eccentric, myocardial, non-branching, calcified plaque (arrow). (c) Two orthogonal curved MPR images are shown of the same vessel demonstrating an eccentric, lateral, non-branching, mixed plaque (arrow in image on top). The plaque is not visible in the view perpendicular to the myocardium (bottom image). The best way to evaluate the spatial orientation of these plaques is analyzing the two orthogonal views of the vessel. (d) Curved MPR of an eccentric, epicardial, non-branching, non-calcified plaque (arrow). Further distally (small arrow), there is a small calcified plaque in the myocardial side of the vessel
Regarding plaque composition, 25.1% of all plaques were completely calcified, 72.3% were mixed, and 2.6% were purely non-calcified. Of all non-calcified and mixed plaques, a significant majority of 45.1% (p < 0.05) were myocardial, whereas only 14.3% were lateral, 20.6% epicardial, and 19.9% concentric.
Of all plaques, 51.3% had a side-branch location (Fig. 3). Of these, 79.6% were mixed, 17.3% were completely calcified, and 3.1% were non-calcified. Thus, 82.7% had non–calcified components.
Distribution of plaques with regard to location (side branches), configuration (eccentric vs. concentric), and composition. The numbers inside the ovals are expressed as percentages of the total number of plaques, while the numbers referring to the configuration and composition are expressed as the percentage of either the side-branching or the non-side-branching lesions
Of the non-side-branch plaques (48.7% of all plaques), 64.5% were mixed (p < 0.001), 2.2% were non-calcified, and 33.3% were completely calcified. Thus, when compared to side-branch lesions, more non-side-branch lesions were completely calcified.
Of all eccentric plaques, 71.3% were mixed, and of these, 52.1% were near a side branch, while 25.4% of eccentric plaques were completely calcified, and most of them (62.8%) had a non-side-branch location. Only 3.3% of the eccentric plaques were non-calcified, and 60% of these were near a side branch.
However, 76% of the concentric plaques were mixed, and 73.3% of them had a side-branch location (compare to 52.1% of eccentric mixed plaques above); 72.2% of the completely calcified concentric plaques had a non-side-branch distribution. Interestingly, all concentric plaques had some calcified component; in other words, none of them was purely non-calcified.
The most frequently observed (p < 0.0001) Medina pattern among the bifurcation lesions was 1.1.0 (Fig. 4). Figure 5 shows examples for each of the Medina classes found in this study.
Distribution of plaques according to the Medina classification. The graph shows the percentage distribution of all side-branching plaques with regard to the various Medina classes. The significant majority of lesions were classified as 1.1.0, which means that the parent vessel is affected before and after the bifurcation, while the side branch is spared
Discussion
The observations in our study suggest that the current understanding of local hemodynamic factors in the pathogenesis of coronary atherosclerotic plaque, which has been established based on invasive or ex vivo methods, finds its correlate in vivo at non-invasive contrast-medium-enhanced cCTA. While this seems intuitive, the convergence of existing knowledge and classifications concerning coronary atherosclerotic plaque deposition and their morphological surrogates at cCTA has not been systematically demonstrated to date. The etiology and pathophysiology of atherosclerotic plaque formation, despite fervent research, is incompletely understood, highly complex, and multifactorial, and local hemodynamic conditions are only one mechanistic facilitator of plaque deposition. However, starting with Virchow’s triad [13], substantial effort has been directed at understanding, classifying, and quantifying the influence of physical, mechanical principles on vascular disease. Automated 4D segmentation, display, and analysis algorithms based on invasive and non-invasive imaging are currently under development [14, 15], which enable tracking of regional coronary artery motion patterns and simulate vascular fluid dynamics in order to study their relationship to plaque formation. With our work we intended to lay the descriptive groundwork for future research in this field, emphasize the utility of non-invasive imaging methods such as cCTA for studying these factors, and illustrate the morphological correlates of their role in the pathogenesis of atherosclerosis at cCTA.
