Abstract
(1) To assess the degree of focal cartilage abnormalities in physically active and sedentary healthy subjects as well as in patients with early osteoarthritis (OA). (2) To determine the diagnostic value of T2 and T1rho measurements in identifying asymptomatic physically active subjects with focal cartilage lesions. Thirteen asymptomatic physically active subjects, 7 asymptomatic sedentary subjects, and 17 patients with mild OA underwent 3.0-T MRI of the knee joint. T1rho and T2 values, cartilage volume and thickness, as well as the WORMS scores were obtained. Nine out of 13 active healthy subjects had focal cartilage abnormalities. T1rho and T2 values in active subjects with and without focal cartilage abnormalities differed significantly (p < 0.05). T1rho and T2 values were significantly higher (p < 0.05) in early OA patients compared to healthy subjects. T1rho measurements were superior to T2 in differentiating OA patients from healthy subjects, yet T1rho was moderately age-dependent. (1) Active subjects showed a high prevalence of focal cartilage abnormalities and (2) active subjects with and without focal cartilage abnormalities had different T1rho and T2 composition of cartilage. Thus, T1rho and T2 could be a parameter suited to identify active healthy subjects at higher risk for developing cartilage pathology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is anticipated that by the year 2020 an estimated 18% of the population in the US will be affected by osteoarthritis (OA) [1]. It is the most common joint disease and the second most common cause of long-term chronic disability among subjects over the age of 50 [2]. Therefore, it is associated with substantial cost to the individual and the society [3].
Improved treatment options like cartilage resurfacing procedures and disease-modifying drugs require the development of non-invasive techniques that allow early diagnosis of OA [4, 5]. Additionally, it is most likely that patients benefit from the early identification of significant risk factors.
Magnetic resonance imaging (MRI) is the most important noninvasive diagnostic technique for the assessment of cartilage. Up to now clinical MRI has focused mostly on the visualization of the advanced disease [6, 7]. With the development of MR sequences, which best delineate the cartilage from the surrounding tissue, it has also become possible to quantify cartilage morphology: loss of volume and average thickness could be demonstrated during the progress of OA [8, 9]. However, since cartilage does not regenerate and cartilage loss is irreversible, it is crucial to diagnose degeneration before cartilage loss occurs. Early events in the development of cartilage matrix breakdown are the loss of proteoglycans (PG), changes in water content, and molecular level changes in collagen [10]. Recently, high-field MRI imaging techniques at 3.0 T have been introduced that improve visualization of subtle cartilage morphologic changes [11, 12]. Additionally, MR-based methods like delayed gadolinium-enhanced MRI of cartilage (dGEMRIC, [13]) or T1rho and T2 relaxation times are currently being established to quantify cartilage matrix composition regarding PG concentration and collagen integrity.
T1rho and T2 are parameters describing different MR relaxation mechanisms, but both are suited to probe slow motion of water protons.
T2 is spin-spin relaxation related with the energy changes between proton spins themselves. It reflects the ability of free water proton molecules to move and to exchange energy. The estimation of cartilage T2 relaxation times is sensitive to a wide range of water interactions in tissue including macromolecular concentration [14–17], structure of the macromolecules [18], and bulk organization of the extracellular matrix [19]. In particular, it therefore also depends on the content, orientation, and anisotropy of collagen [20, 21]. Consequently, findings from several studies indicate higher T2 relaxation times in cartilage of OA patients compared to healthy controls [22–24], and there is evidence that they are correlated with the severity of the disease [25, 26].
T1rho describes the spin-lattice relaxation in the rotating frame [27]. It is related with the energy changes between proton spins and the environment. T1rho measurements can be used to probe the slow motion interactions between motion-restricted water molecules and their local macromolecular environment [28]. The extracellular matrix in the articular cartilage provides a motion-restricted environment to water molecules. Changes to the extracellular matrix, such as PG loss, may be reflected in altered T1rho values. Therefore, in vitro studies showed correlations between changes in PG and T1rho [29–31] and between changes in T1rho and mechanical properties of bovine cartilage explants [32]. In vivo studies showed increased cartilage T1rho values in OA subjects compared to controls [33–35], which reflects the potential for T1rho imaging for non-invasive evaluation of diseased cartilage.
Many sports are associated with a potential risk for long-term sequelae, such as joint degeneration related to a high mechanical stress [22]. Chronic increased joint load can cause damage and degeneration of the joint structures that initiate or accelerate OA [36]. Consequently, studies have shown that subjects engaged in certain professional sports have an accelerated course of this disease [37, 38]. However, little is known how vigorous recreational activity reflects joint function.
The aims of our study were therefore (1) to examine MR-based parameters of the biochemical cartilage matrix composition of the knee joint by determining T1rho and T2 values in physically active and sedentary healthy subjects as well as in patients with early OA, (2) to assess the prevalence and degree of focal cartilage abnormalities in these groups, and (3) to determine the diagnostic value of T2 and T1rho measurements in identifying asymptomatic physically active subjects with focal cartilage abnormalities.
