Abstract
The aim of this study was to investigate the potential of dose reduction in multidetector computed tomography (MDCT) by current-modulated automatic exposure control (AEC) and to test the reliability of the dose estimation by the conventional CT dosimetry program CT-EXPO, when an average tube current is used. Phantom measurements were performed at a CT system with 64 detector rows for four representative examination protocols, each without and with current-modulated AEC. Organ and effective doses were measured by thermoluminescence dosimeters (TLD) at an anthropomorphic Alderson phantom and compared with those given by the calculation with CT-EXPO. The application of AEC yielded dose reductions between 27 and 40% (TLD measurements). While good linearity was observed between measured and computed effective dose values both without and with AEC, the organ doses showed large deviations between measurement and calculation. The dose to patients undergoing a MDCT examination can be reduced considerably by applying a current-modulated AEC. Dosimetric algorithms using a constant current–time product provide reliable estimates of the effective dose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The benefits of medical imaging are immense and have revolutionized the practice of medicine [1]. On the other hand, the increasing use of imaging modalities utilizing ionizing radiation has resulted in a rise of the collective radiation exposure. A detailed analysis reveals that this increase is mainly caused by CT examinations. In Germany, for example, they account for about 7% of all X-ray examinations performed in the year 2004 but for more than 54% of the resultant collective effective dose [2]. Even higher numbers are reported for the USA and Japan [1, 3, 4].
In order to compensate for this trend—at least in part—the dose per CT examination should be reduced as far as reasonably achievable. An effective approach is the application of an automatic exposure control (AEC), which can reduce the patient dose in multidetector computed tomography (MDCT) to a considerable extent without affecting the image quality [5–8]. When utilizing this promising technology, however, the estimation of the patient dose by the radiologist and thus the optimisation of the CT protocols becomes problematic, because the dosimetric algorithms implemented in software tools widely used for dose estimation do not take into account the varying tube current during the course of a tube rotation on the dose distribution within the patient. The software rather uses a constant tube current.
The aim of this study was thus twofold: (a) to investigate the potential of dose reduction in MDCT by using a ‘state-of-the-art’ current-modulated AEC for different body regions and (b) to assess for these regions the reliability of the dosimetry program CT-EXPO [9] when using the average tube current displayed on the operator’s console. To this end, dose measurements were performed with thermoluminescent dosimeters (TLDs) at an anthropomorphic Alderson phantom. The influence of the AEC on the image quality was not investigated. This has been done in previous studies [5–8] and is not of relevance here.
Methods and materials
CT system and automatic exposure control
All measurements were performed on a MDCT system with 64 detector rows (Somatom Sensation 64; Siemens, Erlangen, Germany) equipped with an up-to-date AEC module (Care Dose 4D). This tube current-modulated AEC adapts the current to the patient’s individual anatomy and modulates the current both along and perpendicular to the longitudinal (z) axis of the patient.
In the first step, the attenuation along the z-axis is analysed by a scout view, and then the tube current is adapted for each rotation of the X-ray tube. This is done relative to a reference current, which would be applied without AEC for an average patient to achieve an adequate image quality (z-axis AEC). In the second step, the attenuation for all projection angles is measured during each CT tube rotation in order to modulate the tube current in real time to compensate for differences in attenuation between lateral and anterior-posterior projections (rotational AEC). The current modulation during rotational AEC reflects the patients asymmetry, with less modulation occurring in regions where the patient is more circular [10].
CT protocols
Measurements were performed for four selected body regions at an anthropomorphic Alderson phantom: a whole-body, thoracic and pelvic CT examination, as well as a carotid CT angiography (CTA; see Fig. 1). Typical CT protocols used in the particular hospital were applied, each with constant tube current (reference current) and with the current-modulated AEC. All measurements were performed with the AEC degree “weak”, which is appropriate to slim patients. The parameters of the CT examinations carried out are given in Table 1. For each body region, the axial field-of-view was defined on the basis of a scout view. As mentioned above, the scout view was also used by the AEC to determine the attenuation along the z-axis. Scout views were acquired with identical parameters—and thus the same radiation exposure—for measurements performed without and with AEC. For this reason, their relatively low dose contribution (less than 6% of the total dose [11]) is not explicitly taken into account when investigating the dose reduction potential by AEC.
