Abstract
This study was designed to define the current role of multislice spiral computed tomography (MSCT) for the diagnosis of coronary in-stent restenosis using a meta-analytic process. Restenosis remains a limitation after coronary stent implantation and contributes to a substantial number of coronary re-assessments by conventional invasive coronary angiography (CA). We identified 15 studies (807 patients) evaluating in-stent restenosis by means of both MSCT (≥16 slices) and conventional CA until February 2007. After data extraction the analysis was performed according to a random-effects model. The analysis pooled the results from 15 studies with a total of 1,175 stents. A substantial number of unassessable stents (13%) were excluded from the analysis underscoring the shortcomings of MSCT. With this major limitation the diagnostic performance of MSCT for in-stent restenosis detection can be summarized as follows: the sensitivity and specificity were 84% [95% confidence interval (CI) 77–89%] and 91% (95% CI 89–93%), respectively, with positive and negative likelihood ratios of 12.2 (95% CI 6.6–22.6) and 0.23 (95% CI 0.17–0.31), respectively, and with a diagnostic odds ratio of 67.9 (95% CI 34.4–134.1). MSCT has shortcomings difficult to overcome in daily practice for in-stent restenosis detection and continues to have moderately high sensitivity and specificity. The diagnostic role of this emerging technology as an alternative to CA for in-stent restenosis detection remains limited.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary artery disease requiring revascularization is increasingly treated by percutaneous coronary intervention (PCI) due to the successful introduction of coronary stent implantation [1]. Indeed, by reducing acute major complications and the incidence of restenosis compared with conventional balloon angioplasty, stents are used in almost all PCIs currently [2]. Following the recent introduction of drug eluting stents associated with even further reduction in the occurence of in-stent restenosis, we can anticipate a substantial increase in the number of patients considered for PCIs and with subsequent stent implantation in the future. However, in-stent thrombosis and excessive neo-intimal hyperplasia may still occur causing partial or complete in-stent obstruction [3, 4]. Whereas the clinical diagnosis of acute in-stent thrombosis is frequently easy, that of in-stent restenosis remains sometimes more difficult and needs invasive diagnosis by means of conventional coronary angiography. Because a substantial number of these invasive coronary angiograms are not followed by intervention, multislice spiral computed tomography (MSCT) of coronary arteries has been thought to be able to play a role as a non-invasive tool to exclude in-stent restenosis and thus act as a gatekeeper before considering conventional invasive diagnostic procedures [5, 6]. Using a meta-analytic process, we have conducted a systematic review of all studies comparing MSCT and conventional invasive coronary angiography for the diagnosis of in-stent restenosis, in order to define the current diagnostic performance of MSCT in this setting.
Materials and methods
Search strategy
Database searches for English-language articles published until November 2006 were performed in MEDLINE, Cochrane librabry and BioMed Central databases. We combined the medical subject headings for computed tomography, multislice computed tomography (MSCT), and coronary angiography, with the exploded terms stent and restenosis and scanned references in retrieved articles and reviews. The retrieved studies were carefully examined to exclude potentially duplicate or overlapping data. Meetings abstracts were excluded, as they could not provide adequately detailed data and their results might not be final. Only papers evaluating the presence of in-stent restenosis by both conventional invasive coronary angiography (CA) and MSCT in the same subjects were included.
Study eligibility
We included a study if (1) it used MSCT as a diagnostic test for in-stent restenosis, with >50% diameter stenosis selected as the cut-off criterion for significant restenosis, using conventional invasive angiography and quantitative coronary angiography as the reference standard; (2) it used the latest-generation of MSCT (≥16 slices); (3) and it reported cases in absolute numbers of true positive (TP), false positive (FP), true negative (TN), and false negative (FN) results or presented sufficiently detailed data for deriving these figures. Studies were excluded if they were performed (1) only in patients after coronary artery bypass graft surgery, (2) in a subset of patients with prior heart transplant.
Data extraction
The following information was extracted from each study: first author, year of publication, and journal; study population characteristics, including sample size (number of subjects evaluated with both tests, number of patients excluded); number of stents evaluated and excluded from the analysis; gender; mean age (and standard deviation); mean heart rate (and standard deviation); relative timing of the two imaging procedures and whether or not evaluation of one test was blind to the result of the other; technical characteristics of the MSCT, including type and brand of machine used; and rate of beta-blocker usage. Two investigators performed the data extraction independently. Discrepancies were solved by a third investigator and global consensus. The study quality conformed to the QUADAS guidelines [7, 8].