Materials and methods
Patients and clinical assessment
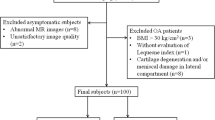
Seventeen patients with mild osteoarthritis (OA) (54.00 ± 9.98 years, 8 male, 9 female) and 20 healthy controls (33.65 ± 9.44 years, 10 male, 10 female) were recruited for the study. Inclusion criteria for all subjects were good health by medical history, physical examination, and clinical laboratories, as well as no contraindications for the use of MRI. Additional inclusion criteria for the patients were mild radiographic signs of OA [grade 1–2 based on the Kellgren-Lawrence (KL) score [39]] and clinical symptoms of knee OA (according to the ACR criteria [40]). Patients with inflammatory arthritis were excluded and also those with knee OA secondary to other causes (acute or chronic infection, metabolic abnormalities, previous surgery or history of intra-articular fracture).
Controls had no clinical evidence of knee OA. They were classified into a physically ‘sedentary’ control group and into a physically ‘active’ control group by use of the Tegner score [41]. The Tegner activity level score is a quantitative grading method, which was originally developed to document activity levels of patients with anterior cruciate ligament insufficiency. It reflects the amount of biomechanical loading on the knee joint. The amount of physical activity is rated numerically on a scale from 0 to 10. Lowest values are assigned to subjects who are disabled (score 0) or only able to perform sedentary work (score 1) because of knee problems. Participation in national or international contact sports yields the highest score of 10 points. Seven subjects were assigned to the ‘sedentary’ group (range of Tegner score 1–5, 35.43 ± 10.66 years, 4 male, 3 female); the remaining 13 subjects formed the ‘active’ group (range of Tegner score 6–10, 32.69 ± 9.02 years, 6 male, 7 female, performing recreational sports like long-distance running, biking, skiing, hiking). Given the assignement criteria, the Tegner score in active controls [median value (25th percentile, 75th percentile): 6 (6, 6)] was significantly higher (p < 0.001) than in sedentary controls [4 (4, 5)]. The average duration of regular physical exercise was 13.6 ± 8.41 years in the active group. Sedentary controls had a mean sedentary life style of 16.3 ± 10.16 years.
A standardized questionnaire (Western Ontario and McMaster Universities Pain, Stiffness, and Physical Function scales, WOMAC) for measuring the degree of pain, functional impairment, and stiffness in all subjects through a 5-point scale (none, slight, moderate, severe, and extreme) was received before the MRI examination [42]. Written informed consent was obtained from all subjects after the nature of the examinations had been fully explained. All MR examinations were performed in accordance with the rules from the local Committee on Human Research.
Imaging
In the OA patients, the following radiographs of the knee were obtained to determine the Kellgren-Lawrence score: (1) bilateral standing flexion weight-bearing view, (2) 30° flexion lateral, and (3) bilateral patello-femoral, sunrise views.
MRI imaging of the signal knee joint in OA patients and of the dominant knee joint in controls was performed on a 3.0-T system (Signa, GE Medical Systems, Waukesha, WI) using a transmit/receive quadrature knee coil (Clinical MR Solutions, Brookfield, WI).
The MRI protocol included eight pulse sequences. The morphology of cartilage and other knee structures was assessed with three standard sequences and three sagittal cartilage dedicated sequences: (1) a coronal T1-weighted fast spin echo sequence with TR/TE = 667/10 ms, echo train length of 5, matrix of 384 × 256, FOV of 13 cm, two signals acquired, bandwith of 31.25 kHz, acquisition time of 6 min 32 s and section thickness of 3 mm, (2) and (3) a coronal and axial fat-saturated intermediate-weighted fast spin-echo sequence (IM-w FSE) with TR/TE = 4,300/49 ms, echo train length of 9, matrix of 320 × 256, FOV of 13 cm, two signals acquired, bandwith of 31.25 kHz, acquisition time of 8 min 46 s, and section thickness of 4 mm. The cartilage dedicated sequences included: (4) a fat-saturated IM-w FSE with the following parameters: TR/TE 4,300/51 ms, echo train length of 9, number of excitations 2 and acquisition time of 12 min 42 s, 45 sections, FOV of 16 cm (matrix 512 × 256) with an in-plane spatial resolution of 0.293 × 0.293 mm2, a slice thickness of 2 mm, and a section gap of 0.5 mm; (5) a T1-weighted 3D high-spatial-resolution volumetric fat-suppressed spoiled gradient-echo (SPGR) sequence with TR/TE 20/7.5 ms, flip angle of 12°, matrix of 512 × 512, in-plane resolution of 0.293 × 0.293 mm2, FOV of 16 cm, number of excitations 0.75, acquisition time of 7 min 37 s, and slice thickness of 1 mm, and (6) a 3D FIESTA-C (fast imaging employing steady-state acquisition with constructive interference in steady state, [43]) sequence. Parameters for the FIESTA-C sequence were: TR/TE 9/4.1 ms, flip angle of 15°, matrix size 320 × 320, in-plane resolution of 0.254 × 0.254 mm2, slice thickness of 1 mm, FOV of 13 cm, number of excitations 1, and acquisition time of 8 min 42 s.
The biochemical composition of the cartilage matrix was assessed with a T1rho- and a T2-mapping sequence: multi-slice T1rho-weighted images were obtained using spin-lock techniques and spiral image acquisition [34]. The acquisition parameters were as follows: 14 interleaves/slice, 4,096 points/interleaf, FOV of 16 cm, effective in-plane spatial resolution of 0.6 × 0.6 mm, slice thickness of 3 mm, skip of 1 mm, number of slices 16, TR/TE of 2,000/5.8 ms, time of spin-lock (TSL) 20/40/60/80 ms, and a spin-lock frequency of 500 Hz. The total acquisition time was 12 min 42 s. The T2 quantification sequence was also based on spiral sequence [44] with TR/TE of 2,000/6.7, 12, 28, 60 ms. All other prescription parameters of the T2 sequence were the same as the T1rho sequence with a total acquisition time of 10 min 36 s.