Dose estimation
Dose measurements were performed at an anthropomorphic whole-body Alderson RANDO phantom (Alderson Research Laboratories Inc; Long Island City, New York, USA) consisting of a human skeleton embedded in plastic material that is radio-equivalent to soft tissue (length of trunk, 68 cm; length of neck and head, 23 cm). The phantom is transected into transaxial cross sections (thickness, 2.5 cm) with holes drilled on a 3 cm × 3 cm grid. The holes were plugged either by tissue-equivalent pins or by holder pins for thermoluminescent dosimeters (TLDs). Dose measurements were performed with lithium fluoride (TLD-100; Bicron-Harshaw, Cleveland, Ohio, USA) rods (size, 1 × 1 × 6 mm3) and chips (size, 3.2 × 3.2 × 0.9 mm3). The TLDs were calibrated for absorbed dose in water using conventional X-ray equipment with a tube potential of 120 kV and a filter of 5-mm aluminium to approximate the radiation quality of the CT system. This is an appropriate approach since mass energy absorption coefficients for soft tissues differ by less than 4% from the corresponding value for water in the range of photon energies used for CT imaging [12]. The resulting error in the dose estimated for bone surface can be neglected because this quantity contributes to the effective dose only with a tissue weighting factor of w = 0.01.
Individual calibration, annealing and readout of the TLDs were performed following a standard procedure [13]. For each measurement on the Alderson phantom, 180 TLD rods were suitably distributed inside and more than 83 TLD chips at the surface of the phantom to sample the nonuniform dose distribution.
To relate the position of the TLDs in the Alderson phantom to the various tissues and organs of the human body, pictures of gross anatomical sections of the ‘visible human’ [14] were matched to the size of the 36 transaxial cross sections of the Alderson phantom using structures of the spine as landmarks. The size and position of the relevant organs were transferred to transparent paper which was fixed on the corresponding phantom sections. For smaller organs, equivalent doses were obtained by taking the mean of the dose values recorded by the TLDs within the specified organs, whereas for extended organs (lung, skin, bone and red bone marrow) equivalent doses were estimated using the scheme presented by Huda and Sandison [15]. Finally, the effective dose was calculated from the tissue and organ equivalent doses using the tissue weighting factors given in ICRP publication 60 [16].
Uncertainties in the TLD measurements were determined according to the “Guidelines for evaluating and expressing the uncertainty of NIST measurement results” [17]. The combined uncertainty for a single TLD dose measurement was estimated to be 9%, taking into account the statistical uncertainty of repeated TLD readings (3%) as well as systematic uncertainties arising from the energy dependence of the TLDs for the photon energies used for CT imaging CT (3%), the dependence of the TLD response on the direction of the incident radiation (3%) and the uncertainty in the calibration of the ionisation chamber (2%). Uncertainties in the organ and effective doses were calculated from the corresponding TLD uncertainties according to the laws of propagation of uncertainty. An additional uncertainty (3%) was taken into account, which arises when deriving absorbed doses to individual organs of the Alderson phantom from TLD doses measured directly at a only a few reference points [18].
For each CT examination performed on the Alderson phantom, the effective dose was also computed with CT-EXPO (version V1.5; Hamburg/Hannover, Germany; module ‘calculate’). This PC program is based on Monte Carlo data published by the Helmholtz Center Munich [19] and has been described in detail elsewhere [20, 21]. In contrast to the program version evaluated in a previous phantom study [22], version V1.5 takes into account the z-overscanning effect. As already mentioned, for examinations performed with AEC the variability of the tube current is neglected by CT-EXPO. Instead, the mean current–time product (mAs) was used for dose estimation, which means that all organ doses are reduced by the same factor, corresponding to the reduction of the tube current.
Correlation between measured and computed effective dose values was tested by calculating Spearman’s rank correlation coefficient (r S) using the program package SigmaStat (version 2.03; SPSS Science Software, Erkrath, Germany). In addition, a linear regression analysis was performed.