Data synthesis and statistical analysis
Categorical variables from individual studies are presented as n/N(%) and continuous variables are presented as mean with (standard deviation) SD. Measures of diagnostic accuracy are reported as point estimates [with 95% confidence intervals (CIs)]. By means of TP, TN, FP, and FN rates, we computed sensitivity, specificity, positive and negative likelihood ratios and diagnostic odds ratios as previously described [9]. We computed all statistics for individual studies, and then combined them using a random-effects model. Between-study statistical heterogeneity was also assessed using the Cochran Q chi-square tests. Weighted symmetric summary receiver operating characteristic plots, with pertinent areas under the curve, were computed using the Moses-Shapiro-Littenberg method [10, 11].
Sources of clinical and statistical heterogeneity were explored by means of subgroup analyses and meta-regression [12]. Specifically, we performed stratified analyses according to publication year, sample size, number of interpretable stents, and 16 vs 64 slices. Statistical computations were performed with SPSS 13.0 (SPSS, Chicago, Ill.) and Meta-DiSc [13], and significance testing was at the two-tailed 0.05 level.
Results
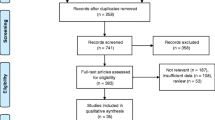
The reviewing process is described in Fig. 1. Database searches identified 199 potentially relevant citations. After title/abstract assessment, we retrieved 35 studies as complete reports, from which 20 were excluded because (1) they did not employ MSCT or ≥16 slices MSCT; (2) they looked only at grafts or at atherosclerotic plaque assessment; (3) they had overlapping data; (4) they were in a language other than English; (5) it was impossible to find or calculate absolute figures from presented data; overlapping or duplicated publication was obvious; or (6) no systematic angiographic control was performed. Thus, we included 15 of these studies in the systematic review [14–28]. All studies were published between January 2004 and February 2007, or were in press, or ahead of publication and then available on a dedicated journal website in February 2007. Table 1 presents demographic data and details on included studies.
In-stent restenosis meta-analysis
As shown in Figs. 2, 3, 4, 5 and 6, the analysis pooled the results from 15 studies with 807 patients, corresponding to 1,175 stents and showed after exclusion of the analysis of 13% of unassessable stents that, in comparison with invasive coronary angiography, MSCT for in-stent restenosis detection had a sensitivity of 84% (95% CI 77–89%), a specificity of 91% (95% CI 89–93%), a positive likelihood ratio of 12.2 (95% CI 6.6–22.6), a negative likelihood ratio of 0.23 (95% CI 0.17–0.31), and a diagnostic odds ratio of 67.9 (95% CI 34.4–134.1). The summary receiver operator characteristic curves are shown in Fig. 7. Statistical heterogeneity was found for specificity (P < 0.001) and positive likelihood ratio (P < 0.001).
Plot of symmetric summary receiver operator characteristic of MSCT-CA in comparison with CA for in-stent restenosis detection. The receiver operator characteristic curve provides a graphical display of diagnostic accuracy, by plotting 1-specificity on the horizontal axis and sensitivity on the vertical axis. The pertinent area under the curve (AUC) and Q* statistic (the point where sensitivity and specificity are maximal), both with standard errors (SE), are also included
Additional analyses
We explored sources of clinical and statistical heterogeneity by performing subgroup analysis for the number of slices in each CT scan. While a 64-slice CT scan should be more accurate than a 16-slice one, we did not find significant results by interaction testing. Specifically, for 16-slice CT scans, we found a sensitivity of 82% (95% CI 72–89%), a specificity of 92% (95% CI 88–94%), a 16.1 (95% CI 5.1–50.6) positive likelihood ratio, a 0.25 (95% CI 0.16–0.37) negative likelihood ratio, and a 69.9 (95% CI 30.3–161.4) diagnostic odds ratio. For >16-slice CT, we found a sensitivity of 85% (95% CI 76–92%), a specificity of 91% (95% CI 88–94%), a 10 (95% CI 5.5–18.2) positive likelihood ratio, a 0.20 (95% CI 0.11–0.33) negative likelihood ratio, and a 67.7 (95% CI 21.2–215.8) diagnostic odds ratio.