MRI scans were performed in one contiguous session without removing the subject from the scanner. Measurements were conducted in the morning hours from 9 to 11 a.m.
Post processing
Cartilage segmentation was performed using in-house software [45] developed with Matlab (Mathworks, Natick, MA). Based on the sagittal SPGR images, articular cartilage was segmented using a semi-automatic technique based on Bezier splines and edge detection, and was defined in six distinct regions: medial/lateral tibia, trochlea, medial/lateral femur, and patella. Subsequently, the medial and lateral femoral condyles were partitioned into weight-bearing and non-weight-bearing portions as described in [46].
The above-mentioned regions were combined to larger units: medial and lateral femoral condyles and trochlea were summarized as “femur,” lateral (medial) tibial plateau and lateral (medial) femoral condyle as “lateral (medial) compartment,” lateral and medial tibial plateau as “tibia,” trochlea and patella as “patello-femoral compartment,” and the whole segmented cartilage of the knee joint as “all.”
Following segmentation, cartilage was transformed into a 3D binary mask with isotropic voxels by using shape-based interpolation with distance fields. Finally, the cartilage thickness was determined by calculating the minimum Euclidean distance from each point on the articular surface to the bone-cartilage interface. The average thickness was calculated for each slice and then averaged for all the slices. The cartilage volume was determined by multiplying the total number of voxels encompassing the cartilage by the actual volume of each voxel.
Studies have shown that variations in joint size have a larger effect on cartilage volume than on cartilage thickness [47]. Therefore, cartilage volume was normalized by dividing it by the maximal epicondylar distance to minimize variation due to joint size.
The T1rho maps were reconstructed by fitting the image intensity pixel-by-pixel to the equation below using a Levenberg-Marquardt mono-exponential fitting algorithm developed in-house [33]:
where TSL is time of spin-lock, S is the signal intensity in T1rho-weighted image with a certain TSL, and S0 is the signal intensity when TSL = 0.
The T2 maps were reconstructed by fitting the image intensity pixel-by-pixel to the equation:
where S is the signal intensity in T2-weighted image with a certain TE, and S0 is the signal intensity when TE = 0.
Both T1rho and T2 maps were automatically registered to the SPGR images with a rigid-body registration technique to reduce effects of knee movement. Cartilage segmentations were then resampled and superimposed on the T1rho and T2- maps to define the regions of interest for T1rho and T2 assessment. Areas of partial volume effects due to fluid and areas of cartilage lesions appeared as visible clusters with elevated values and were manually excluded from the respective maps.
The segmentation and analysis were performed by a single observer (R.S.).
Assessment of focal cartilage abnormalities
Pathologic findings at the knee joint were analyzed using a modified WORMS score [48] by two radiologists (TML and RS with 19 and 5 years’ experience in musculoskeletal imaging, respectively) in a consensus reading. They incorporated all MRI sequences except the T1rho- and T2-mapping sequences. Since the original WORMS score was developed for knees with osteoarthritic changes and we expected at the most only mild degenerative changes in our control subjects, the 15 previously described compartments were merged to a total of 7 compartments: the medial and lateral tibia, trochlea, medial and lateral femur, as well as medial and lateral patella. Flattening or depression of the articular surfaces were not evaluated. The scoring of cartilage signal and morphology, marginal osteophytes, medial and lateral meniscal integrity, synovitis/effusion, intra-articular loose bodies and peri-articular cysts/bursitis, anterior and posterior cruciate ligament integrity, as well as medial and lateral collateral ligament integrity were performed as suggested by the original WORMS score. To characterize the extent of the regional involvement of bone marrow edema and subchondral cysts, the original partitions of the femoral condyles and tibial plateaus in anterior, central, and posterior regions were used. Any other lesions not included in a WORMS category score were also noted. The seven above-mentioned compartments were combined to larger units similiar to those used during the segmentation process (femur, lateral/medial compartment, tibia, patello-femoral compartment, all).
Statistical analysis
Data were initially assessed for normality with the Kolmogorov-Smirnov test. On the basis of these results, we decided to use parametric procedures to compare age, body-mass index (BMI), T1rho and T2 values, as well as cartilage thickness and volume among the three groups of participants. A one-way analysis of variance (ANOVA) and Scheffé post hoc comparisons were used to test for equality of mean values in each data group.
Inter-group differences in WORMS and WOMAC scores as well as in MRI-derived cartilage matrix composition parameters among active subjects with cartilage lesions, active subjects without cartilage lesions, and sedentary subjects were tested with the Kruskal-Wallis test. When statistically significant differences occurred, single posttest comparisons were performed by using the Mann-Whitney U test with Bonferroni correction for multiple comparisons.
The differences in morphological features (WORMS score, cartilage volume, and thickness) between OA patients and healthy asymptomatic subjects were additionally analyzed by combining active and sedentary subjects to one group.