Results
The effective doses calculated for the eight different CT examinations are plotted in Fig. 2 versus the corresponding dose values determined experimentally on the basis of TLD measurements on the Alderson phantom. Figure 2 reveals that the line-of-identity is within the predicted 95% confidence bands of the regression line. Statistical analysis yielded a high correlation (P < 0.001; r S = 0.976) between calculated and measured effective dose values. For the AEC module investigated in this study, the dose reduction factors determined for the four CT protocols are summarized in Table 2. The TLD measurements yielded reduction factors for the effective dose between 27% (thoracic CT) and 40% (pelvic CT). The PC program CT-EXPO provided comparable results, with dose reduction factors between 26 and 45%. Somewhat larger deviations between TLD measurements and CT-EXPO occurred only for the carotid CTA performed in the neck-shoulder region of the Alderson phantom. For this region, CT-EXPO overestimated the dose reduction compared with the TLD measurements (45% vs. 38%).
Correlation between the effective dose values determined by the program CT-EXPO and TLD measurements for the four CT examinations (circles whole-body; diamonds pelvis; squares thorax; triangles carotid CTA) performed in this study without (filled symbols) and with (open symbols) current-modulated AEC. The solid line gives the result of a linear regression analysis (slope, 0.913; regression coefficient, r S = 0.976) and the dotted curves the 95% confidence interval
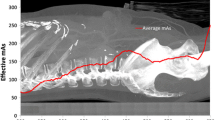
Figure 3 shows the dose profiles (derived from TLD measurements) along the longitudinal axis of the Alderson phantom for whole-body CT examinations carried out with and without AEC. As expected, the amount of the dose reduction varies considerably along the phantom with stronger reduction in the head-neck region compared with the rest of the body.
Axial dose profile in the Alderson phantom (TLD dose in the centre of each slice) for a whole-body CT examination, performed with and without AEC. The CT parameters are summarized in Table 1
For the four CT protocols considered, dose values for tissues and organs with high tissue weighting factors are plotted in Fig. 4. The highest organ doses were observed for the whole-body CT examination performed without AEC. For this type of examination, the organ doses estimated from the TLD measurements varied between 35.4 ± 2.0 mGy (thyroid) and 5.7 ± 0.4 mGy (testes). The organ doses calculated by CT-EXPO differed markedly from the corresponding dose values derived from the TLD measurements. For organs located fully in the volume of directly irradiated tissue, deviations were between −22 and +35%. Larger deviations, in individual cases up to a factor of 2, were observed for organs at the border or outside the axial body region directly irradiated. Averaged over all organs considered, the over- and underestimation of the individual organ doses compensated one another to some extent, and the mean organ dose deviation between TLD measurement and calculation with CT-EXPO was −4% for the whole-body CT examination, +13% for the thorax −1% for the pelvis and +3% for carotid CT angiography. The application of AEC enlarged the mean deviation between organ doses only slightly to −6% for the whole-body CT examination, +14% for the thorax, −2% for the pelvis and −6% for the CT carotid angiogram. In the case of the CT carotid angiogram, the change in the mean deviation from +3% without AEC to −6% with AEC results mainly from the overestimation of the reduction of the lung dose by CT-EXPO (46%) compared with the TLD measurements (14%).
Comparison of organ doses estimated by TLD measurements on the Alderson phantom and the program CT-EXPO for four different CT examinations performed without and with AEC. a whole-body CT examination, b CT carotid angiography, c thoracic CT and d pelvic CT (cf. Fig. 1). (The ‘missing’ legend in a is identical to that shown in the other figures. The error bars give the uncertainties in the TLD measurements)
Discussion
Nowadays all vendors of CT systems offer AEC modules in order to reduce radiation exposure to patients undergoing a CT examination. Parallel to this encouraging development, however, it is necessary to provide reliable dosimetry programs to the practitioner that can be used to make a rough estimate of the patient dose as a measure for the optimization of CT examinations.