Finally, we performed meta-regression analyses exploring the impact of sample size and publication year on the diagnostic performance of MSCT. While the latter did not disclose significant results, we found a significant interaction between changes in sample size and diagnostic odds ratios in the individual studies (P < 0.04).
Pooled summary estimates are given in Table 2. Quality assessment for all included studies is shown in Table 3.
Discussion
In the present study, we focused on the diagnostic performance of the newest generation of MSCT (≥16 slices) for the detection of in-stent restenosis. First of all, the rate of scanned stents judged unassessable by the investigators was very high 13% (ranging from 0% to 45.6%) and constitutes a major limitation of these analyses, which is important to keep in mind while interpreting the relatively high specificity (91%) and sensitivity (84%) of MSCT for in-stent restenosis detection presented in our pooled estimates. Furthermore, these figures were provided in highly selected patients, favoring again the positive perception of MSCT as a valuable diagnostic tool. These results are in keeping with recent recommendations that patients with previous coronary stenting should not routinely undergo CT coronary angiography, to avoid unjustified radiation exposure [29].
Overall subtle in-stent hyperplasia quantification remains impossible and only qualitative assessment of coronary stents is feasible [6, 19]. It has been suggested that MSCT could reduce the need for invasive diagnostic procedures by non-invasively excluding in-stent restenosis [14–28]. The present meta-analysis identifies that this optimistic view could be envisioned only in highly selected patients and mainly in patients with proximal and large stents, as frequently commented on in the individual studies [22, 23]. Indeed small stent diameter has been identified by several groups as a major factor for failure of in-stent restenosis assessment, with a consensus that only stents with diameter >3 mm are routinely interpretable [6, 14–28]. However, in routine clinical settings, many patients are treated with relatively small stents, having diameters of 2.5 mm or 3.0 mm. Therefore, further improvement in spatial resolution and temporal resolution is required for MSCT to become a realistic routine diagnostic procedure for the evaluation of in-stent restenosis [30].
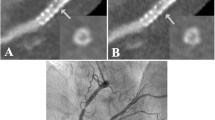
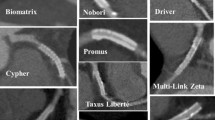
Imaging coronary stents using CT is a technically more demanding task than imaging native coronary arteries. The degree of metal artifacts of stents, including partial volume effects is related to stent material, the size of the diameter, the thickness and strut design [6, 31–34]. The metal artifact of tantalum is so severe in comparison with other materials that it is considered to be impossible to assess the lumens of the stents made of tantalum by MSCT. The gold markers of some stents also cause severe artifacts, making the lumen at the edges of these stents difficult to evaluate. Other patient factors that might limit proper assessment of stent lumen as well as the native coronary artery lumen include cardiac motion artifacts, more frequent in the second segment of RCA, and severe coronary calcification [5, 9].
In-stent restenosis remains a limitation for the long-term efficacy of coronary stenting in complex lesions like bifurcation or left main stenting. In case of left main restenosis patient outcome is threatened. Because in-stent restenosis is often asymptomatic, its detection with non-invasive technology, especially when a large amount of myocardium is concerned, is critical and clinically relevant. Proximal or left main in-stent restenosis is easier to assess given the larger diameter of the stents implanted in these coronary segments. Recently, dedicated MSCT studies for in-stent restenosis assessment have suggested that, using the latest generation MSCT, these patients may be suitable for non-invasive angiographic follow-up [26].
Study limitations
As mentioned previously, substantial statistical heterogeneity has been documented, casting caution on the results and interpretation of the estimates of comprehensive, pooled effects, although the use of the random-effects model should still provide relatively robust results. The well-known tendency towards publication bias favoring studies with positive and encouraging results also complicates comprehensive evaluation. In this meta-analysis, data abstraction and quality assessment were done by independent reviewers and, in the case of any divergences, resolution was made by consensus. Thus, the inter-operator agreement could not be quantitatively assessed. We should also acknowledge that not all reports provided details regarding technically important issues like kernel convolution filter use, which has been shown to substantially enhance the stent lumen depiction [6, 31, 34].