Pearson correlation analysis was employed to examine the correlation of T1rho-/T2 values with age, cartilage volumes, and cartilage thickness within and across the three subject groups. Correlations between T1rho/T2 values and Tegner, WOMAC, and WORMS scores were performed across all subjects and within the studied groups with the Spearman rank correlation coefficient.
All evaluations were performed with the SPSS statistical package (version 14; SPSS, Chicago, IL). A level of significance of P < 0.05 for comparative measurements was used throughout the study.
Results
Patient characteristics and clinical parameters of OA
Table 1 shows the baseline characteristics of all subjects. While sedentary and active controls did not show significant differences concerning age (p > 0.01), mild OA patients were significantly older than both control groups (p < 0.05). Weight and body mass indexes (BMI) were not different between the groups. No significant differences in WOMAC scores were found between active and sedentary controls (p > 0.05). Mild OA patients had significantly higher (p < 0.001) values in the WOMAC total score as well as in the WOMAC subscores than active controls. Compared to sedentary controls they had significantly higher (p < 0.05) WOMAC total scores and WOMAC functionality subscores.
T1rho relaxation time
Mild OA patients had significantly higher (p < 0.05) T1rho relaxation times than both control groups in the femoral compartment, particularly in the non-weight bearing parts of the lateral femur condyle, in the trochlea, in the patello-femoral compartment, and in the overall whole cartilage (Table 2).
Active controls presented with significantly lower (p < 0.05) values in the patella than mild OA patients. Inter-group comparison indicated that at this location also sedentary controls showed a tendency for lower T1rho values than mild OA patients. However, this observation was not significant when assessed with post-hoc comparison. No significant differences in T1rho relaxation time were found between active and sedentary controls (p > 0.05).
T2 relaxation time
Mild OA patients exhibited significantly higher (p < 0.05) T2 relaxation times in the weight-bearing parts and in the non-weight-bearing parts of the medial femur condyle than active and sedentary control subjects (Table 3). Therefore, they also had significantly higher (p < 0.05) T2 values in the medial compartment. Again no significant differences were found between active and sedentary controls.
Cartilage morphometry
Applying the analysis of variance, sedentary controls showed a trend (p = 0.035) for higher average cartilage volume (1.84 ± 0.42 cm3) in the medial tibia plateau than mild OA patients (1.43 ± 0.43 cm3) and active controls (1.41 ± 0.26 cm3). However, this did not remain significant in the post-hoc inter-group comparison. No differences in cartilage volume between the studied groups could be observed in the remaining compartments. The mean values of the whole knee cartilage were 17.29 ± 4.31 cm3 for mild OA patients, 18.87 ± 5.98 cm3 for sendentary controls, and 17.46 ± 2.92 cm3 for active controls.
Comparison of cartilage thickness did not reveal any significant inter-group differences: the average values for the whole knee were 1.73 ± 0.36 mm for sedentary controls, 1.70 ± 0.20 mm for active controls, and 1.65 ± 0.29 mm for mild OA patients. Combining active and sedentary subjects to one group of healthy subjects, there was an average cartilage volume of 17.95 ± 4.14 cm3 and an average cartilage thickness of 1.71 ± 0.25 mm in the whole knee.
Focal MRI pathology
Nine out of 13 subjects in the active control group and 2 out of 7 subjects in the sedentary control group had abnormal cartilage and/or bone marrow findings (Fig. 1). This resulted in a trend to higher WORMS score in the active controls compared to the sedentary controls, particularly at the patella [median value (25th percentile, 75th percentile): active controls: 1 (0, 1.5); sedentary controls: 0 (0, 1)]. However, due to the small patient numbers, these findings were not statistically significant.
Cartilage lesions (arrows) in two asymptomatic active subjects (female marathon runners, Tegner score of 6) on the sagittal IM-w FSE sequence (TR/TE: 4,300/51 ms). (a) shows a partial thickness focal defect at the trochlea, smaller than 1 cm in greatest width, resulting in a WORMS score of 2.0 in a 38-year-old asymptomatic woman. (b) demonstrates abnormal signal at the patella and a cartilage fissure in a 36-year-old asymptomatic woman. This lesion had a diameter of more than 1 cm in subsequent horizontal sections resulting in a WORMS score of 3.0
Combining active and sedentary subjects to a group of healthy subjects and comparing their WORMS scores to those of the mild OA patients, they had statistically significant lower (p < 0.05) values in the medial femur condyle [mild OA: 0 (0, 1.5); controls 0 (0, 0)], in the medial compartment [mild OA: 3 (1, 6); controls 0 (0, 0)], in the trochlea [mild OA: 1 (0, 5.5); controls 0 (0, 0)], in the patella [mild OA: 6 (2, 8.5); controls 0.5 (0, 1)], and in the whole knee [mild OA: 7 (4.5; 19); controls 0 (0, 2)].
Cartilage matrix parameters in relation to age, clinical parameters, and WORMS scores
The total T1rho relaxation times showed a positive correlation with age (r = 0.467, p < 0.01) across all examined subjects. Within the studied groups no significant correlation between age and the T1rho relaxation times were observed. T2 values were not correlated with age.
No significant correlations were found between T1rho/T2 values and cartilage volumes and thickness as well as with Tegner and WOMAC scores (p > 0.05). Total T1rho values were moderately positively correlated with the total WORMS scores across all subjects (r = 0.324, p < 0.05). No significant correlation between T1rho/T2 values and the WORMS score was found within the subjects groups.