As shown in a previous study for ten different CT systems [22] and in the present study (Fig. 2), the effective doses estimated with the PC-program CT-EXPO correspond quite well to the results of TLD measurements performed on the Alderson phantom. However, this does not apply to organ doses, which can differ considerably between measurement and calculation (see Table 2). This is mainly due to differences in the size, form, density and arrangement of the relevant organs in the mathematical phantoms “Adam” and “Eva” used in CT-EXPO and in the Alderson phantom. In particular, considerable differences can occur for organs at the border or outside of the examined body region, as the arrangement, size and shape of the organs in the mathematical phantom often do not correspond to the real anatomy of man [23]. Organs situated in the Alderson phantom at the border or partly inside the body region directly irradiated may be located in the mathematical model completely inside or outside of this region, so that the doses are over- or underestimated, respectively. A reliable comparison of organ doses is thus only possible for organs that lie in both models completely within the body region directly irradiated. This methodological problem, however, has no substantial effect on the determination of effective dose values as the relatively low doses to organs outside the directly irradiated body region do not contribute considerably to the effective dose. Furthermore, the over- and underestimation of the individual organ doses compensate one another when the effective dose is computed by the averaging process. In this study, deviations of organ doses were observed up to 35% for organs located completely inside the examination volume and up to a factor of 2 for organs at the border or outside of the examination volume. In spite of these deviations, the effective doses estimated with CT-EXPO corresponded sufficiently well to the results of the TLD measurements.
Radiation exposure of patients undergoing CT examinations can be reduced considerably by the use of current-modulated AEC. For the CT system and the CT protocols investigated in this study, the dose reduction factor determined for the effective dose was between 27 and 40%.
Although the variable tube current leads to a different dose distribution in the individual organs of the phantom for examinations performed with AEC versus examinations with constant tube current, this has no marked effect on the determined effective dose. Only for CT examinations carried out in the asymmetric neck-shoulder region does CT-EXPO overestimate the dose reduction slightly because of disregarding the variable tube current. The TLD measurements reflect the real decrease in the organ doses due to the variable tube current, which is stronger for the thyroid in the thin neck region, but less for the lungs and other organs in the thicker body region. The calculation with CT-EXPO neglects this difference and all organ doses are reduced by the same factor, which leads to an overestimation of the achieved dose reduction relative to the measurement. In the case of CT examinations of real patients, the relative reduction of the effective dose achieved by the implemented AEC module is—to a good approximation—given by the ratio of the effective and the reference tube current displayed on the operator’s console.
As organ doses computed by conventional CT dosimetry programs can deviate considerably from that occurring in real patients, this aspect needs to be carefully addressed when performing risk analyses. When the total radiation risk to patients from CT examinations has to be estimated, we recommend the use of the organ doses given by the dosimetry program as best dose estimates, since errors in organ doses compensate one another to a large extent when computing the total risk. In contrast, when the risk to the embryo from an MDCT examination of a female patient in the very early stages of pregnancy—conducted either based on a stringent clinical indication or due to the unawareness of pregnancy—has to be determined, a conservative estimate of the uterine dose, that is used as surrogate for the dose to the embryo, should be used that takes into account the uncertainty budgets of the dosimetry program documented in the user manual.
Conclusion
Current-modulated AEC should be applied as far as possible as it can markedly reduce radiation exposure to patients undergoing MDCT examinations. For CT examinations performed with an AEC, conventional dosimetry programs yield—at least for the body regions investigated in this study—appropriate estimates of the effective dose when using the average tube current displayed after the examination on the operator’s console. In contrast, computed organ doses may diverge largely from measured values.