Many studies have been aimed at better understanding the relationship between the preferential distribution of atherosclerosis and endothelial shear stress. The most widely accepted theory is that the blood moving across the arterial wall produces a tangential drag force, which is a function of blood velocity and viscosity. This force seems to be responsible for morphological changes of the endothelial cells, leading to increased permeability to atherogenic lipoprotein particles [16]. During the cardiac cycle the vessel wall is subjected to diastolic and systolic pressure producing an oscillating shear stress, which potentially promotes atherosclerotic plaque formation [17]. In non-branching vessel segments, the lowest shear stress typically prevails in the myocardial side of the vessel wall [2, 16], which is where we observed the significant majority of eccentric plaques based on cCTA. More than half of coronary plaques in our study were found at or close to the level of vessel bifurcations. That turbulent flow at vessel bifurcations renders these regions susceptible to disease formation has been know for over a century [13] and is thought to be related to mechanical damage to the vessel endothelium [9, 10, 16, 17]. While our investigation lacks data to directly link plaque distribution with (local) hemodynamics, we believe that our results illustrate the manifestation of this well-established disease mechanism at cCTA and demonstrate that observations obtained at CT can be categorized according to taxonomies initially established based on invasive techniques, such as the Medina classification [11]. Such classifications were established to facilitate planning and predicting the success of percutaneous coronary interventions. Our observation that findings at non-invasive cCTA directly translate into pertinent classification systems may further enhance the nascent role of cCTA as a helpful means for planning complex interventions [18].
The non-invasive characterization of coronary artery plaques with CT has been a topic of intense research. It has been repeatedly demonstrated that CT can reliably detect non-calcified plaque [19–21]. However, further differentiation into more lipid-rich versus predominantly fibrous lesions has been recognized as challenging because of the substantial overlap in HU attenuation of these tissues [19] and the many confounding factors interfering with reliable plaque attenuation measurements, such as volume averaging or the influence of intra-arterial contrast medium attenuation [22]. While much attention has been paid to tissue characterization at CT for identifying lesions at risk of rupture, basic observations such as plaque location have been neglected to date. In our investigation, we made no attempt to further characterize non-calcified plaque components into more lipid-rich or fibrous tissue because of the aforementioned limitations. However, we find it intriguing that the vast majority of plaques with non-calcified components were observed in areas of the vessel wall that are most exposed to the above-discussed factors promoting initial plaque deposition, i.e., the myocardial vessel wall with low endothelial shear stress and vessel bifurcations with turbulent flow. Thus, it appears that the factors that are involved in the de novo initiation of atherogenesis are also partially responsible for the perpetuation and escalation of the atherosclerotic response as non-calcified plaque components are ordinarily considered a sign of greater disease activity as compared to purely calcified lesions [23]. Such notions are in good agreement with recent invasive in vivo serial studies using IVUS [24] that demonstrate that areas with low endothelial shear stress promote plaque progression over time. These authors [24] conclude that early in vivo identification of arterial subsegments likely to develop high-risk plaque characteristics may allow for selective interventions to avoid adverse cardiac outcomes. This implies pre-emptive intervention (e.g., with stent placement) on non-obstructive lesions that exhibit high-risk features [25], a concept that is unlikely to move into the clinical mainstream in the foreseeable future. Extrapolating pertinent invasive findings, however, it is conceivable that one time or (in select cases) serial assessment with non-invasive cCTA of high-risk individuals may be utilized to assess for high-risk plaque characteristics based on spatial distribution and composition of lesions, among other features. This may contribute to the quest for using non-invasive testing to identify not necessarily the “vulnerable” plaque, but more fundamentally the individual vulnerable to major adverse cardiac events [26] for better risk stratification, risk factor management, and disease prevention, which is more in accord with the systemic nature of atherosclerotic disease.