Cartilage matrix parameters in active subjects with and without focal cartilage abnormalities
In an additional analysis, subjects in the active control group were differentiated according to presence or absence of focal cartilage abnormalities on dedicated morphological MRI sequences. T1rho and T2 values of active subjects without cartilage lesions (n = 4) were compared to those of subjects with cartilage lesions (n = 9) in this subgroup. Active controls with cartilage lesions demonstrated significantly higher (p < 0.05) T1rho values in the lateral femur condyle, in the trochlea, in the lateral tibia, in the patella and in the patello-femoral compartment, as well as in the whole knee compared to subjects without lesions (Figs. 2 and 3a). T2 values were significantly elevated (p < 0.05) only at the tibia (Fig. 3b).
Color-coded T1rho maps overlaid on SPGR images (TR/TE: 20/7.5 ms) from a central section of the patello-femoral cartilage. (a) demonstrates the T1rho map of an asymptomatic active (25-year-old male) subject without focal cartilage abnormalities anywhere in the knee. The average T1rho of the patello-femoral compartment was 33.7 ± 0.73 ms. (b) shows the T1rho map of an asymptomatic (36-year-old female) active subject at the central femoro-patellar joint who had a cartilage lesion at the lateral patella. The average T1rho of the patello-femoral compartment was 45.9 ± 1.68 ms. Interestingly, compared to subject (a), the T1rho values at the central patella and at the trochlea are globally increased, not only in the region of the defect, yet have a similar distribution
Comparison of (a) T1rho- and (b) T2 values among active healthy subjects without cartilage lesions (n = 4), active healthy subjects with cartilage lesions (n = 9), and sedentary healthy subjects (n = 13). “*” indicates a statistically significant inter-group difference (p < 0.05) between active healthy subjects with and active healthy subjects without cartilage lesions. Comp: compartment
T1rho values in active subjects with cartilage lesions were higher than in sedentary controls, while T1rho values in subjects without cartilage lesions were lower than those of sedentary controls in all examined compartments (Fig. 3a). However, these findings were not statistically significant.
T2 values were not statistically different between active subjects with/without cartilage lesions and sedentary subjects (Fig. 3b).
Discussion
In our study we sought to assess MRI-derived measurements of knee cartilage biochemical composition (T1rho and T2) and morphometry (volume and thickness) in physically active healthy subjects with a potentially increased risk of OA and compare the findings with those in sedentary healthy subjects and in patients with clinically and radiographically mild OA.
Patients with mild OA presented with elevated T1rho and T2 relaxation times in some compartments compared to both healthy control groups. This is in agreement with previous findings that reported higher T1rho [33–35, 49] and T2 [23, 26, 46] values in the cartilage of these kind of patients. In compartments with significant inter-group differences, the ratio of elevated values between OA patients and healthy subjects was comparable (T1rho: 1.06–1.16; T2: 1.08–1.16). However, significant inter-group differences existed in more compartments in T1rho values compared to T2 values (T1rho: femur, patella, patella-femoral compartment, non-weight-bearing parts of the lateral femur condyle, and in the whole knee; T2: medial femur condyles and in the medial compartment). Since all compartments in the OA patients except the tibia plateaus had visually impaired knee cartilage and the number of affected compartments regarding significantly higher inter-group differences was higher in T1rho values than in T2 values, we conclude that T1rho is more sensitive to cartilage degeneration than T2. This is concordant with Regatte et al. [24] who showed substantially elevated T1rho relaxation times of about 30–40% in specimens of early OA patients in the entire cartilage from the superficial region to the subchondral bone. T2 values were only increased about 5–8% in the same regions. This could be explained by the fact that T1rho is highly correlated with the PG content of cartilage [29] and loss of PG is an initiating event in early OA, while neither the content nor the type of collagen is altered in early OA [10]. Since the T2 relaxation time of the cartilage is a parameter that is mainly influenced by collagen content and orientation [50, 51], this lack of sensitivity could be due to the later onset of collagen breakdown during the course of the disease. Additionally, T2 relaxation times are affected by the orientation of the collagen [52], an effect that is not known for T1rho relaxation times. However, it remains unclear why T1rho and T2 values between patients and controls were not different in the medial tibia plateau since structural changes occur frequently at the medial compartment including both tibia and femur during the early course of the disease [53].
We observed a moderate relationship between T1rho and age. This is in agreement with previous findings [33] and suggests that the mean T1rho values exhibit similar changes as seen in studies on T2 relaxation times [54, 55]. Further efforts are necessary to elaborate the influence of senescent changes on cartilage proteoglycan and the influence of osteoarthritic changes on the T1rho values.
We did not find a significant difference in cartilage volume or thickness between the studied groups except a trend for reduced cartilage volume at the medial tibia plateau in mild OA patients compared to sedentary controls. Reviewing the literature, quantification of cartilage morphometry yields a variety of results: some studies have demonstrated that MRI of the knee cartilage is a sensitive way of quantifying cartilage loss in OA [8, 9, 53, 56–63], whereas other authors could not demonstrate a volume or thickness loss in any of the knee cartilage compartments within 1 year [46, 64]. This might be in part explained by the increase of water content and consequently swelling of the cartilage in the early stages of OA leading to an enlargement of the cartilage volume. These findings suggest that cartilage morphometry may lag behind biochemical and molecular changes, which can be measured quantitatively with T1rho and T2 values.