References
Amis ES Jr, Butler PF, Applegate KE, Birnbaum SB, Brateman LF, Hevezi JM, Mettler FA, Morin RL, Pentecost MJ, Smith GG, Strauss KJ, Zeman RK (2007) American College of Radiology. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol 4:272–284
Umweltradioaktivität und Strahlenbelastung im Jahr 2006. Deutscher Bundestag Drucksache 16/6835, 2007, http://dip21.bundestag.de/dip21/btd/16/068/1606835.pdf. Accessed 15 May 2008
Mettler FA (2007) Magnitude of radiation uses and doses in the United States: NCRP Scientific Committee 6-2 Analysis of Medical Exposures. In: Forty-third annual meeting of the National Council on Radiation Protection and Measurement (NCRP) Advances in radiation protection in medicine, pp 9−10
Nishizawa K, Matsumoto M, Iwai K, Maruyama T (2004) Survey of CT practice in Japan and collective effective dose estimation. Nippon Acta Radiol 64:151–158
Greess H, Wolf H, Suess C, Kalender WA, Bautz W, Baum U (2004) Dosisautomatik bei der Mehrzeilen-CT: Phantommessungen und klinische Ergebnisse. Fortschr Röntgenstr 176:862–869
Graser A, Wintersperger BJ, Suess C, Reiser MF, Becker CR (2006) Dose reduction and image quality in MDCT colonography using tube current modulation. AJR:187 September 2006:695–701
Russel MT, Fink JR, Rebeles F, Kanal K, Ramos M, Anzai Y (2008) Balancing radiation dose and image quality: clinical application of neck volume CT. AJNR Am J Neroradiol. doi:10.3174/ajnrA0891
Kalender WA, Buchenau S, Deak P, Kellermeister M, Langner O, van Straten M, Vollmar S, Wilharm S (2008) Technical approaches to the optimisation of CT. Phys Med 24(2):71–79
Stamm G, Nagel HD (2002) CT-Expo − ein neuartiges Programm zur Dosisevaluierung in der CT. Fortschr Rontgenstr 174:1570–1576
Keat N (2005) CT scanner automatic exposure control systems. MHRA Report 05016
Brix G, Lechel U, Glatting G, Ziegler S, Münzing W, Müller S, Beyer T (2005) Radiation exposure of patients undergoing whole-body dual modality 18F-FDG PET/CT examinations. J Nucl Med 46:608–613
ICRU Publication 17 (1970) Radiation dosimetry: X rays generated at potentials of 5 to 150 kV. ICRU, Washington, DC
European Commission (2000) Report EUR 19604 EN: recommendations for patient dosimetry in diagnostic radiology using TLD
Jastrow W (2008) Atlas of human sections in [sic] the internet. Labelling of sections from the visible human project. http://www.uni-mainz.de/FB/Medizin/Anatomie/workshop/englWelcome.html. Accessed 15 May 2008
Huda W, Sandison GA (1984) Estimation of mean organ doses in diagnostic radiology from Rando phantom measurements. Health Physics 47:463–467
ICRP Publication 60 (1991) 1990 recommendations of the International Commission on Radiological Protection. Annals of the ICRP vol 21/1-3. Elsevier Science, Oxford
Taylor B, Kuyatt C (2001) Guidelines for evaluating and expressing the uncertainty of NIST measurement results. National Institute of Standards and Technology, Gaithersburg, MD. http://physics.nist.gov/Pubs/guidelines/TN1297/tn1297s.pdf. Accessed 15 May 2008
Aschan C (1999) Applicability of thermoluminescent dosimeters in x-ray organ dose determination and in the dosimetry of systemic and boron neutron capture radiotherapy. University of Helsinki HU-P-D77
Zankl M, Panzer W, Drexler G (1991) The calculation of dose from external photon exposures using reference human phantoms and Monte Carlo methods. Part IV. Organ doses from tomographic examinations. GSF report 30/91. Neuherberg
Galanski M, Nagel HD, Stamm G (2001) CT-Expositionspraxis in der Bundesrepublik Deutschland. RoFo 173:R1–R66
Nagel HD, Galanski M, Hidajat N, Maier W, Schmidt T (2002) Radiation exposure in computed tomography – fundamentals, influencing parameters, dose assessment, optimization, scanner data, terminology, 4th edn. CTB, Hamburg
Brix G, Lechel U, Veit R, Truckenbrodt R, Stamm G, Coppenrath EM, Griebel J, Nage HD (2004) Assessment of a theoretical formalism for dose estimation in CT: an anthropomorphic phantom study. Eur Radiol 14:1275–1284
Hidajat N, Vogel T, Schröder RJ, Felix R (1996) Berechnete Organdosen und effektive Dosis für die computertomographische Untersuchung des Thorax und Abdomens: Sind diese Dosen realistisch. RoFo 164:382–387
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lechel, U., Becker, C., Langenfeld-Jäger, G. et al. Dose reduction by automatic exposure control in multidetector computed tomography: comparison between measurement and calculation. Eur Radiol 19, 1027–1034 (2009). https://doi.org/10.1007/s00330-008-1204-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-1204-6