With this preliminary study, we aimed at investigating the predilection sites for plaque initiation using cCTA in the context of currently accepted paradigms concerning the influence of mechanical factors in atherogenesis. Our study is purely descriptive, and the immediate relevance for current clinical patient management is limited. However, in review of the available literature, which is only very selectively referenced in this current contribution, the role of local coronary artery motion patterns and fluid dynamics in plaque deposition and progression is a topic of intense research. While current investigations mainly use invasive means, such as IVUS, the development of techniques for analyzing these factors based on non-invasive imaging is currently underway [14, 15], so that our observations may lay the very basic groundwork for future investigations in this field. For instance, our preliminary description of preferential deposition of certain plaque types (e.g., eccentric versus concentric, purely calcified versus mixed or non-calcified) in distinct vascular micro-environments as assessed by cCTA may lend itself to the non-invasive study of mechanisms of vascular remodeling [27, 28], which dictate the propensity of atherosclerotic lesions to manifest as chronic obstructive versus acute occlusive disease [29]. Similarly, patterns of plaque formation in correlation to severity and duration of exposure to cardiovascular risk factors or changes in response to specific therapy [30] may be other worthwhile aspects of future research, but were not investigated in more detail within the limited scope of this initial description.
Despite these limitations, we believe that we were able to confirm earlier findings obtained in invasive or ex-vivo fashions regarding mechanisms of atherosclerotic plaque formation and to demonstrate the ability of cCTA to study these mechanisms non-invasively with the potential to enhance our understanding of the pathogenesis of coronary atherosclerosis.
References
Sabbah HN, Khaja F, Hawkins ET et al (1986) Relation of atherosclerosis to arterial wall shear in the left anterior descending coronary artery of man. Am Heart J 112:453–458
Jeremias A, Huegel H, Lee DP et al (2000) Spatial orientation of atherosclerotic plaque in non-branching coronary artery segments. Atherosclerosis 152:209–215
Bruschke AV, Wijers TS, Kolsters W, Landmann J (1981) The anatomic evolution of coronary artery disease demonstrated by coronary arteriography in 256 nonoperated patients. Circulation 63:527–536
Meier B, Gruentzig AR, Hollman J, Ischinger T, Bradford JM (1983) Does length or eccentricity of coronary stenoses influence the outcome of transluminal dilatation. Circulation 67:497–499
Mintz GS, Popma JJ, Pichard AD et al (1996) Limitations of angiography in the assessment of plaque distribution in coronary artery disease: A systematic study of target lesion eccentricity in 1,446 lesions. Circulation 93:924–931
Wang JC, Normand SL, Mauri L, Kuntz RE (2004) Coronary artery spatial distribution of acute myocardial infarction occlusions. Circulation 110:278–284
Farb A, Burke AP, Tang AL et al (1996) Coronary plaque erosion without rupture into a lipid core: A frequent cause of coronary thrombosis in sudden coronary death. Circulation 93:1354–1363
Kolodgie FD, Virmani R, Burke AP et al (2004) Pathologic assessment of the vulnerable human coronary plaque. Heart 90:1385–1391
Katritsis DG, Pantos J, Efstathopoulos E (2007) Hemodynamic factors and atheromatic plaque rupture in the coronary arteries: from vulnerable plaque to vulnerable coronary segment. Coron Artery Dis 18:229–237
Finet G, Ohayon J, Rioufol G et al (2007) Morphological and biomechanical aspects of vulnerable coronary plaque. Arch Mal Coeur Vaiss 100:547–553
Medina A, Suarez de Lezo J, Pan M (2006) A new classification of coronary bifurcation lesions. Rev Esp Cardiol 59:183
Nikolaou K, Sagmeister S, Knez A et al (2003) Multidetector-row computed tomography of the coronary arteries: predictive value and quantitative assessment of non-calcified vessel-wall changes. Eur Radiol 13:2505–2512