Physically active subjects had a high prevalence of focal cartilage abnormalities in particular at the femoro-patellar joint compartment, indicating a high mechanical burden of the knee in this group. T1rho and T2 relaxation times between active subjects and sedentary controls were not different; however, a significant difference in these parameters in active subjects with and without cartilage defects was found: similiar to the results observed between mild OA patients and healthy controls, the T1rho values in active subjects with lesions were elevated in more compartments than the T2 values and showed the same pattern by affecting mainly the patello-femoral complex. These findings could be secondary to increased exposure to chronic trauma with more advanced cartilage damage. We consider this as a further indication that T1rho is more sensitive than T2 to changes in the cartilage matrix composition.
On the other hand, average T1rho values in active subjects with defects were consistently higher than in sedentary controls, while average T1rho values in subjects without defects were consistently lower than those of sedentary controls. Since we included only healthy-appearing cartilage in our analysis, we conclude that these findings may indicate that higher T1rho values are associated with higher risk for development of morphological lesions and thus T1rho may assess cartilage “quality.” Differences in cartilage “quality” may be explained in part by genetic factors. It is also possible that during the process of focal cartilage lesion development, some substances are released into the synovial fluid that serve as mediators and initiate cartilage breakdown in areas that are not visually affected by OA. Further studies are clearly necessary to determine the incidence of OA in follow-up examinations of healthy subjects with high T1rho values and to compare it to those with low T1rho values.
A limitation of our study is the partitioning of our healthy subjects into an active and a sedentary group since it is dependent on the information given by the participants. To compensate for that we applied the Tegner score. It not only incorporates the kind of activity, but also considers how frequently this activity is performed. However, only a relatively small number of subjects was assigned to the sedentary group. In addition, even though the period of active or sedentary lifestyle was comparable between the groups, this information is highly subjective. Another limitation is that there was no true gold standard in the study; none of the study subjects underwent arthroscopy, which also could not be ethically justified in healthy subjects. However, we were not interested in examining the T1rho/T2 values of cartilage lesions themselves, but in those of the surrounding, “macroscopic” healthy cartilage. Since there is evidence that cartilage lesion detection with the cartilage dedicated sequences used in our study has a high sensitivity [65] and we excluded cartilage lesions during the postprocessing steps, we believe that we incorporated only cartilage areas with normal, healthy cartilage.
In conclusion, our findings suggest that active subjects with and without focal cartilage abnormalities have different T1rho compositions of cartilage. T1rho may therefore be a parameter suited to identify active healthy subjects at higher risk for developing cartilage degeneration and OA, but future studies will be required to address this issue. T1rho is well suited to differentiate healthy subjects and early OA patients and is more sensitive than T2 relaxations times, yet T1rho is also dependent on age. We believe that T1rho measurements may have the potential to be used in daily clinical routine as–in contrast to other MRI-based methods of determining cartilage matrix composition like dGEMRIC-they do not require special preparations such as application of contrast agents plus the total examination time is relatively short.
References
Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder GG, Liang MH, Pillemer SR, Steen VD, Wolfe F (1998) Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 41:778–799
Peyron JG (1984) The epidemiology of osteoarthritis. WB Sanders, Philadelphia, pp 9–27
Sharma L, Kapoor D, Issa S (2006) Epidemiology of osteoarthritis: an update. Curr Opin Rheumatol 18:147–156
Brandt KD, Mazzuca SA, Katz BP, Lane KA, Buckwalter KA, Yocum DE, Wolfe F, Schnitzer TJ, Moreland LW, Manzi S, Bradley JD, Sharma L, Oddis CV, Hugenberg ST, Heck LW (2005) Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum 52:2015–2025
Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O (2007) A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at 5 years. J Bone Joint Surg Am 89:2105–2112
Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, Li W, Hill C, Gale D (2003) Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med 139:330–336
Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, Majumdar S (2003) Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology 226:373–381
Bruyere O, Genant H, Kothari M, Zaim S, White D, Peterfy C, Burlet N, Richy F, Ethgen D, Montague T, Dabrowski C, Reginster JY (2007) Longitudinal study of magnetic resonance imaging and standard X-rays to assess disease progression in osteoarthritis. Osteoarthr Cartil 15(1):98–103, Epub 2006 Aug 4
Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Beaudoin G, Choquette D, Haraoui B, Tannenbaum H, Meyer JM, Beary JF, Cline GA, Pelletier JP (2006) Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: correlation with clinical symptoms and radiographic changes. Arthritis Res Ther 8:R21
Dijkgraaf LC, de Bont LG, Boering G, Liem RS (1995) The structure, biochemistry, and metabolism of osteoarthritic cartilage: a review of the literature. J Oral Maxillofac Surg 53:1182–1192
Lammentausta E, Kiviranta P, Nissi MJ, Laasanen MS, Kiviranta I, Nieminen MT, Jurvelin JS (2006) T2 relaxation time and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) of human patellar cartilage at 1.5 T and 9.4 T: relationships with tissue mechanical properties. J Orthop Res 24:366–374
Link TM, Sell CA, Masi JN, Phan C, Newitt D, Lu Y, Steinbach L, Majumdar S (2006) 3.0 vs 1.5 T MRI in the detection of focal cartilage pathology-ROC analysis in an experimental model. Osteoarthr Cartil 14:63–70
Bashir A, Gray ML, Hartke J, Burstein D (1999) Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med 41:857–865
Fragonas E, Mlynarik V, Jellus V, Micali F, Piras A, Toffanin R, Rizzo R, Vittur F (1998) Correlation between biochemical composition and magnetic resonance appearance of articular cartilage. Osteoarthr Cartil 6:24–32
Liess C, Lusse S, Karger N, Heller M, Gluer CC (2002) Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthr Cartil 10:907–913
Lusse S, Claassen H, Gehrke T, Hassenpflug J, Schunke M, Heller M, Gluer CC (2000) Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging 18:423–430
Mlynarik V, Trattnig S, Huber M, Zembsch A, Imhof H (1999) The role of relaxation times in monitoring proteoglycan depletion in articular cartilage. J Magn Reson Imaging 10:497–502
Menezes NM, Gray ML, Hartke JR, Burstein D (2004) T2 and T1rho MRI in articular cartilage systems. Magn Reson Med 51:503–509
Goodwin DW, Wadghiri YZ, Zhu H, Vinton CJ, Smith ED, Dunn JF (2004) Macroscopic structure of articular cartilage of the tibial plateau: influence of a characteristic matrix architecture on MRI appearance. AJR Am J Roentgenol 182:311–318
Goodwin DW, Zhu H, Dunn JF (2000) In vitro MR imaging of hyaline cartilage: correlation with scanning electron microscopy. AJR Am J Roentgenol 174:405–409
Xia Y, Moody JB, Burton-Wurster N, Lust G (2001) Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthr Cartil 9:393–406
Bachmann GF, Basad E, Rauber K, Damian MS, Rau WS (1999) Degenerative joint disease on MRI and physical activity: a clinical study of the knee joint in 320 patients. Eur Radiol 9:145–152
Blumenkrantz G, Lindsey CT, Dunn TC, Jin H, Ries MD, Link TM, Steinbach LS, Majumdar S (2004) A pilot, 2-year longitudinal study of the interrelationship between trabecular bone and articular cartilage in the osteoarthritic knee. Osteoarthr Cartil 12:997–1005
Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R (2006) T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging 23:547–553
David-Vaudey E, Ghosh S, Ries M, Majumdar S (2004) T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging 22:673–682
Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S (2004) T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology 232:592–598
Redfield AG (1969) Nuclear Spin Thermodynamics in the Rotating Frame. Science 164:1015–1023
Makela HI, Grohn OH, Kettunen MI, Kauppinen RA (2001) Proton exchange as a relaxation mechanism for T1 in the rotating frame in native and immobilized protein solutions. Biochem Biophys Res Commun 289:813–818
Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, Leigh JS, Reddy R (2001) Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med 46:419–423
Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS, Nugent AC, Johnson GA, Makela HI, Grohn OH, Kettunen MI, Kauppinen RA (1997) T1rho-relaxation in articular cartilage: effects of enzymatic degradation T1rho imaging using magnetization-prepared projection encoding (MaPPE) Proton exchange as a relaxation mechanism for T1 in the rotating frame in native and immobilized protein solutions. Magn Reson Med 38:863–867
Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R (2002) Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol 9:1388–1394
Wheaton AJ, Dodge GR, Elliott DM, Nicoll SB, Reddy R (2005) Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magn Reson Med 54:1087–1093
Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S (2007) In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthr Cartil 15:789–797
Li X, Han ET, Ma CB, Link TM, Newitt DC, Majumdar S (2005) In vivo 3T spiral imaging based multi-slice T(1rho) mapping of knee cartilage in osteoarthritis. Magn Reson Med 54:929–536
Regatte RR, Akella SV, Wheaton AJ, Lech G, Borthakur A, Kneeland JB, Reddy R (2004) 3D-T1rho-relaxation mapping of articular cartilage: in vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad Radiol 11:741–749
Buckwalter JA, Martin JA (2004) Sports and osteoarthritis. Curr Opin Rheumatol 16:634–639
Kaplan LD, Schurhoff MR, Selesnick H, Thorpe M, Uribe JW (2005) Magnetic resonance imaging of the knee in asymptomatic professional basketball players. Arthroscopy 21:557–561
Major NM, Helms CA (2002) MR imaging of the knee: findings in asymptomatic collegiate basketball players. AJR Am J Roentgenol 179:641–644
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M et al (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 29:1039–1049
Tegner Y, Lysholm J (1985) Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 43–49