Conclusions
In highly selected patients with proximal large stents, the use of the newest MSCT with 64 slices provide adequate diagnostic performance. However, the use of MSCT for the detection of in-stent restenosis has shortcomings difficult to overcome in daily practice and the diagnostic accuracy remains moderate. In the future, the detection of in-stent restenosis might be possible with clinically useful accuracy, but the currently high rate of unevaluable stents does not permit the recommendation of MSCT for stent assessment in unselected patients.
References
Serruys PW, Kutryk MJ, Ong AT (2006) Coronary-artery stents. N Engl J Med 354:483–495
Agostoni P, Valgimigli M, Biondi-Zoccai GG et al (2006) Clinical effectiveness of bare-metal stenting compared with balloon angioplasty in total occlusions: insights from a systematic overview of randomized trials in light of the drug-eluting stent era. Am Heart J 151:682–689
McFadden EP, Stabile E, Regar E et al (2004) Late thrombosis in drug-eluting coronary stents after discontinuation on antiplatelet therapy. Lancet 364:1519–21
Valgimigli M, Malagutti P, van Mieghem CA, Vaina S, Lightart JM, Sianos G, serruys PW (2006) Persistent of neointimal growth 12 months after intervention and occurence of delayed restenosis in patients with left main coronary artery disease treated with drug-eluting stents. J Am Coll Cardiol 47:1491–1494
Achenbach S (2006) Computed tomography coronary angiography. J Am Coll Cardiol 48:1919–1928
Pugliese F, Cademartiri F, van Mieghem C, Meijboom WB, Malagutti P, Mollet NRA, Martinoli C, de Feyter PJ, Krestin GP (2006) Multidetector CT for visualization of coronary stents. Radiographics 26:887–904
Whiting P, Harbord R, Kleijnen J (2005) No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Med Res Methodol 5:19
Deville WL, Buntinx F, Bouter LM et al (2002) Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2:9
Hamon M, Biondi-Zoccai GG, Malagutti P et al (2006) Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography. J Am Coll Cardiol 48:1896–1910
Moses LE, Shapiro D, Littenberg B (1993) Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 12:1293–1316
Walter SD (2002) Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med 21:1237–1256
Lijmer JG, Bossuyt PM, Heisterkamp SH (2002) Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med 21:1525–1537
Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A (2006) Meta-Disc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 6:31
Schuijf JD, Bax JJ, Jukema JW, Lamb HJ, Warda HMA, Vliegen HW, de Roos A, van der Wall EE (2004) Feasibility of assessment of coronary stent patency using 16-slice computed tomography. Am J Cardiol 94:427–430
Cademartiri F, Marano R, Runza G, Mollet N, Nieman K, Luccichenti G, Gualerzi M, Brambilla L, Coruzzi P, Galia M, Midiri M (2005) Non-invasive assessment of coronary artery stent patency with multislice CT: preliminary experience. Radiol Med 109:500–507
Kitagawa T, Fujii T, Tomohiro Y, Maeda K, Kobayashi M, Kunita E, Sekiguchi Y (2006) Noninvasive assessment of coronary stents in patients by 16-slice computed tomography. Int J Cardiol 109:188–194
Gilard M, Cornily JC, Rioufol G, Finet G, Pennec PY, Mansourati J, Blanc JJ, Boschat J (2005) Noninvasive assessement of left main coronary stent patency with 16-slice computed tomography. Am J Cardiol 95:110–112
Kefer JM, Coche E, Vanoverschelde JL, Gerber BL (2007) Diagnostic accuracy of 16-slice multidetector-row CT for detection of in-stent restenosis vs detection of stenosis in nonstented coronary arteries. Eur Radiol 17:87–96
Chabbert V, Carrie D, Bennaceur M, Maupas E, Lauwers V, Mhem M, Lhermusier T, Elbaz M, Joffre F, Rousseau H, Puel J (2007) Evaluation of in-stent restenosis in proximal coronary arteries with multidetector computed tomography (MDCT). Eur Radiol 17:1452–1463