Virchow RLK. (1856) “Thrombose und Embolie. Gefässentzündung und septische Infektion”, Gesammelte Abhandlungen zur wissenschaftlichen Medicin. Frankfurt am Main: Von Meidinger & Sohn, 219–732
Chandran KB, Wahle A, Vigmostad SC, Olszewski ME, Rossen JD, Sonka M (2006) Coronary arteries: imaging, reconstruction, and fluid dynamic analysis. Crit Rev Biomed Eng 34:23–103
Wahle A, Lopez JJ, Olszewski ME et al (2006) Plaque development, vessel curvature, and wall shear stress in coronary arteries assessed by X-ray angiography and intravascular ultrasound. Med Image Anal 10:615–631
Gibson CM, Diaz L, Kandarpa K, Sacks FM, Pasternak RC, Sandor T, Feldman CL, Stone PH (1993) Relation of vessel wall shear stress to atherosclerosis progression in human coronary arteries. Arterioscler Thromb 13:310–315
Ku DN, Giddens DP, Zarins CK, Glagov S (1985) Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5:293–302
Otsuka M, Sugahara S, Umeda K et al (2008) Utility of multislice computed tomography as a strategic tool for complex percutaneous coronary intervention. Int J Cardiovasc Imaging 24:201–210
Becker CR, Nikolaou K, Muders M et al (2003) Ex vivo coronary atherosclerotic plaque characterization with multi-detector-row CT. Eur Radiol 13:2094–2098
Leber AW, Knez A, Becker A et al (2004) Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: a comparative study with intracoronary ultrasound. J Am Coll Cardiol 43:1241–1247
Ferencik M, Chan RC, Achenbach S et al (2006) Arterial wall imaging: Evaluation with 16-section multidetector CT in blood vessel phantoms and ex vivo coronary arteries. Radiology 240:708–716
Cademartiri F, Mollet NR, Runza G et al (2005) Influence of intracoronary attenuation on coronary plaque measurements using multislice computed tomography: observations in an ex vivo model of coronary computed tomography angiography. Eur Radiol 15:1426–1431
Burke AP, Weber DK, Kolodgie FD, Farb A, Taylor AJ, Virmani R (2001) Pathophysiology of calcium deposition in coronary arteries. Herz 26:239–244
Stone PH, Coskun AU, Kinlay S et al (2007) Regions of low endothelial shear stress are the sites where coronary plaque progresses and vascular remodelling occurs in humans: an in vivo serial study. Eur Heart J 28:705–710
Young JJ, Phillips HR, Marso SP et al (2008) Vulnerable plaque intervention: State of the art. Catheter Cardiovasc Interv 71:367–374
Naghavi M, Libby P, Falk E et al (2003) From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Parts I and II. Circulation 108:1664–1778
Pasterkamp G, Galis ZS, de Kleijn DPV (2004) Expansive arterial remodeling: Location, location, location. Arterioscler Thromb Vasc Biol 24:650–657
Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM (2000) Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: An intravascular ultrasound study. Circulation 101:598–603
Beckman J, Ganz J, Creager M, Ganz P, Kinlay S (2001) Relationship of clinical presentation and calcification of culprit coronary artery stenoses. Arterioscler Thromb Vasc Biol 21:1618–1622
Ramaswamy SD, Vigmostad SC, Wahle A et al (2006) Comparison of left anterior descending coronary artery hemodynamics before and after angioplasty. J Biomech Eng 128:40–48
Author information
Authors and Affiliations
Corresponding author
Additional information
PS is a medical consultant for Bayer-Schering. CT is a medical consultant for Medrad. PC is a medical consultant for Bracco and receives research support from Siemens. UJS is a medical consultant for Bayer-Schering, Bracco, General Electric, Siemens, and TeraRecon and receives research support from Bayer-Schering, Bracco, General Electric, Medrad, and Siemens.
Rights and permissions
About this article
Cite this article
Enrico, B., Suranyi, P., Thilo, C. et al. Coronary artery plaque formation at coronary CT angiography: morphological analysis and relationship to hemodynamics. Eur Radiol 19, 837–844 (2009). https://doi.org/10.1007/s00330-008-1223-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-1223-3