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Krug R, Han ET, Banerjee S, Majumdar S (2006) Fully balanced steady-state 3D-spin-echo (bSSSE) imaging at 3 Tesla. Magn Reson Med 56:1033–1040
Oh J, Cha S, Aiken AH, Han ET, Crane JC, Stainsby JA, Wright GA, Dillon WP, Nelson SJ (2005) Quantitative apparent diffusion coefficients and T2 relaxation times in characterizing contrast enhancing brain tumors and regions of peritumoral edema. J Magn Reson Imaging 21:701–708
Carballido-Gamio J, Bauer JS, Keh-Yang L, Krause S, Majumdar S. Combined Image Processing Techniques for Characterization of MRI Cartilage of the Knee. In: IEEE-EMBS. 2005. Shanghai
Stahl R, Blumenkrantz G, Carballido-Gamio J, Zhao S, Munoz T, Hellio Le Graverand-Gastineau MP, Li X, Majumdar S, Link TM (2007) MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthr Cartil 15(11):1225–1234, Epub 2007 Jun 11
Faber SC, Eckstein F, Lukasz S, Muhlbauer R, Hohe J, Englmeier KH, Reiser M (2001) Gender differences in knee joint cartilage thickness, volume and articular surface areas: assessment with quantitative three-dimensional MR imaging. Skeletal Radiol 30:144–150
Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK (2004) Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartil 12:177–190
Duvvuri U, Charagundla SR, Kudchodkar SB, Kaufman JH, Kneeland JB, Rizi R, Leigh JS, Reddy R (2001) Human knee: in vivo T1(rho)-weighted MR imaging at 1.5 T-preliminary experience. Radiology 220:822–826
Nissi MJ, Rieppo J, Toyras J, Laasanen MS, Kiviranta I, Jurvelin JS, Nieminen MT (2006) T(2) relaxation time mapping reveals age- and species-related diversity of collagen network architecture in articular cartilage. Osteoarthr Cartil 14(12):1265–1271, Epub 2006 Jul 14
Nissi MJ, Toyras J, Laasanen MS, Rieppo J, Saarakkala S, Lappalainen R, Jurvelin JS, Nieminen MT (2004) Proteoglycan and collagen sensitive MRI evaluation of normal and degenerated articular cartilage. J Orthop Res 22:557–564
Akella SV, Regatte RR, Wheaton AJ, Borthakur A, Reddy R (2004) Reduction of residual dipolar interaction in cartilage by spin-lock technique. Magn Reson Med 52:1103–1109
Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Labonte F, Beaudoin G, de Guise JA, Bloch DA, Choquette D, Haraoui B, Altman RD, Hochberg MC, Meyer JM, Cline GA, Pelletier JP (2004) Quantitative magnetic resonance imaging evaluation of knee osteoarthritis progression over two years and correlation with clinical symptoms and radiologic changes. Arthritis Rheum 50:476–487
Mosher TJ, Dardzinski BJ, Smith MB (2000) Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2-preliminary findings at 3 T. Radiology 214:259–266
Mosher TJ, Liu Y, Yang QX, Yao J, Smith R, Dardzinski BJ, Smith MB (2004) Age dependency of cartilage magnetic resonance imaging T2 relaxation times in asymptomatic women. Arthritis Rheum 50:2820–2828
Baysal O, Baysal T, Alkan A, Altay Z, Yologlu S (2004) Comparison of MRI graded cartilage and MRI based volume measurement in knee osteoarthritis. Swiss Med Wkly 134:283–288
Cicuttini F, Hankin J, Jones G, Wluka A (2005) Comparison of conventional standing knee radiographs and magnetic resonance imaging in assessing progression of tibiofemoral joint osteoarthritis. Osteoarthr Cartil 13:722–727
Cicuttini F, Wluka A, Hankin J, Wang Y (2004) Longitudinal study of the relationship between knee angle and tibiofemoral cartilage volume in subjects with knee osteoarthritis. Rheumatology (Oxford) 43:321–324
Cicuttini FM, Wluka AE, Wang Y, Stuckey SL (2004) Longitudinal study of changes in tibial and femoral cartilage in knee osteoarthritis. Arthritis Rheum 50:94–97
Ding C, Cicuttini F, Scott F, Boon C, Jones G (2005) Association of prevalent and incident knee cartilage defects with loss of tibial and patellar cartilage: a longitudinal study. Arthritis Rheum 52:3918–3927
Wluka AE, Forbes A, Wang Y, Hanna F, Jones G, Cicuttini FM (2006) Knee cartilage loss in symptomatic knee osteoarthritis over 4.5 years. Arthritis Res Ther 8:R90
Wluka AE, Stuckey S, Snaddon J, Cicuttini FM (2002) The determinants of change in tibial cartilage volume in osteoarthritic knees. Arthritis Rheum 46:2065–2072
Wluka AE, Wolfe R, Stuckey S, Cicuttini FM (2004) How does tibial cartilage volume relate to symptoms in subjects with knee osteoarthritis? Ann Rheum Dis 63:264–268
Gandy SJ, Dieppe PA, Keen MC, Maciewicz RA, Watt I, Waterton JC (2002) No loss of cartilage volume over three years in patients with knee osteoarthritis as assessed by magnetic resonance imaging. Osteoarthr Cartil 10:929–937
Yoshioka H, Stevens K, Hargreaves BA, Steines D, Genovese M, Dillingham MF, Winalski CS, Lang P (2004) Magnetic resonance imaging of articular cartilage of the knee: comparison between fat-suppressed three-dimensional SPGR imaging, fat-suppressed FSE imaging, and fat-suppressed three-dimensional DEFT imaging, and correlation with arthroscopy. J Magn Reson Imaging 20:857–864
Acknowledgments
This work was supported by the Research Evaluation and Allocation Committee (REAC) of the University of California, San Francisco, CA, USA, through the Clough G. Memorial Endowment Fund and by Glaxo Smith Kline (GSK) Inc., Research and Development, London, UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stahl, R., Luke, A., Li, X. et al. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients—a 3.0-Tesla MRI study. Eur Radiol 19, 132–143 (2009). https://doi.org/10.1007/s00330-008-1107-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-1107-6