Watanabe M, Uemura S, Iwama H, Okayama S, Takeda Y, Kawata H, Horii M, Nakajima T, Hirohashi S, Kichikawa K, Ookura A, Saito Y (2006) Usefulness of 16-slice multislice spiral computed tomography for follow-up study of coronary stent implantation. Circ J 70:691–697
Ohnuki K, Yoshida S, Ohta M, Shimizu M, Mochizuki S, Nishioka M, Sakuma T, Fukuda K, Ishizaki M, Hirakawa E, Andou T (2006) New diagnostic technique in multi-slice computed tomography for in-stent restenosis: pixel count method. Int J Cardiol 108:251–258
Gilard M, Cornily JC, Pennec PY, Le Gal G, Nonent M, Mansourati J, Blanc JJ, Boschat J (2006) Assessment of coronary artrey stents by 16 slice computed tomography. Heart 92:58–61
Gaspar T, Halon DA, Lewis BS, Adawi S, Schliamser JE, Rubinstein R, Flugelman MY, Peled N (2005) Diagnosis of coronary in-stent restenosis with multidetector row spiral computed tomography. J Am Coll Cardiol 46:1573–1579
Van Mieghem C, Cademartiri F, Mollet NR et al (2006) Multislice spiral computed tomography for the evaluation of stent patency after left main coronary artery stenting. A comparison with conventional coronary angiography and intravascular ultrasound. Circulation 114: 645–653
Rixe J, Achenbach S, Ropers D, Baum U, Kuettner A, Ropers U, Bautz W, Daniel WG, Anders K (2006) Assessment of coronary artery stent restenosis by 64-slice multi-detector computed tomography. Eur Heart J 27:2567–2572
Rist C, von Ziegler F, Nikolaou K, Kirchin MA, Wintersperger BJ, Johnson TR, Knez A, Leber AW, Reiser MF, Becker CR (2006) Assessment of coronary artery stent patency using 64-slice computed tomography. Acad radiol 13:1465–1473
Ehara M, Kawai M, Surmely J-F et al (2007) Diagnostic accuracy of coronary in-stent restenosis using 64-slice computed tomography. Comparison with invasive coronary angiography. J Am Coll Cardiol 49:951–959
Oncel D, Oncel G, Karaca M (2007) Coronary stent patency and in-stent restenosis: determination with 64-section multidetector CT coronary angiography. Initial experience. Radiology 242:403–409
Budoff MJ, Achenbach S, Blumenthal RS et al (2006) Assessment of coronary artery disease by cardiac computed tomography. A scientific statement on cardiovascular imaging and intervention, council on cardiovascular radiology and intervention, and committee on cardiac imaging, council on clinical cardiology. J Am Coll Cardiol 114:1761–1791
Groen JM, Greuter MJ, van Ooijen PM, Oudkerk M (2007) A new approach to the assessment of lumen visibility of coronary artery stent at various heart rates using 64-slice MDCT. Eur Radiol 17:1879–1884
Schepis T, Koepfli P, Leschka S, Desbiolles L, Husmann L, Gaemperli O, Eberli FR, Wildermuth S, Marincek B, Luscher TF, Alkadhi H, Kaufmann PA (2007) Coronary artery stent geometry and in-stent contrast attenuation with 64-slice computed tomography. Eur Radiol 17:1464–1473
Utsunomiya D, Awai K, Sakamoto T, Hazeyama H, Nishiharu T, Urata J, Yamashita Y (2006) In vitro evaluation of metallic coronary artery stents with sub-millimeter multi-slice computed tomography using an ecg-gated cardiac phantom: relationship between in-stent visualization and stent type. Cardiology 107:254–260
Mahnken AH, Buecker A, Wildberger JE, Ruebben A, Stanzel S, Vogt F, Gunther RW, Blindt R (2004) Coronary artery stents in multislice computed tomography: in vitro artifact evaluation. Invest Radiol 39:27–33
Maintz D, Seifarth H, Raupach R, Flohr T, Rink M, Sommer T, Ozgun M, Heindel W, Fischbach R (2006) 64-slice multidetector coronary CT angiography: in vitro evaluation of 68 different stents. Eur Radiol 16:818–826
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamon, M., Champ-Rigot, L., Morello, R. et al. Diagnostic accuracy of in-stent coronary restenosis detection with multislice spiral computed tomography: a meta-analysis. Eur Radiol 18, 217–225 (2008). https://doi.org/10.1007/s00330-007-0743-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-007-0743-6